Abstract
Beer is one of the most consumed beverages in the world. Advances in instrumental techniques have allowed the analysis and characterization of a large number of beers. However, review studies that outline the methodologies used in beer characterization are scarce. Herein, a systematic review investigating the molecular targets and sensometric techniques in beer characterization was performed following the PRISMA protocol. The study reviewed 270 articles related to beer analysis in order to provide a comprehensive summary of the recent advances in beer analysis, including methods using sensors and sensing systems. The results revealed the use of various techniques that include several technologies, such as nanotechnology and electronics, often combined with scientific data analysis tools. To our knowledge, this study is the first of its kind and provides the reader with a faithful overview of what has been done in the sensor field regarding beer characterization.
1. Introduction
Beer is one of the most popular and widely consumed beverages in the world [1]. It is a complex mixture that contains more than 7700 volatile and non-volatile compounds that are responsible for the taste and smell of beer [2]. Beer is obtained from the fermentation of malted cereals in the presence of hops as a flavoring agent [3]. The brewing process includes several steps that will determine the composition and the characteristics of beer [4]. Malting is the first step by which the barley (or other cereals) is germinated and dried and/or roasted to form the malt. It is followed by the milling and mashing steps that will allow the saccharification and the formation of the wort. Then, the wort is separated from the grain during the lautering step. Hops are then added, and the mixture is brought to a boil allowing the release of the bitterness and aromas. After cooling, the wort is brought into a fermenter in order to start the fermentation [5]. Depending on the fermentation temperature and the type of yeast that is used, three main types of beers can be identified. Top fermented or “ale” beers are produced using the warm fermentation method by adding Saccharomyces cerevisiae at 15–20 °C. Bottom fermented or “lager” beers, which represent the majority of the worldwide production, are produced by adding Saccharomyces pastorianus at cooler temperatures (7–12 °C). The third type of beers are naturally fermented beers which rely on the spontaneous fermentation without yeast inoculation. The next steps are conditioning, filtering, and finally packaging [4,5]. Besides being thirst-quenching, beer is a natural dietary source of natural antioxidants such as polyphenols and flavonoids and could present a beneficial effect in countering diseases such as coronary heart disease and cancer [6,7].
During the past two decades, a large number of studies were published that covered the characterization of foodstuffs and beverages using different approaches and methodologies [3]. From an analytical point of view, authenticating beer samples was rarely considered in the literature until recent years, when the use of new instrumental techniques allowed the analysis and characterization of a large number of beers [5]. However, review studies that outline the methodologies used in beer characterization are scarce, such as describing LC-MS and NMR methods used for beer characterization and quality control [3,8]. Moreover, due to the growing needs of the rapidly expanding beverage industry, there is a need for the development of novel cost-effective tools to monitor the bioprocess. In order to fill this gap and provide an updated and comprehensive review of the recent advances in beer analysis, this study aims to deliver a meticulous summary of the sensometric methods for the characterization of beers in the last 15 years, targeting different compounds present in beer.
2. Methods
A systematic review of the recent advances in the analytical characterization of beer was performed in order to identify methods used to investigate beer in the last 15 years. This systematic review was conducted by following the reporting checklist of the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA). A comprehensive literature search was conducted to identify and categorize the articles published in the different journals present in the Web of Science Core Collection (WoS). The choice of the Web of Science lies in the fact that it provides a large volume of scientific literature specifically in technical sciences such as analytical chemistry. Moreover, a variety of sorting options allows browsing through large volumes of data quickly. Other databases such as PubMed, Scopus, and Google Scholar were not used since PubMed focuses more on clinical and biomedical sciences; several technical and non-medicine journals were not indexed. The citation analysis that WoS presents provides better graphics and is more detailed than the citation analyses presented on Scopus and Google Scholar, which offer results with inconsistent accuracy [9].
A protocol was developed in advance to access the analysis method and inclusion criteria. The website webofscience.com was used to search the Web of Science Core Collection for articles published in the different journals present in the platform using the query: TS = (Beer*) AND (TS = (*sensor) OR TS = (*sensors) OR TS = (e-nose*) OR TS = (e-tongue) OR TS = (biosens*) OR TS = (chemosens*) OR TS = (electronic nose) OR TS = (electronic tongue)) NOT (TS = (*beers law) OR TS = (*beer law) OR TS = (*Lamberts law) OR TS = (*lambert law)), thus searching only in the titles, abstracts, and/or keywords of the literature. The search query excluded articles with the Lambert–Beer law as it found a lot of false positive matches. A date restriction of 15 years was applied; only articles published from 1 January 2007 until 31 December 2022 were accepted. Research articles published only in English were accepted, and review papers, book chapters, and proceedings were not included. The last search was run on 4 August 2022.
The review was conducted using the Covidence platform. The authors’ names, title, source, abstract, journal name, and year of publication of the recorded articles were first imported to Covidence. Then, two independent reviewers screened the titles and abstracts of the articles independently, and papers that clearly were not about beer analysis were discarded. During this phase, the conflicts were discussed between the two reviewers until a consensus was reached. The screened articles were then classified according to the methods used for the analysis into four groups and distributed to four reviewers for full-text screening. The inclusion criterion was that an article had to be on the use of sensors and sensing systems to characterize and analyze beer samples. The exclusion criteria were raw materials of beer such as hops, or components such as ethanol, without any reference to beer. Papers that did not provide access to the full text were also eliminated. If the reviewer had any doubt about the eligibility of an article, it was discussed with other authors until a consensus was found.
3. Results and Discussion
3.1. Study Selection
The current study included 270 research articles. The study selection process has been summarized in the PRISMA chart in Figure 1. The literature search resulted in 418 records, of which 145 were eliminated in line with the inclusion criteria. Among them, many articles contained the word “beer”, but it was referring to Beer’s law and not to beer as a beverage. The remaining 273 articles were carefully screened, and one paper was excluded in line with the exclusion criteria. Two additional studies were discarded because we did not have access to the full text.
Figure 1.
PRISMA flow chart of study selection process.
After analyzing 270 articles, several technologies were found to be used to design a multitude of sensors, including nanotechnology using nanomaterials such as carbon nanotubes and metallic nanoparticles, and biotechnology using apta-biosensors and electronics such as E-noses and E-tongues. These technologies are often combined with scientific data analysis tools.
