Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Titrable Acidity and Total Soluble Solids
2.3. Grape Skin Extracts
2.4. Sequential Extraction of Cell Wall Material
2.5. Quantification of Total Phenolic Compounds
2.6. Total Flavonoid Content
2.7. Anthocyanin’s Identification and Separation
2.8. Total Tannins Quantification
2.9. Data Analysis
3. Results
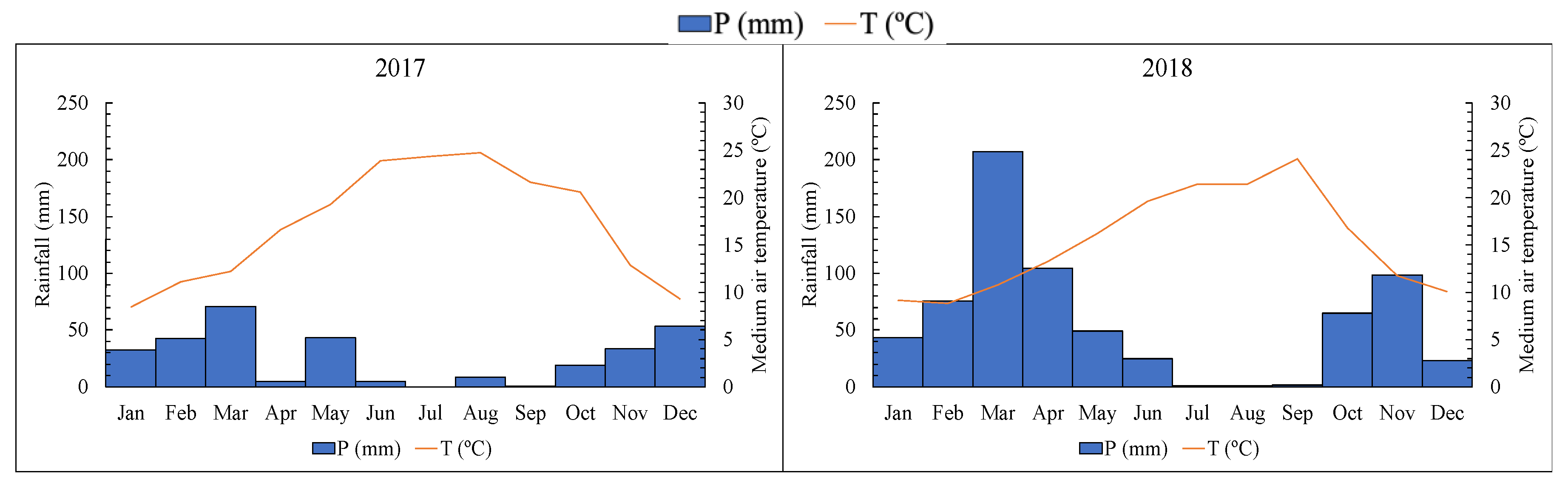
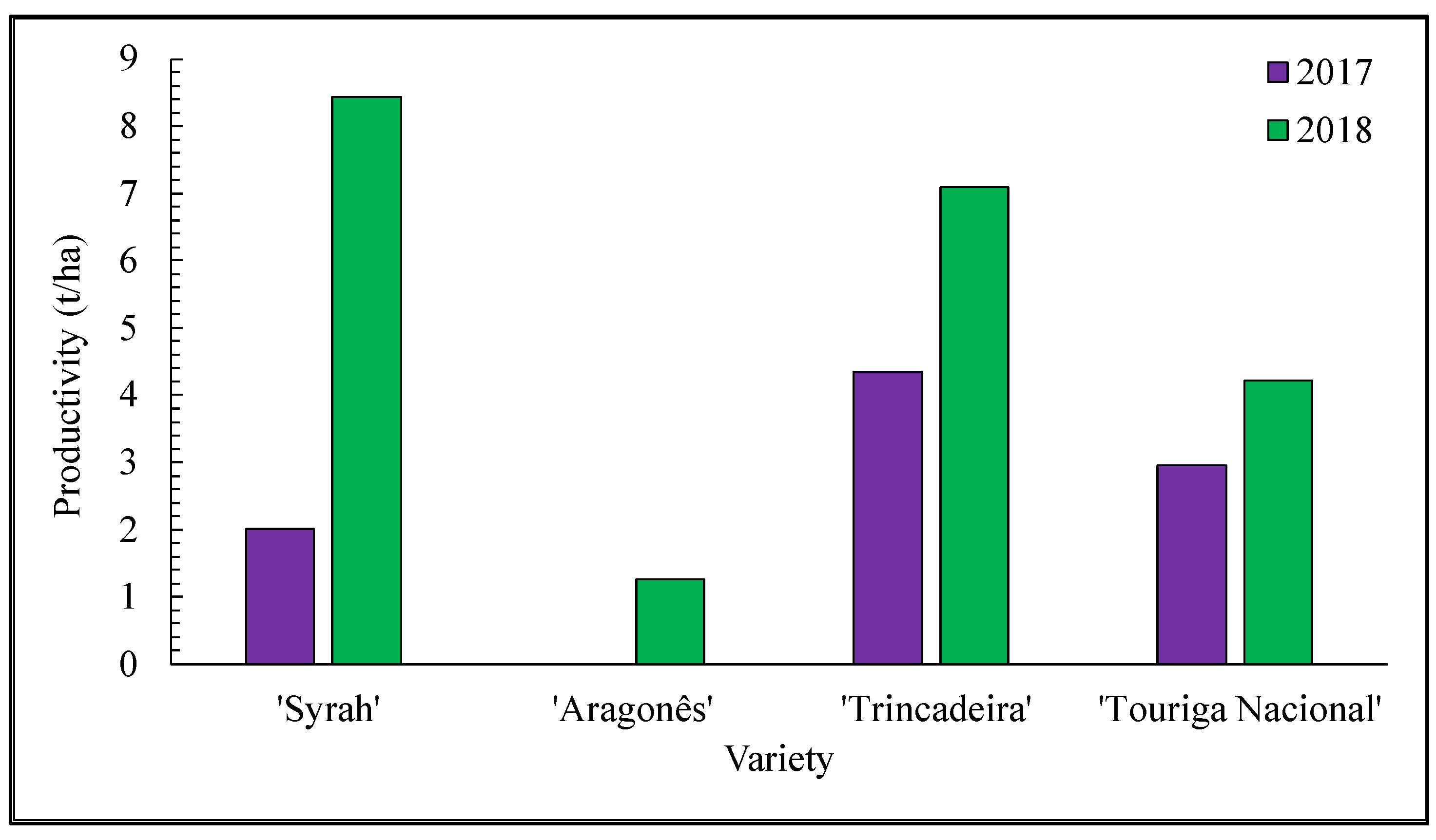
3.1. Influence of Climate in the Quality of Grape Berries
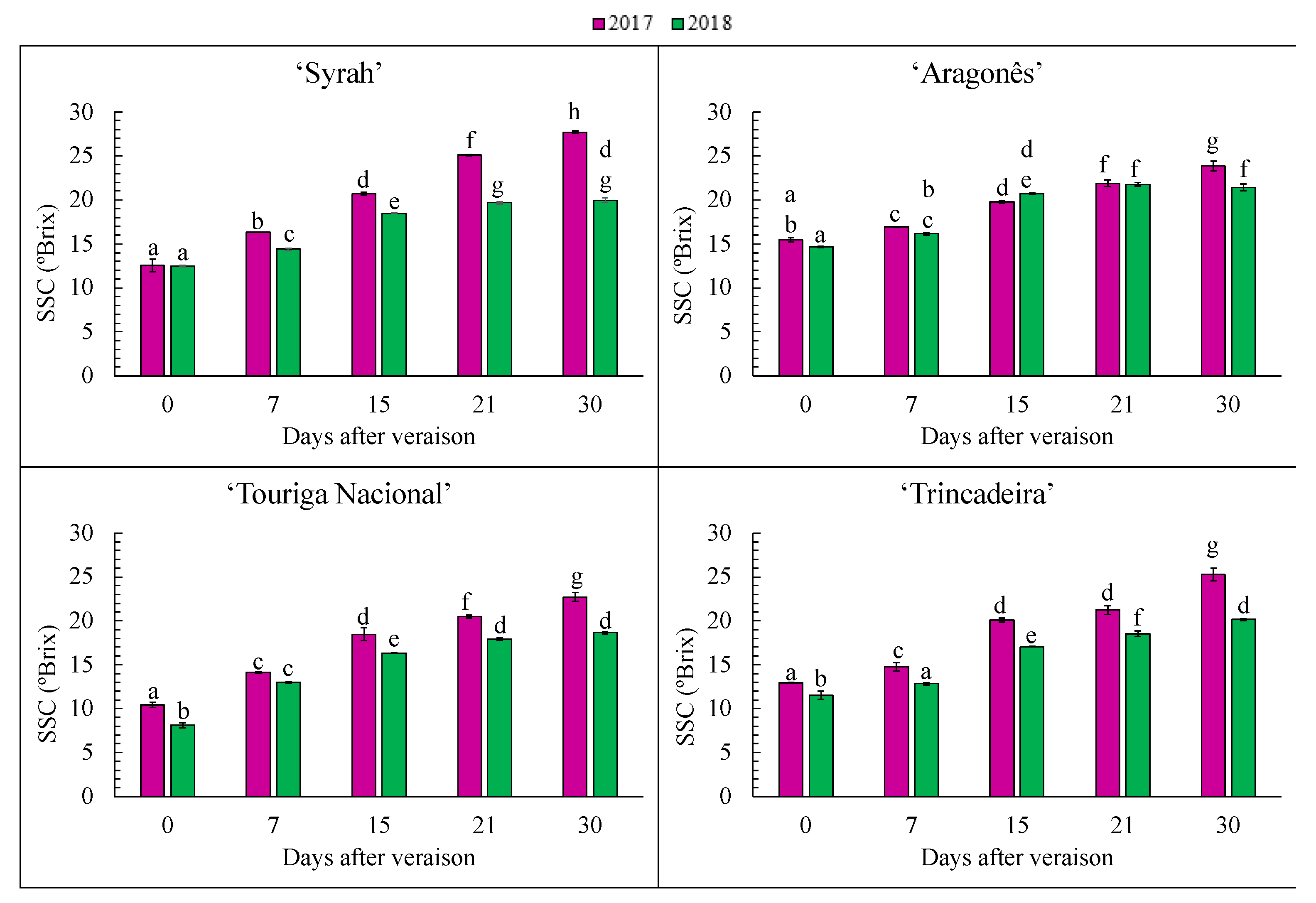
3.2. Evaluation of Acidity and Total Soluble Solids
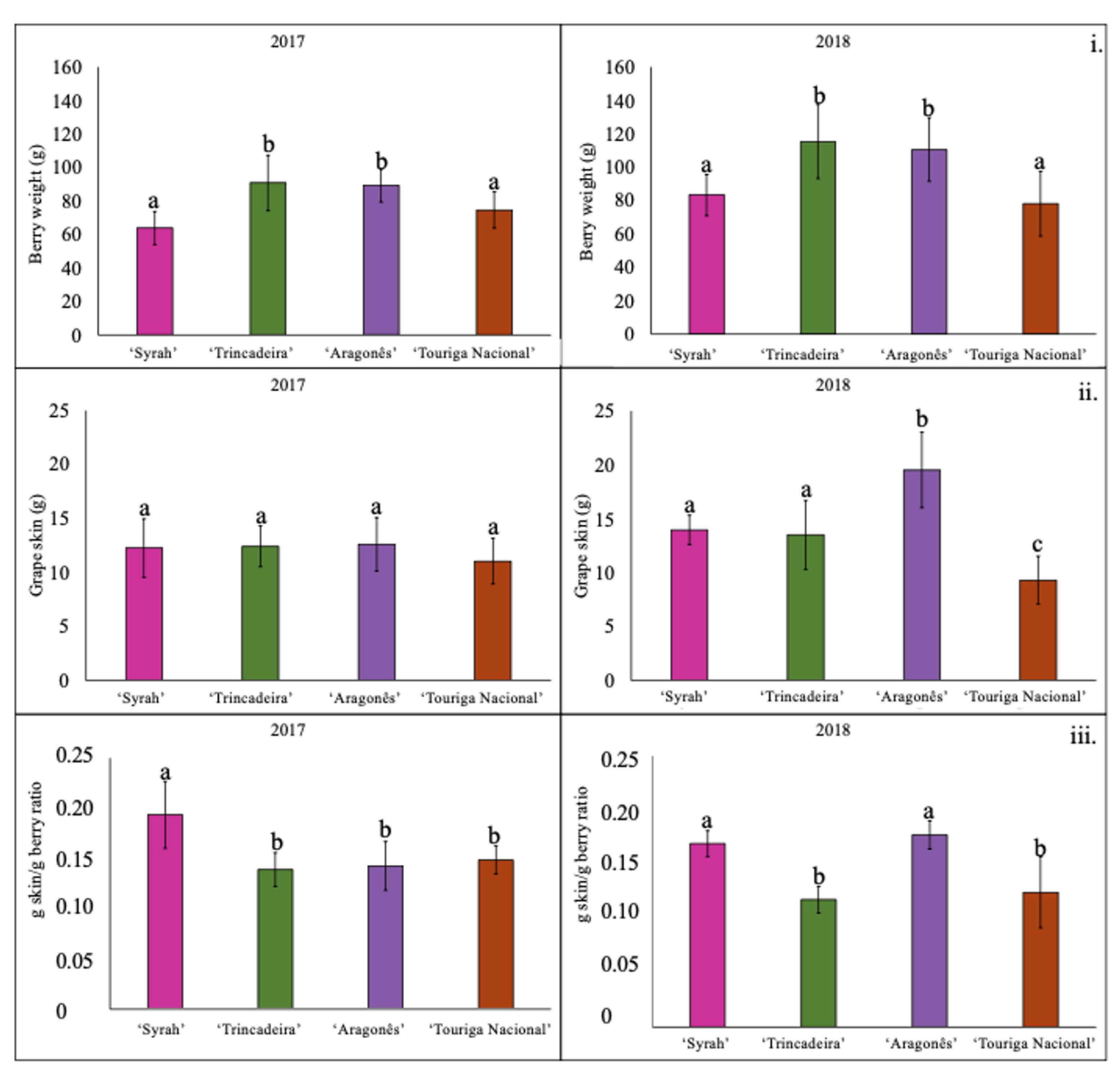
3.3. Grape Berry Composition
3.4. Skin Cell Wall
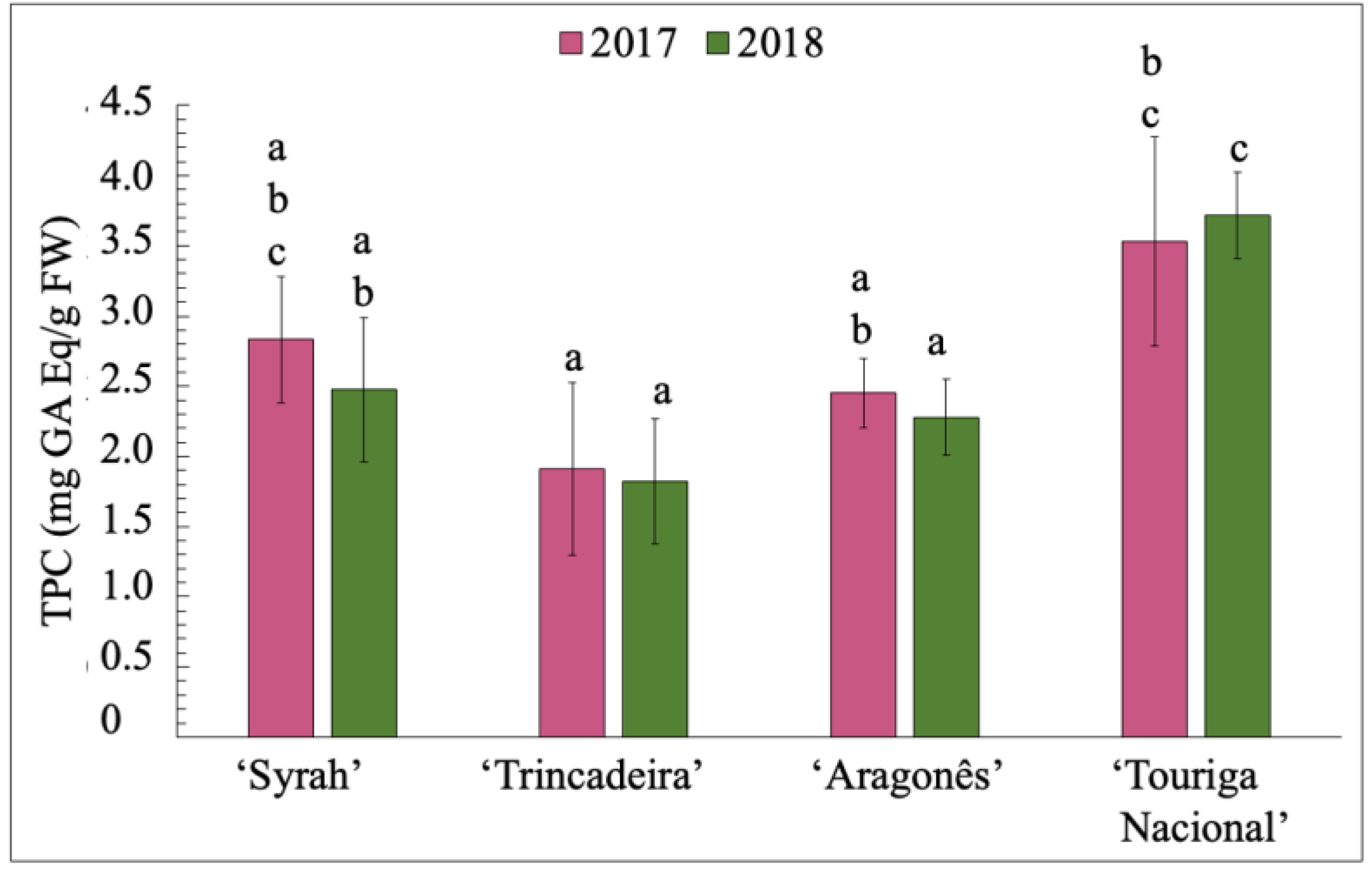
3.5. Total Phenolic Content
3.6. Total Flavonoids
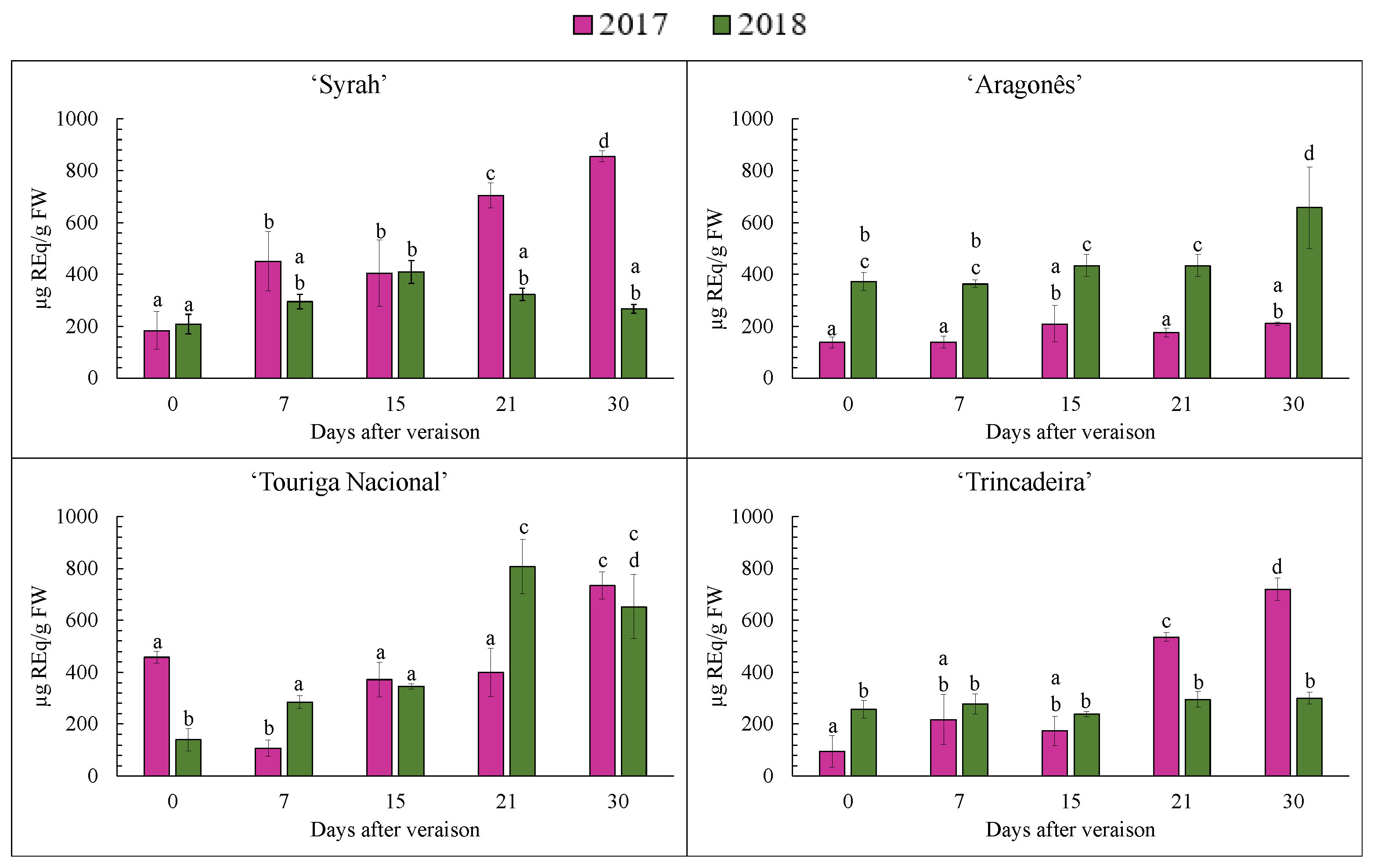
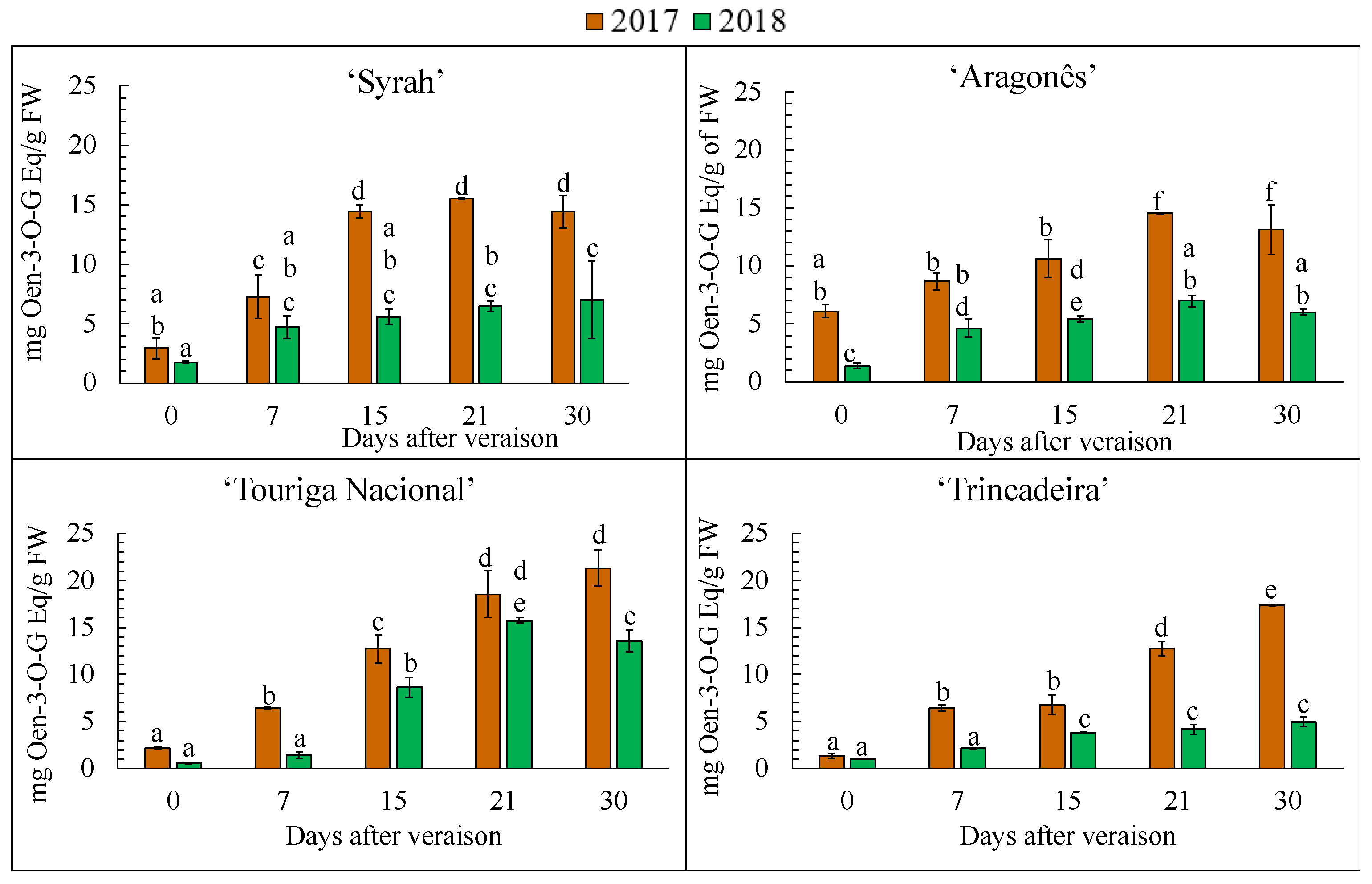
3.7. Anthocyanin Separation, Identification and Quantification
3.8. Total Tannins
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Rouxinol, M.I.; Martins, M.R.; Murta, G.C.; Mota Barroso, J.; Rato, A.E. Quality Assessment of Red Wine Grapes through NIR Spectroscopy. Agronomy 2022, 12, 637. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The Evolution of Phenolic Compounds in Vitis vinifera L. Red Berries during Ripening: Analysis and Role on Wine Sensory—A Review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Tomasi, D.; Gaiotti, F.; Jones, G.V. Organoleptic Characteristics of the Wines. In The Power of the Terroir: The Case Study of Prosecco Wine; Springer: Basel, Switzerland, 2013; pp. 149–166. ISBN 978-3-0348-0627-5. [Google Scholar]
- Hosu, A.; Cristea, V.-M.; Cimpoiu, C. Analysis of Total Phenolic, Flavonoids, Anthocyanins and Tannins Content in Romanian Red Wines: Prediction of Antioxidant Activities and Classification of Wines Using Artificial Neural Networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Giménez-Bañón, M.J.; Cebrían-Pérez, A.; Férnandez-Férnandez, J.I.; Gómez-Martínez, J.C.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Cell Wall Characterization of New Monastrell Hybrid Descendants and Their Phenolic Wine Composition. Eur. Food Res. Technol. 2022, 248, 1253–1265. [Google Scholar] [CrossRef]
- Chang, B.-M.; Keller, M. Cuticle and Skin Cell Walls Have Common and Unique Roles in Grape Berry Splitting. Hortic. Res. 2021, 8, 168. [Google Scholar] [CrossRef]
- Gao, Y.; Zietsman, A.J.J.; Vivier, M.A.; Moore, J.P. Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology. Molecules 2019, 24, 165. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Application of Elicitors at Two Maturation Stages of Vitis vinifera L. Cv Monastrell: Changes in Skin Cell Walls. Chemistry 2022, 4, 98–111. [Google Scholar] [CrossRef]
- Deloire, A.; Rogiers, S.; Šuklje, K.; Antalick, G.; Zeyu, X.; Pellegrino, A. Grapevine Berry Shrivelling, Water Loss and Cell Death: An Increasing Challenge for Growers in the Context of Climate Change: Original Language of the Article: English. Tech. Rev. 2021. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Determination of Phenolic Compounds of Grape Skins during Ripening by NIR Spectroscopy. LWT—Food Sci. Technol. 2011, 44, 847–853. [Google Scholar] [CrossRef]