We have briefly summarized the focal molecular targets for the beer analysis in Figure 2. As observed from Figure 2, most of the sensometric work has been focused on toxins, especially ochratoxins (OTA, OTB, and OTC), followed by ethanol, amines, polyphenols, sugars, organic acids, and heavy metals. The order in the discussion is different in Figure 2 due to the type of sensors, e.g., sugars and ethanol were tested using similar/same sensors. Therefore, in order to obtain a cohesive text flow, we discussed compounds better suited with flow.
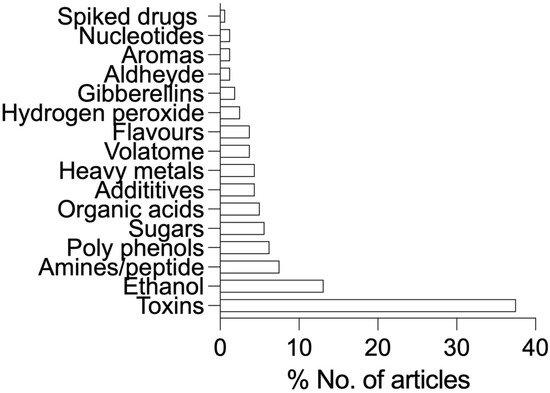
Figure 2.
A representative overview of compound classes detected by sensometric techniques in beer.
3.2. Sensors to Detect Toxins
Beers typically contain a high water content and are therefore susceptible to microbial (bacterial and fungal) contamination. This has been reviewed by Xu and coworkers [10]. The beers become contaminated not only by microbes but also by several mycotoxins that are present in the grains and survive the harsh brewing conditions due to their thermal stability [11]. The most common mycotoxins that are carried from grains (barley, wheat) to beer are ochratoxin (OT), aflatoxins (AF), fumonisins (FN), and zearalenone (ZEN) [11]. Herein, we will discuss different sensometric techniques utilized to detect these toxins in beer (See abbreviations).
3.2.1. Carbon Nanotubes
An aptamer specific to OTA was combined with carboxyfluorescein and single-walled carbon nanotubes (SWCNTs) to develop a selective fluorescent aptasensor for OTA detection. The fluorescence of the free OTA (fluorescein) aptamer was quenched by SWCNTs. This strategy was selective towards OTA with a limit of detection (LOD) of 24.1 nM [12]. Similar results were also observed when exonuclease III was coupled to fluorescein and SWCNTs [13]. An electrochemical biosensor based on a glassy carbon electrode (GCE) modified with multiwalled carbon nanotubes (MWCNTs) was developed for OTA. The electrode was electropolymerized with a molecularly imprinted polymer composed of pyrrole monomers that form hydrogen bonds between the oxygen groups of OTA. A detection limit of 1.7 µg/L was obtained with minimal sample preparation [14]. The sensitivity of the sensor improved when GCE was modified with 2D titanium carbide nanosheets (LOD of 0.028 µM) [15].
An electrochemical aptasensor based on MWCNTs doped with polyethyleneimine (PEI)-functionalized molybdenum disulfide (MoS2) was designed for the sensitive detection of ZEN. The nanocomposite was coupled with an ultra-sensitive ZEN aptamer and immobilized into a gold and silver electrode. The method showed promising results for ZEN determination in real beer samples with a linear concentration range from 0.5 pg/mL to 50 ng/mL and a LOD of 0.17 pg/mL [16]. Similarly, an aptasensor based on silver nanoparticles (AgNPs) also detected ZEN in a low picomolar concentration with a LOD of 0.32 pg/mL [17].
3.2.2. Metallic Nanoparticles
The colorimetric quantification of OTA was performed by the release of gold nanoparticles (AuNPs) that were preloaded in a DNA hydrogel. The sensor proved to be highly selective towards OTA and could even detect other toxins such as OTB [18,19]. Hao and coworkers developed a fluorescent DNA hydrogel aptasensor for OTA detection in beer [20]. Similarly, AuNPs and AgNPs were also utilized for the luminescence resonance energy transfer OTA quantification in beer with a LOD in the lower nanomolar range (0.5 nM) [21,22]. Furthermore, OTA aptamer-functionalized persistent luminescence nanorod (PLNR) Zn2GeO4:Mn2+ and aptamer complementary DNA-modified gold nanoparticles (AuNPs) were utilized, which further enhanced the sensitivity of the sensors [23]. Similar results were observed when nitrogen-doped carbon dots were utilized in combination with AgNPs [24]. Carbon paste electrodes (CPE) modified with AuNPs were used for a sensitive determination of OTA in different food samples. The developed sensor was tested on spiked beer samples with a LOD of 0.2 nM [25]. Iron-gold nanoparticles were coupled to silver core-shell nanoparticles to design a novel surface-enhanced Raman spectroscopy (SERS) aptasensor for the sensitive detection of ZEN and showed enhanced sensitivity compared to Ag/AuNPs-based sensors alone with a sensitivity of 0.001 ng/mL [26]. Similar results were also obtained when a gold and silver nano dumbbell assembly was formed and used to fabricate SERS probes to detect OTA [27].
A multicolor exonuclease-assisted aptasensor for the visual detection of OTA was obtained by etching gold nanorods (AuNRs) using a G-quadruplex-hemin DNAzyme. The multicolor platform utilized exonuclease I (Exo I) to differentiate the conformational change between OTA-bound and free aptamers. The method was successfully applied to the detection of OTA in spiked beer samples [28]. The sensitivity of DNAzyme-based sensors was improved when AuNPs were treated with graphene and manganese oxide nanocomposite when tested on T2 toxin [29].
Self-propelled micromotors based on graphene oxide/nickel/platinum nanoparticles were developed for the mycotoxin analysis. The nanomachines were used for fast and simultaneous detection of mycotoxins in several beverages including beer. The results showed a high sensitivity of the proposed strategy towards FN-B1 with a LOD of 0.70 ng/mL [30].
An electrochemical aptasensor based on a Y-shaped DNA structure and gold nanorods (AuNRs) was designed for OTA and FN-B1 detection. AuNRs were used as carriers for aptamers and as signal probes to achieve signal amplification. The sensor allowed the simultaneous detection of OTA and FN-B1 in spiked beer samples in the 1.0 pg/mL to 100 ng/mL concentration ranges, with LODs of 0.47 and 0.26 pg/mL for OTA and FB1, respectively [31]. A CPE modified with AuNPs was used for a sensitive determination of OTA in different food samples. The developed sensor was tested on spiked beer samples having a linear concentration range from 0.5 to 100 nM and a LOD of 0.2 nM [25].
A sensitive biosensor for AF-B1 determination was constructed using a copper-based metal-organic framework (Cu-MOF) and gold nanoparticles (AuNPs) with Exonuclease III (Exo III). The Cu-MOF was used to amplify the electrochemical signals, while Exo III played an important role in the recycling process. The biosensor was applied to determine aflatoxin B1 in beers [32].
A novel electrochemical aptasensor based on carbon dots/α-Fe2O3-Fe3O4 nanocomposite for the ultrasensitive determination of AF-B1 was developed. The carbon dots improved the aptasensor’s sensitivity due to their good electrical conductivity and large specific surface areas, while the α-Fe2O3-Fe3O4 nanoparticles were used for their magnetic properties and to improve the catalytic performance of the aptasensor. The results revealed an excellent LOD of 0.5 pM for the determination of AF-B1 in beer [33].
A novel chemiluminescence method based on dual aptasensors using Fe3O4 and SiO2 nanoparticles as carriers was developed. The sensor performed a simultaneous detection of OTA and adenosine triphosphate (ATP) in spiked beer samples with nanomolar sensitivities [34].
3.2.3. Upconverting Nanoparticles
An ultrasensitive aptasensor based on hexagonal core/shell upconverting nanoparticles (CS-UCNPs) and graphene oxide (GO) was developed for OTA detection. The CS-UCNPs, coupled to an OTA aptamer, acted as energy donor, and GO acted as energy acceptor in a luminescence resonance energy transfer (LRET) process. CS-UCNPs were brought in close proximity to the GO surface resulting in quenching of the luminescence of UCNPs. The aptasensor showed good specificity and high sensitivity towards OTA in beer samples with a LOD of 1 pg/mL [35]. On the other hand, fluorescence- and carbon electrode-based aptasensors [36,37] have limited sensitivities. Upconverted nanoparticles (UCNPs) coupled to magnetic nanoparticles or metal organic frameworks were used to design novel sensors for the determination of OTA and T2. These sensors either utilized FRET or IR for the upconversion. The sensitivity of OTA was 5 pg/mL and 87 pg/mL for T2 [38,39].
A highly sensitive immunosensing platform based on zearalenone-conjugated bovine serum albumin immobilization on a screen-printed carbon electrode was designed for ZEN detection. An anti-zearalenone monoclonal antibody was added for indirect competitive immunoreaction with ZEN followed by an alkaline phosphatase-labeled antibody and 1-naphthyl phosphate as a substrate, allowing the determination of ZEN concentrations via differential pulse voltammetry. The immunosensor showed good specificity, reproducibility, and sensitivity with a LOD of 0.25 ng/mL, and good recovery values in the range of 89–97% against beer samples [40].
A novel aptasensor for OTA detection based on UCNPs assembled with gold nanourchins (AuNUs) was described. UCNPs acted as a luminescence label for upconversion luminescence (UCL), and AuNUs acted as an enhancing substrate for SERS. This dual-mode aptasensor was applied for OTA detection in spiked beer samples with high recoveries of 95.2−103% for UCL and 92.4−108% for SERS [41].
Screen-printed carbon electrodes (SPCEs) were modified with two aptamers using a diazonium salt as a binder. The aptasensors were used for the detection of AF-B1 in beer and wine samples. The quantification of AF-B1 was achieved by using electrochemical impedance spectroscopy, and the results showed good recovery levels in the range of 92–100% and the LODs were 0.12 ng/mL and 0.25 ng/mL for the two sequences [42]. The SPCE was modified with the OTA aptamer using diazonium salt and click chemistry for the design of a sensitive OTA aptasensor. The detection of OTA in spiked beer samples was performed in a range between 1.25 ng/L and 500 ng/L with a LOD of 0.25 ng/L [43]. Similarly, when the SPCE was modified with horseradish peroxidase by pyrrole, electropolymerization was used to fabricate a sensitive biosensor for OTA with the same amounts of recovery as discussed above [44]. The SPCE combined with immunosensing platforms and magnetic beads improved the performance of immunosenors and decreased the LOD to 33 μM [37]. An automated flow-based aptasensor for OTA based on SPCE modified with functionalized magnetic beads was prepared. This method was fully automated avoiding human errors, and a short analysis time of 20 min was achieved [45]. Selectivity of OTA was improved by using horseradish peroxidase immobilized in a poly pyrrole matrix onto disposable screen-printed carbon electrodes with a LOD of 0.1 ng/mL. It was successfully applied on beer and the concentration of OTA was found to be 1 μM [46].
For T-2 toxin detection in beer, the SELEX method aided by graphene oxide was used to create a high-affinity ssDNA aptamer that exclusively binds to the T-2 toxin. The LOD was 0.4 μM using a specific aptamer sequence as the recognition element [47]. Their findings suggest that the graphene oxide SELEX method was suitable for the screening of aptamers against small molecule toxins, providing a potential application for aptamer-based biosensors. Similarly, Zhao with his team created a fluorescence aptasensor using an aptamer that was tagged with the Cy3-aptamer for the detection of T-2 toxin in beer [48]. The fluorescence of the Cy3-aptamer based on the fluorescence resonance energy transfer (FRET) and photoinduced energy transfer (PET) was quenched by NH2-UiO-66. They found that the NH2-UiO-66/Cy3-aptamer combination disintegrated as a result of T-2 toxin recognition and binding to Cy3-aptamer. A highly sensitive response to T-2 toxin was made possible by the restoration of fluorescence intensity after blocking the energy transfer mechanism. They also observed a strong linear correlation between toxin concentration and the fluorescence intensity [48].
Goud and colleagues have established an aptasensing platform for the detection of toxin analytes in alcoholic beverages by comparing the analytical performance of two aptamer sequences [49]. They established an interaction between the TAMRA-labeled Q-DNA strand and FAM (carboxyfluorescein)-labeled aptamer with and without the target analyte using an epitope structure-switching signaling aptamer for the high sensitivity detection of AF-B1. Their approach demonstrated high selectivity and sensitivity (LOD of 0.2 ng/mL) [49]. Similarly, a gold electrode-modified methylene blue-labeled aptamer was used for the binding with either cDNA or AF-B1. The features of an electrochemical aptasensor were simple operation, quick evaluation, better restoration, and minimal cost. With a 5 min sample incubation, their electrochemical aptasensor allowed for the detection of AF-B1 at 2 nM [50]. Since additional aflatoxins (AF-B2, AF-G1, and AF-G2) can also bind with the aptamer itself, an additional sample preparation step will improve the selectivity for the targeted AF [51].