- Inoue, E.; Kobayashi, H.; Hoshino, R.; Hisamoto, M.; Watanabe-Saito, F.; Okuda, T. Adsorption Properties of Grape Phenolics to Grape Insoluble Cell Wall Materials. FSTR 2019, 25, 863–869. [Google Scholar] [CrossRef]
- Gladstones, J. Viticulture and Environment; Trivinum Press Pty Ltd.: Adelaide, Australia, 2021; Volume 1, ISBN 978-0-9945016-0-8. [Google Scholar]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines. Agriculture 2022, 12, 491. [Google Scholar] [CrossRef]
- Cameron, W.; Petrie, P.R.; Barlow, E.W.R.; Patrick, C.J.; Howell, K.; Fuentes, S. Advancement of Grape Maturity: Comparison between Contrasting Cultivars and Regions. Aust. J. Grape Wine Res. 2020, 26, 53–67. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin Profile and Antioxidant Activity from 24 Grape Varieties Cultivated in Two Portuguese Wine Regions. J. Int. Des Sci. De La Vigne Et Du Vin 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Nunes, C.; Rato, A.E.; Barros, A.S.; Saraiva, J.A.; Coimbra, M.A. Search for Suitable Maturation Parameters to Define the Harvest Maturity of Plums (Prunus domestica L.): A Case Study of Candied Plums. Food Chem. 2009, 112, 570–574. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of Polyphenols and Evaluation of Antioxidant Capacity in Grape Pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- dos Santos Costa, D.; Oliveros Mesa, N.F.; Santos Freire, M.; Pereira Ramos, R.; Teruel Mederos, B.J. Development of Predictive Models for Quality and Maturation Stage Attributes of Wine Grapes Using Vis-Nir Reflectance Spectroscopy. Postharvest Biol. Technol. 2019, 150, 166–178. [Google Scholar] [CrossRef]