Efforts were also made to detect OTA using the optoelectronics system [52]. The technique utilizes the fluorescent properties of the compounds of interest. The proposed prototype was based on real-time thin-layer chromatography, where hydrogenated amorphous silicon photosensors were deployed to observe the fluorescent properties of the compound OTA. The system consisted of three modules. A UV (ultraviolet) source, a high performance thin-layer chromatographic plate, and photo-detectors made up of silicon. The operating principle was that during the chromatographic run, the OTA is separated from other compounds, and when its fluorescent bands synchronize with the photosensors, a current peak is detected which corresponds to the amount of that compound present in the sample. This obtained peak is then compared with a standard reference to determine if the amount is within the range. An OTA filter between the chromatographic plate and photosensors was used to filter out unwanted wavelengths coming from the UV source [52].
Furthermore, an image sensor exploiting the RGB model was developed for the analysis of OTA. It was a low cost, portable fluorescence detecting device which was developed to design a colorimetric assay for the detection of various analytes. One of the applications was to detect OTA in red wine and beer with a low nanomolar LOD and acceptable recoveries [53]. An indirect competitive enzyme-linked immunosorbent assay strategy conjugated by an analytical system was developed for chemiluminescence detection, and it was used for the detection of OTA. The U-shaped microchannel performed better than straight channels, and the LOD was reported to be 0.1 ng/mL for beer and 2 ng/mL for wine [54].
Conclusively, aptasensors coupled to nanoparticles, carbon nanotubes, or optoelectronics systems offer reliable, quick, and accurate testing methods for the quantitative assessment of a mycotoxin in beer and wine samples. The detection of toxins in beer and wine through aptamer-based biosensors may serve as a stepping-stone for considerable improvements in the quality control of the alcoholic beverage market. Overall, sensors that are developed to detect different toxins possess high sensitivities, and with the introduction of an appropriate sample preparation strategy, the selectivity can be enhanced further.
3.3. Detection of Ethanol and Sugars
It is very well known that the yeast fermentation of sugars (from barley or wheat) produces ethanol. This process is very well established and has been used for ages [55]. This also means there will be a need for sensors simultaneously detecting both sugars and ethanol. Hence, both will be discussed together in this section.
A polyvinyl alcohol–multiwalled carbon nanotube–alcohol dehydrogenase (PVA–MWCNT–ADH) biocomposite was used to develop an amperometric ethanol biosensor. The MWCNT was used for electron transfer, PVA was used as a backbone binder, and ADH as a catalyst that facilitated the conversion of ethanol into acetaldehyde. The proposed biosensor presented a low electrochemical oxidation potential of NADH, making possible the detection of ethanol in real samples such a beer with high sensitivity and a fast response [56].
A novel ethanol biosensor was developed by incorporating alcohol dehydrogenase (ADH) into a colloidal gold (Aucoll)–MWCNTs composite electrode using Teflon as the binder. The biosensor was applied for the determination of ethanol in commercial beer samples with a LOD of 4.7 µM, which was better than those reported for other carbon nanotube-based ADH biosensors [57]. GCE was modified with a nanocomposite film to elaborate an ethanol biosensor. The film was made up of MWCNTs, ADH, and chitosan as a polymeric binder. The MWCNTs improved the electrons transfer, while ADH facilitated the conversion of ethanol into acetaldehyde. The developed ethanol biosensor exhibited high sensitivity, and a LOD of 0.52 µM was observed [58]. Similarly, when the ADH enzyme was entrapped in SWCNT with reduced graphene oxide, the detection efficiency was slightly better than MWCNT [59]. An enzymatic electrode for the sensitive amperometric detection of ethanol was designed. The electrode was integrated with alcohol dehydrogenase and chloranil in liquid-crystalline cubic phases on single-walled carbon nanotubes. The composite showed good electrocatalytic oxidation of NADH at low-anodic potential and exhibited good analytical performances and good stability for ethanol determination in complex samples such as beer [60]. Similarly, tin oxide-based nanosheets were turned into 3D hierarchical nanoflowers to build a sensitive gas sensor that successfully monitored the existence of ethanol by a simple integrated device [19].
Glucose oxidase was immobilized into a nanocomposite formed with AuNPs, N,N-dimethylformamide (DMF), and 1-butyl-3-methylimidazolium hexafluorophosphate (BMIMPF6). The composite film was modified on a GCE for glucose detection in beer and human plasma. The results revealed that the average recovery of the spiked beer samples was 99% [61]. Au/SiO2 nanoparticles were electrodeposited onto a GCE to construct a novel ethanol biosensor. The nanosensor showed excellent catalytic activity in the oxidation of NADH and was successfully applied for the determination of ethanol in real samples including beer [62]. AuNPs coated with polydopamine, using localized surface plasmon resonance, were used as a sensing platform to monitor fermentable sugars in beer wort. The proposed strategy was successfully tested in real samples [63].
A paper-based screen-printed biosensor for ethanol determination in beers was developed for the first time. The paper-based screen-printed electrode was modified with a nanocomposite formed by carbon black and Prussian blue nanoparticles for enhanced hydrogen peroxide (H2O2) detection. Ethanol was indirectly quantified by monitoring the concentration of the produced H2O2. The nanomodified device was successfully used for ethanol determination in commercial beer samples [64].
An electrochemical method for ethanol determination using a nanoporous carbon electrode in the presence of NAD+ and ADH was developed. The electrode was obtained by treating a GCE in 0.1 mol/L NaOH by electrochemical oxidation at 1.80 V followed by reduction at −1.00 V, resulting in the formation of nanoporous cavities on the electrode surface. The electrocatalytic activity of the obtained electrode towards the oxidation of NADH was excellent and was successfully utilized for ethanol determination in real beer samples [65]. A flow injection analysis integrated with an amperometric ADH graphene-based composite biosensor was designed for ethanol determination in alcoholic drinks. The amperometric device was compared to a portable Raman spectrometer, and the results revealed several limitations of the two tools, mainly in the analysis of samples with complex matrices, such as dark beers and red wines [66]. Based on a semiconductive polymer-nickel oxide nanocomposite, the sensor showed good sensing of ethanol and a good reproducibility at room temperature for real samples such as beer and wine [67].
Screen-printed electrodes (SPEs) equipped with gold working electrodes were modified by coating their probe surface with a paper crown which was soaked with two different room temperature ionic liquids used for the flow injection amperometric detection of ethanol in alcoholic beverages. It was found to be a rapid ethanol detection method in drinks suitable for untrained operators, and easy to transport [68]. For simultaneous quantification and discrimination of methanol and ethanol, a dual biosensor analysis system was developed where both ADH and AOX (alcohol oxidase) were used because ADH can oxidize only ethanol from the mixture, and AOX can oxidize preferentially methanol but also ethanol [69]. The methanol “fuel cell” was used directly for detecting methanol and ethanol. Two different open circuit voltage formats and a potentiometric format were used. However, the findings showed that for commercial beverages it takes a long time, at least two–three months, to obtain the response within the desired limit. Analysis times were reduced when oxidoreductive enzymes were used in the fuel cells [70].
An ADH/peroxidase-based amperometric enzyme biosensor for the detection of ethanol in alcoholic beverages was developed with a LOD of 2.85 mM [71]. By the application of the eukaryotic double mediator (EDM) system, ethanol in alcoholic drinks was detected in a fast, direct, and small-sample measurement method but with poor selectivity [72]. Based on Methylobacterium organophilum, an immobilized eggshell membrane electrode and an oxygen electrode were used to develop an ethanol biosensor with a LOD of 25 nM [73]. A low-cost method using natural indicators and digital images in a flatbed scanner was used for the detection of ethanol in beer. It was the first method to quantify ethanol in beer containing ethanol < 2% [74]. A low-cost wireless amperometric system for the detection of glucose and ethanol was developed with the glucose and ethanol amperometric biosensor integrated with a wireless telemetry system. The results obtained in this method were compared with the enzymatic spectrophotometric kit for glucose and the modular measurement system for ethanol detection, and it was found that it included ease and speed of construction with the cheapness of materials and the detection of the main fermented materials with sensitivity and repeatability [75]. During small-scale fermentation, an online microdetector containing an amperometric biosensor was developed for the high temporal resolution monitoring of glucose and ethanol concentration. It was found that the developed microdetector indicated an increase in ethanol productivity [76]. An ultrasound analysis was successfully used to determine ethanol and sugar content throughout the fermentation process of beer [77]. Analytical characteristics of a bi-enzyme amperometric sensor were investigated to detect ethanol by the role and influence of alcohol dehydrogenase and horseradish peroxidase. For wine and beer samples, the LODs were 2.94 mM and 9.79 mM, respectively [78].
Alpha-glucosidase and pyranose oxidase were immobilized onto a graphite electrode to construct a maltose biosensor. The immobilization was carried out using carbon nanotubes, chitosan, and glutaraldehyde. The novel bi-enzymatic sensor was used to detect maltose in complex matrices such as beer and revealed to have a good sensitivity and accuracy [79]. A non-enzymatic sensor for electrochemical glucose monitoring was developed. The sensor was based on graphene oxide decorated with AgNPs on a GCE. The proposed sensor was revealed to be a reliable tool for glucose determination during the glycolytic process in fermented food such as beer [80].
A sensitive and selective method was developed for maltose determination in beverages using a carbon nanostructured screen-printed electrode modified with CuO/glucose oxidase/maltase/SiO2 biocomposite film. The dispositive was successfully used for the maltose determination in beer samples with a linear concentration range from 0.01 to 0.1 mmol/L and a LOD of 5 nM [81]. A maltose biosensor was developed comprising a maltose-binding protein where the green fluorescent protein was flanked at the N-terminus and the Renilla luciferase variant at the C-terminus. This bioluminescence resonance energy transfer system (BRET) showed a 30% increase in the BRET ratio, and the equivalent FRET showed only a 10% increase. This method was used for maltose detection in beer, and the concentration of maltose in beer was found to be 10.6 ± 0.5 mM. This sensor was sensitive with an EC50 of 0.37 µM for maltose [82]. The same maltose-binding protein biosensor was combined with a microfluidic system for the detection of maltose in beer and water. By this method, the quantification of maltose at the mL scale was completed in <100 s with an EC50 13 nM which had not been reported previously. The concentration of beer found was 2.2 mM, where the enzyme-linked colorimetric assay showed 0.96 mM [83].
3.4. Amines and Peptides
Amino acids, peptides, and amines are present in the raw ingredients (barley, hops, wort) utilized in the production of the beers. Yeast utilizes these resources as a source of carbon and nitrogen to carry out the fermentation process [84]. The presence of these amines in the raw materials have been reviewed by Koller and coworkers [85]. The non-sensometric methods for the determination of amines and amino acids have been reviewed previously [86]. This present manuscript will focus on how different sensors have been used to measure biogenic amines.
CPE modified with SWCNT was used to design an electrochemical sensor for histamine determination. The proposed sensor was successfully applied for histamine detection in alcoholic beverages including beer samples, thus offering a promising analytical tool for routine quality control. The current response of histamine was linearly proportional to its concentration in the range from 4.5 to 720 µM with a LOD of 1.26 µM [87]. The optical properties of SWCNTs were used to develop a novel paper-based near-infrared optical nose. The SWCNTs were encapsulated with a wide variety of peptides on a paper substrate, and the emitted fluorescence was continuously imaged via a complementary metal oxide semiconductor camera. Machine learning tools used the fluorescence images to discriminate between the aromas of red wine, beer, and vodka. A slow and low response of the sensors was noticed during the sniffing of beer, and most of them did not reach a plateau [88]. The specific recognition and determination of histamine was performed using an electrochemical sensor based on AuNPs. The AuNPs were electrodeposited onto a GCE surface, followed by molecularly imprinted polymer layer immobilization. The proposed methodology proved to be appropriate for histamine detection in beer samples [89].