- Tramontini, S.; van Leeuwen, C.; Domec, J.-C.; Destrac-Irvine, A.; Basteau, C.; Vitali, M.; Mosbach-Schulz, O.; Lovisolo, C. Impact of Soil Texture and Water Availability on the Hydraulic Control of Plant and Grape-Berry Development. Plant Soil 2013, 368, 215–230. [Google Scholar] [CrossRef]
- Cockell, C.S.; Horneck, G. The History of the UV Radiation Climate of the Earth—Theoretical and Space-Based Observations. Photochem. Photobiol 2001, 73, 447. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.-Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. To What Extent Are the Effects of UV Radiation on Grapes Conserved in the Resulting Wines? Plants 2021, 10, 1678. [Google Scholar] [CrossRef]
- Boulet, J.C.; Ducasse, M.A.; Cheynier, V. Ultraviolet Spectroscopy Study of Phenolic Substances and Other Major Compounds in Red Wines: Relationship between Astringency and the Concentration of Phenolic Substances. Aust. J. Grape Wine Res. 2017, 23, 193–199. [Google Scholar] [CrossRef]
- Palma, P.; Fialho, S.; Lima, A.; Catarino, A.; Costa, M.J.; Barbieri, M.V.; Monllor-Alcaraz, L.S.; Postigo, C.; de Alda, M.L. Occurrence and Risk Assessment of Pesticides in a Mediterranean Basin with Strong Agricultural Pressure (Guadiana Basin: Southern of Portugal). Sci. Total Environ. 2021, 794, 148703. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Coelho, R.; Rato, A.; Samara-Lima, R.; Silva, L.L.; Campostrini, E.; Mota, J.B. Adaptive Strategies of Two Mediterranean Grapevines Varieties (Aragonez Syn. Tempranillo and Trincadeira) Face Drought: Physiological and Structural Responses. Theor. Exp. Plant Physiol. 2016, 28, 205–220. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under Deficit Irrigation: Hints from Physiological and Molecular Data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Influência Da Rega Na Composição Fenólica Das Uvas Tintas Da Casta TOURIGA FRANCESA (Vitis vinifera L.). Cienc. Y Tecnol. Aliment. 1998, 2, 60–73. [Google Scholar] [CrossRef]
- Sugiura, T.; Sato, A.; Shiraishi, M.; Amamiya, H.; Ohno, H.; Takayama, N.; Miyata, N.; Sakaue, T.; Konno, S. Prediction of Acid Concentration in Wine and Table Grape Berries from Air Temperature. Hortic. J. 2020, 89, 208–215. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Petrie, P.; Sadras, V. Advancement of Grapevine Maturity in Australia between 1993 and 2006: Putative Causes, Magnitude of Trends and Viticultural Consequences. Aust. J. Grape Wine Res. 2008, 14, 33–45. [Google Scholar] [CrossRef]
- Boido, E.; García-Marino, M.; Dellacassa, E.; Carrau, F.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterisation and Evolution of Grape Polyphenol Profiles of Vitis vinifera L. Cv. Tannat during Ripening and Vinification: Polyphenolic Profiles of Tannat. Aust. J. Grape Wine Res. 2011, 17, 383–393. [Google Scholar] [CrossRef]