Tyramine oxidase was immobilized onto citric acid-capped AgNPs that were attached to a gold electrode via cysteine self-assembled monolayer to construct a sensitive amperometric biosensor for tyramine detection. The proposed method was used for the tyramine measurement in sauce and beer showing an optimum response within 8 s, when polarized at 0.25 V, 35 °C, and pH 8.5, with a LOD of 0.01 mM [90]. The sensitivity of tyramine determination was improved by utilizing GCE modified with silver-substituted ZnO nanoflowers. The LOD improved to 30 nM [91]. Helicoid AuNPs with intrinsic chirality were synthesized and utilized to construct, for the first time, an electrochemical chiral sensor. The electrode was applied for tyrosine analytes determination, and the sensor recognized L-tyrosine in both beer and milk [92].
A sensitive fluorescent sensor based on the encapsulation of UCNPs into a zinc-based metal-organic framework (ZIF-8) and the incorporation of molecularly imprinted polymer (MIP) was developed for octopamine determination. The ZIF-8 improved the dispersion and emission intensity of UCNPs and reduced the adsorption time of MIP. The proposed biosensor was successfully applied to detect octopamine in spiked beer samples with a recovery range of 81.75–90.63% [93].
An optical sensor based on nanofiber mat functionalized with active vinyl groups and solid surface-room temperature phosphorescence as a transduction system was used to determine tryptamine. This method appears to be a sensitive, economical, and environmentally friendly method for direct tryptamine detection in beer with short run times (<15 min) and a LOD of 6 ng/mL [94].
Diamine oxidase (DAO), as a biocatalytic component of the electrochemical biosensor, was used for the first time for the detection of biogenic amines in wine and beer. According to the results, this process was found to be less efficient for beer comparing with the GC-MS process [95]. A fluorescent system based on nitrogen-doped graphene quantum dots (GQDs) and Mercury (II) was designed for cysteine detection. The fluorescence of the system, quenched by Hg(II) due to the efficient electron transfer between GQDs and Hg(II), recovered gradually upon cysteine addition. The sensor was successfully applied to detect cysteine in beer samples with a LOD of 1.3 nM [96]. All in all, it appears that sensors are efficiently determining amines from beers. Still, a complete profiling of different amines present in the sample is not yet possible.
3.5. Polyphenols
A number of polyphenols are present in beers. They are known to possess antioxidant effects and have been very well investigated and reviewed [97,98]. They are also known to form complexes with proteins in the beer and result in a type of haze. Polyphenols not only possess numerous health benefits but also affect the physical stability of the beers [98].
GCE modified with a SWCNT-tyrosinase-polyalanine nanocomposite was used for polyphenol determination. The sandwich-type biosensor exhibited a good analytical performance when applied for phenolic compound detection in real beer samples, with satisfactory recoveries regarding caffeic acid and catechol. The LODs were 8 nM and 6 nM for catechol and caffeic acid, respectively [99]. Similarly, when the CPE was used for the determination of maltol, the electrochemical response of the electrode for maltol was compared after being doped with carbon nanotubes or graphene. The results showed that there was no advantage to doping the CPE, and the method was sensitive, accurate, cheap, and easy for the determination of maltol in beer [100]. SPCEs modified with AuNPs were used as a platform for enzyme deposition for the design of a rapid and sensitive polyphenols biosensor [101].
An electrochemical sensor based on a carbon cloth electro-deposited with AgNPs and drop-coated by a covalent organic framework (COF) was developed for the simultaneous detection of bisphenol A and S. AgNPs provided a stable reference signal and improved the conductivity, while the COF improved the adsorption and enrichment of the target. The constructed sensor was applied for the simultaneous determination of bisphenols in beer [102].
Nitrogen-doped fluorescent carbon dots were used as a novel fluorescent sensor for the highly sensitive detection of tannic acid. The fluorescence quenching of the carbon dots was observed with the increase in tannic acid concentration. The calibration curve displayed a linear detection range from 0.4 to 9.0 µmol/L with a LOD of 0.12 μM [103]. Fluorescent carbon dots and water-soluble carbon dots synthesized from citrate were also used for quercetin determination [104,105].
An electrode called the sonogel–carbon electrode was utilized in three different studies led by the same group. In the first study, Trametes versicolor laccase and mushroom tyrosinase were immobilized into the electrode to form a bi-enzyme biosensor for polyphenol determination in beer [106]. For the same purpose, three types of phenoloxidases were incorporated individually into a sonogel–carbon electrode to design three types of amperometric biosensors. The employed enzymes were Trametes versicolor laccase, mushroom tyrosinase, and horseradish peroxidase. The comparison between the three sensors showed that the laccase-based biosensor was the most stable, with a relative standard deviation of 3.3%, and thus was selected as the best one to evaluate polyphenols in beers [106,107]. A laccase–sonogel–carbon biosensor was proposed to determine polyphenols in beers for beer stability estimation. The proposed method was used for beer stability estimation instead of the Folin–Ciocalteu assay due to its better sensitivity and selectivity [108]. Resveratrol was quantified in beer by detection of the fluorescent photoproduct of UV radiation, with a LOD of 1.0 ng/mL [109].
Tyrosinase was immobilized on new sonogel–carbon electrodes to prepare three amperometric biosensors for the detection of phenols and polyphenols. It was designed to seek new electrochemical biosensors, and it was observed that the Nafion/Tyr/sonogel carbon biosensor stabilized it, and in real samples it showed a reproducible response and protected the immobilized enzymes from possible inhibitors [110].
3.6. Organic Acids
Organic acids are end products that are obtained after yeast or bacterial fermentation. They add a particular sour flavor to the beers. If this flavor is unintentional, then it might be due to microbial contamination and needs immediate attention. The final composition of the organic acids depends upon the strains [111,112]. Some of the common organic acids are lactic acid, acetic acid, malic acid, tartaric acid, citric, and succinic acid [113].