- Buttrose, M.S.; Hale, C.R.; Kliewer, W.M. Effect of Temperature on the Composition of “Cabernet Sauvignon” Berries. Am. J. Enol. Vitic. 1971, 22, 71–75. [Google Scholar]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The Interplay between Atmospheric Conditions and Grape Berry Quality Parameters in Portugal. Appl. Sci. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Antunes, M.T.; Lehmann, J.; Dias, J.E.E.; Böhm, J. Atlas Das Castas Da Península Ibérica: História, Terroir, Ampelografia; Dinalivros: Lisboa, Portugal, 2011; ISBN 9789725765913. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of Grape Skins: Significance of Plant Cell-Wall Structural Components and Extraction Techniques for Phenol Release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Gómez-Plaza, E.; Terrier, N.; Doco, T.; Ros-García, J.M. The Composition of Cell Walls from Grape Skin in Vitis Vinifera Intraspecific Hybrids: Cell Wall Composition of Skin of Intraspecific Hybrid Grapes. J. Sci. Food Agric. 2017, 97, 4029–4035. [Google Scholar] [CrossRef]
- Silva, L.R.; Queiroz, M. Bioactive Compounds of Red Grapes from Dão Region (Portugal): Evaluation of Phenolic and Organic Profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Garrido, I.; Uriarte, D.; Hernández, M.; Llerena, J.L.; Valdés, M.E.; Espinosa, F. The Evolution of Total Phenolic Compounds and Antioxidant Activities during Ripening of Grapes (Vitis vinifera L.,Cv. Tempranillo) Grown in Semiarid Region: Effects of Cluster Thinning and Water Deficit. Int. J. Mol. Sci. 2016, 17, 1923. [Google Scholar] [CrossRef]
- Younis, E.B.M.; Hasaneen, A.G.M.N.; Abdel-Aziz, H.M.M. An Enhancing Effect of Visible Light and UV Radiation on Phenolic Compounds and Various Antioxidants in Broad Bean Seedlings. Plant Signal. Behav. 2010, 5, 1197–1203. [Google Scholar] [CrossRef]
- Llorens, L.; Neugart, S.; Vandenbussche, F.; Castagna, A. Editorial: Ultraviolet Radiation: Friend or Foe for Plants? Front. Plant Sci. 2020, 11, 10–11. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Zarrouk, O.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Santos, R.R.; Pinheiro, C.; Costa, J.M.; Lopes, C.; Chaves, M.M. Impact of Irrigation Regime on Berry Development and Flavonoids Composition in Aragonez (Syn. Tempranillo) Grapevine. Agric. Water Manag. 2012, 114, 18–29. [Google Scholar] [CrossRef]
- Jordão, A.M.; Correia, A.C. Relationship Between Antioxidant Capacity, Proanthocyanidin and Anthocyanin Content During Grape Maturation of Touriga Nacional and Tinta Roriz Grape Varieties. S. Afr. J. Enol. Vitic. 2012, 33, 214–224. [Google Scholar] [CrossRef]
- Torchio, F.; Cagnasso, E.; Gerbi, V.; Rolle, L. Mechanical Properties, Phenolic Composition and Extractability Indices of Barbera Grapes of Different Soluble Solids Contents from Several Growing Areas. Anal. Chim. Acta 2010, 660, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Dissecting the Polysaccharide-Rich Grape Cell Wall Changes during Winemaking Using Combined High-Throughput and Fractionation Methods. Carbohydr. Polym. 2015, 133, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Anthocyanins and Tannins in Four Grape Varieties (Vitis vinifera L.) Evolution of Their Content and Extractability. J. Int. Des Sci. De La Vigne Et Du Vin 2008, 42, 147–156. [Google Scholar] [CrossRef]


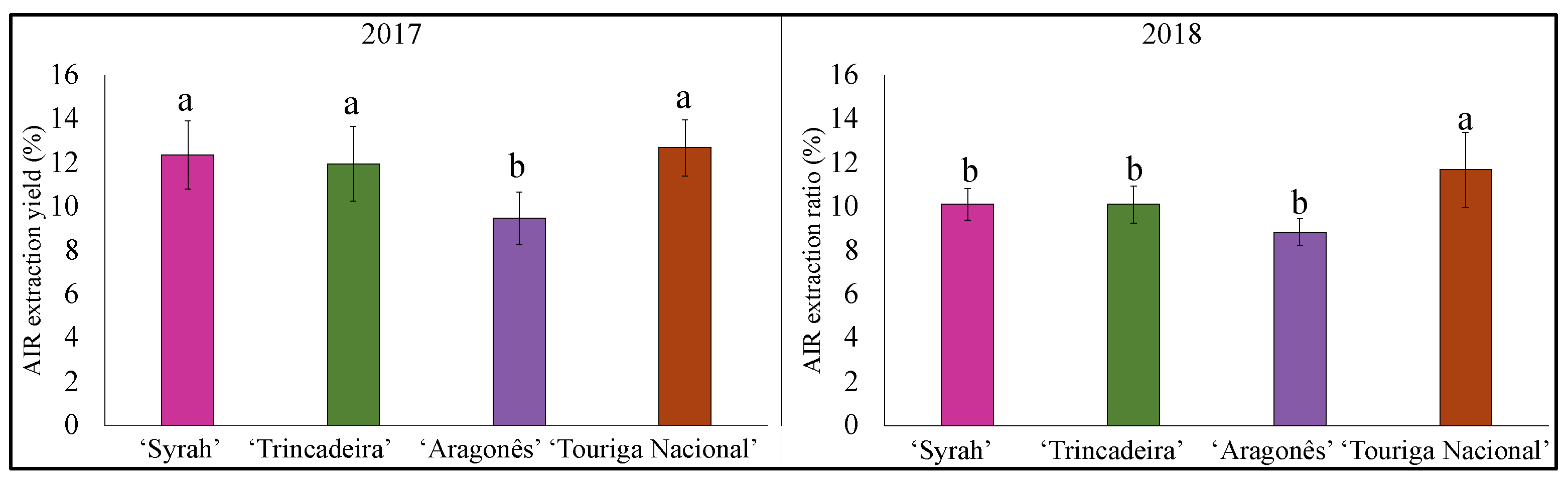