Lactate oxidase and horseradish peroxidase were incorporated into a carbon nanotube-polysulfone membrane using a phase inversion technique and deposited onto a SPE to form a fast and sensitive sensor for an L-lactate analysis. The enzymatic biosensor was applied for L-lactic acid determination in different wine and beer samples showing a good reproducibility LOD of 5 nM [114]. When lactate oxidase was immobilized on a GCE with a hydrogel film composed of laponite and a bioinspired thymine polycation to enhance the analytical response towards L-lactate, the polycation, obtained by copolymerization of 4-vinylbenzyl thymine and 4-vinylbenzyl trimethylammonium chloride, performed a better analysis of lactate in beer than SPEs [115]. Graphene oxide nanoparticles were electrodeposited onto a pencil graphite electrode to construct a lactate dehydrogenase-based biosensor. The sensor measured lactate levels in serum and beer with a LOD of 0.1 µM [116]. An amperometric biosensor was designed to detect D-lactic acid based on the DLDH/DP/TTF (D-lactic acid dehydrogenase/Diaphorase/tetrathiafulvalene) system. The approach involved the integration of D-lactic acid determination with the commercial L-lactic acid biosensor in a semi-automatic FIA (flow injection analysis) system. A successful determination of D-lactic acid from the beer samples was found through this method [117]. An amperometric sensor was also developed with lactate dehydrogenase which was immobilized into a Prussian blue-modified electrode for the detection of lactate with a LOD of 0.84 µM. The lactate detection in beer with this method performed better as compared with other commercial kits [118].
Platinum nanoparticles (PtNPs) were electrodeposited onto SWCNTs that were modified on a GCE to construct a sensitive biosensor for methylglyoxal detection. The proposed method was successfully applied to the quantitative analysis of methylglyoxal in wine and beer samples showing high sensitivity, good reproducibility, and low LOD of 2.8 nM [119]. A new method based on the electrochemical activation of GCE was designed for methylglyoxal detection. The evaluation of the electrode performances showed that the proposed method was able to detect methylglyoxal in low nanomolar concentrations [120].
Hydrophobic interactions between GO and negatively charged enzymes were used for the first time to fabricate CE-integrated immobilized enzyme microreactors (IMERs) by a simple immobilization procedure based on layer-by-layer assembly. It was successfully used to detect pyruvate in beer samples within a 0.2–0.3 mM concentration and a recovery range between 86 and 101% with RSD between 1.9 and 3.2% [121]. Overall, no differences were observed between electrochemical/amperometric sensors utilized to detect lactate, while nanoparticulate systems performed better.
3.7. Hydrogen Peroxide
The utilization of hydrogen peroxide in breweries as an additive to prevent microbial contamination has been known since the last century [122]. Both hydrogen peroxide and ethanol present different stress factors on the yeast during fermentation; therefore, a number of efforts have been made to monitor them simultaneously [123]. Some of the sensometric techniques utilized for the detection of hydrogen peroxide are outlined below.
Ultrathin two-dimension (2D) MOF nanosheets and MWCNTs were used to design a novel electrochemical biosensor for H2O2 determination. The 2D MOF nanosheets and MWCNTs were mixed to form a composite film that was transferred to electrodes as a novel sensing platform. The advanced platform was successfully applied to determine the amount of H2O2 in serum and beer samples with a LOD of 0.70 μM and a wide linear range from 0.001 to 8.159 mM [124].
A paper-based screen-printed biosensor for ethanol determination in beers was developed for the first time. The paper-based SPE was modified with a nanocomposite formed by carbon black and Prussian blue nanoparticles for enhanced H2O2 detection. Ethanol was indirectly quantified by monitoring the concentration of the produced H2O2. The nanomodified device was successfully used for ethanol determination in commercial beer samples [64].
GCE modified with meso-macroporous carbon networks aerogels was used to develop an eco-friendly biosensor for the detection of ascorbic acid and H2O2. The aerogels were made by assembling low-cost carbon nanorods that were derived from apples’ biomass. The proposed method exhibited a better analytical performance than GCE modified with carbon nanotubes. The results were satisfactory for detecting H2O2 in real beer samples, and the modified electrode could be used to develop a novel electrochemical sensing platform for the routine determination of ascorbic acid or H2O2 in food analysis [125].
An electrochemical platform based on phthalocyanine-conjugated polymer nanosheets was constructed for the accurate detection of H2O2. The method exhibited excellent H2O2 determination performance in commercial beers with a wide linear detection range from 0.1 to 1000 μM, a short response time of 0.1 s, and a low LOD of 0.017 μM [126].
For the fast detection of H2O2 in microliter food samples, an immobilized cytochrome C-coated SPE was applied for making an efficient biosensor. This method showed 1.4-fold increased H2O2 detection compared to cytochrome C in mesoporous zeolitic imidazolate framework-8 (ZIF8). With this device, the concentration of H2O2 in milk and beer was accurately determined [127].
3.8. Metals Ions
The bioaccumulation of heavy metals is known to cause a number of diseases [128,129]. The beverages consumed by humans also possess a number of heavy metals, and owing to their toxic effects, these have to be under permissible limits [130]. Therefore, it is very important to have detection strategies to monitor their levels during the product’s life cycle [131].
A colorimetric and turn-on fluorescent sensor for the detection of potassium metabisulphite (KMS) was developed. A colloidal solution of AuNPs stabilized with graphene oxide (GO-AuNPs) was synthetized to determine KMS by colorimetry and spectrophotometry. The surface plasmon resonance properties of AuNPs and the fluorescence property of GO were used to perform the analysis. The method was successfully used for KMS determination in real samples including beer with a LOD of 9.4 µM [132].
A paper-based electrochemical sensor pyrolyzed by a CO2 laser was fabricated for the sulfite analysis. The proposed method showed a LOD of 1 mg/L with good repeatability and reproducibility when applied to beer and wine samples for sulfite determination [133]. Non-modified SPCEs were used for total sulfites determination in beer. The developed method combined gas-diffusion microextraction with square-wave voltammetric detection and showed low LOD (0.050 mg/L) when applied to beer samples [134].
Dual DNA tweezers were used for the simultaneous detection of Cu2+ and Mg2+ in wine and beer. The dual DNAzyme provided two kinds of fluorescent signals that were amplified by the entropy-driven three-dimensional DNA nanomachine for quantitative and simultaneous detection of the two metal ions. The LODs were 2 nM for Mg2+ and 10 pM for Cu2+, respectively [135]. Square wave voltammetry (SWV) and a preconcentration step were used without agitation at a boron-doped diamond SPE modified with bismuth film (SPE-BDD/BiF) to directly detect the amount of lead (II) and mercury (II) in beer. This method was found to be highly sensitive and capable of finding very low amounts of lead and mercury with a LOD of 6.5 and 7.5 µg/L, respectively [136].