| Days after Veraison | Period | Dose UVA (KJm−2) | Dose UVB (KJm−2) | Dose SW (KJm−2) |
|---|---|---|---|---|
| 0 to 6 | 17–23 July 2017 | 9810.9 | 33.4 | 172,877.9 |
| 7 to 14 | 24–30 July 2017 | 9278.2 | 30.9 | 169,268.2 |
| 15 to 22 | 31 July–6 August 2017 | 9653.4 | 31.0 | 171,743.1 |
| 23 to 30 | 7–16 August 2017 | 11,387.3 | 36.2 | 214,957.3 |
| 0 to 6 | 7–12 August 2018 | 7796.7 | 23.3 | 139,616.4 |
| 7 to 14 | 13–22 August 2018 | 12,219.8 | 34.8 | 218,720.0 |
| 15 to 22 | 23–28 August 20188 | 5997.5 | 16.5 | 108,362.6 |
| 23 to 30 | 29 August–4 September 2018 | 6072.7 | 17.0 | 1,114,000.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouxinol, M.I.; Martins, M.R.; Salgueiro, V.; Costa, M.J.; Barroso, J.M.; Rato, A.E. Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera. Beverages 2023, 9, 8. https://doi.org/10.3390/beverages9010008
Rouxinol MI, Martins MR, Salgueiro V, Costa MJ, Barroso JM, Rato AE. Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera. Beverages. 2023; 9(1):8. https://doi.org/10.3390/beverages9010008
Chicago/Turabian StyleRouxinol, Maria Inês, Maria Rosário Martins, Vanda Salgueiro, Maria João Costa, João Mota Barroso, and Ana Elisa Rato. 2023. "Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera" Beverages 9, no. 1: 8. https://doi.org/10.3390/beverages9010008
APA StyleRouxinol, M. I., Martins, M. R., Salgueiro, V., Costa, M. J., Barroso, J. M., & Rato, A. E. (2023). Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera. Beverages, 9(1), 8. https://doi.org/10.3390/beverages9010008