The 2-Salen ligands were used as cobalt-selective electrodes, and polymeric membrane electrode (PME) and coated graphite electrode (CGE) were compared, and it was observed that the CGE showed a limit detection of 7 nM. It was used successfully for the detection of cobalt in soil, beer, and water [137]. A bridge-modified 4-tertbutylthiacalix arene was used as an electroactive material for the preparation of a cobalt-selective sensor. PVC-based membranes using sodium tetraphenylborate as the anion discriminator and bis(2-ethylhexyl) sebacate, chloronaphthalene, tri-n-butylphosphate, o-nitrophenyl octyl ether, and tris (2-ethylhexyl) phosphate as plasticizers were prepared and investigated as a cobalt-selective sensor. A LOD of 300 ppb and a low response time of 10 s were observed [138]. A novel solid cobalt-selective electrode using 4-tertbutylthicalix arene as ionophore was designed for selective cobalt sensing. The membrane, having an ionophore/polyvinyl chloride/sodium tetraphenylborate/nitrophenyl octyl ether ratio of 3.5:33:1.5:62 (w/w; mg), had the best performance characteristics. The constructed biosensor could be an interesting alternative for the routine analysis of cobalt in beer samples [139].
7-nitro-1,2,3-benzoxadiazole-acridone acetyl piperazine was used as a fluorescent probe for the detection and quantification of hydrogen sulfide with a limit of 0.19 μM [140]. The fluorophore 9-anthraldehyde aggregated in water, turning the solution yellow-green. The presence of sulfite anions monomerized the fluorophore, with the monomer being blue. This allowed for the quantification of sulfite with a LOD of 3.19 μM [141]. A similar concept, albeit in reverse, was also used by the same authors to quantify sulfite. The fluorophore, TPE-N2+, which forms an aggregate with polylysine when the polymer is exposed to sulfite anions, was used to quantify with a LOD of 3.6 μM [142]. The fluorophore 6-cyanonaphthalen-2-yl-2,4- dinitrobenzenesulfonate was used as a fluorescent probe for the quantification of hydrogen sulfide with a LOD of 30 nM [143]. Then, 6-(2, 4-dinitrophenoxy)-2-naphthonitrile was used by the same group for the same purpose, and a LOD of 76 nM was determined [144].
A new approach was introduced to substitute the laser-induced fluorescence detection system, and it included a high-performance detection sensor for MEMS-based capillary electrophoresis chips. This method was successfully used to analyze beer, wine, and milk mixed in different buffer solutions. It was also able to differentiate between NH4+, K+, Ca2+, Na+, Mg2+, and Li+ ions, indicating their use in the profiling of metal ions [145]. A combination of cation-selective poly (4-amino-2,1,3-benzothiadiazole) in an array with pH was used with anion-selective electrodes. It was successfully used for recognizing different Czech beers by exhibiting a sensitive response for Zn2+ and Cd2+ in a concentration range of 100 μM–10 mM and in concert with other polymer electrodes that recognized other Czech beers [146].
Different selective and partial selective electrodes were compared for different systems. It was found that independent sources of information are generated by selective electrodes. However, for the juice flow mode, selective electrodes, and for the beer flow mode, partially selective electrodes are recommended. However, the best result will be obtained when they are combined because all the information from two different points of view will be obtained, and it will be easier to analyze the sample [147].
3.9. Gibberellin
Gibberellins are plant growth hormones and are used as additives during the brewing process to improve the quality of the malt for the brewing industry. Gibberellins have been known to positively regulate heavy metal toxicity, and some of the gibberellins themselves are known to be dangerous to humans [148,149]. Therefore, it is important to develop methods to detect and quantify the same in the beers and sometimes in tandem with heavy metals.
A highly sensitive molecularly imprinted electrochemical sensor was designed for gibberellin detection. Gold-doped g-C3N4 nanohybrids were prepared on the surface of a GCE in order to enhance the electrochemiluminescence signal of Ru(bpy)3Cl2 using a resonance energy transfer. The proposed strategy allowed an ultra-sensitive detection of gibberellin in beer samples with a LOD of 1.64 pM [150]. Similarly, when magnetic Fe3O4 nanoparticles and Fe3O4-Au nanoparticles with high affinity were synthesized, and then L-cysteine-GA3 was grafted on the surface of a gold electrode via magnetic self-assembly, they showed similar results [151].
For Gibberellin A3 (GA3) detection, a molecularly imprinted electrochemical luminescence (MIP-ECL) sensor was developed where GA3 was detected by competitive binding between GA3 and Rhodamine-labeled GA3 to the MIP film. The sensitive assays had a LOD of 3 pM [152]. Improvement in the same technique was made by utilizing dendrimer multilabel template molecules. GA3 was labeled by using ferrocene carboxylic acid-modified PDTPA (poly-diethylenetriaminepentaacetic acid). The electrochemical sensor was observed to be sensitive to GA3 detection in a concentration range of 1.0 × 107 M to 2.0 × 109 M. Additionally, the sensor’s viability was confirmed by the accurate examination of beer samples [153].
4. Conclusions
This study was performed to provide an updated systematic review of the recent advances in the analytical characterization of beer using sensors and sensing systems. It provides the reader with an overview of what has been accomplished in the sensing field in recent years. Thus, it provides the foundation for further research that could outline other strategies used for beer characterization.
Conclusively, we have summarized different sensors developed to analyze the contents of the beer. Furthermore, it has been observed that the sensors are very selective for the target molecules; however, to meet the growing demands of the beverage industry, there is a need for sensors able to profile a wide range of compounds in a single shot.
Author Contributions
Conceptualization, B.K.P., M.M.K.N. and K.B.; methodology, S.S., S.F.C., K.J.T., K.B. and M.M.K.N.; data curation, S.S., S.F.C., K.J.T., K.B., M.M.K.N. and B.K.P.; writing, reviewing, and editing, S.S., S.F.C., K.J.T., K.B. and M.M.K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The higher education commission, Pakistan and Erasmus Mundus funding agencies are deeply acknowledged for supporting the studies of S.F.C., S.S., and K.B.
Conflicts of Interest
The authors declare no conflict of interest. All authors have agreed to the final draft of this manuscript.
Abbreviations
| Abbreviations | Definition |
| ADH | Alcohol Dehydrogenase |
| AF | Aflatoxin |
| AgNPs | Silver Nanoparticles |
| AOX | Alcohol Oxidase |
| ATP | Adenosine Triphosphate |
| AuNPs | Gold Nanoparticles |
| AuNUs | Gold Nanourchins |
| BDD | Boron-Doped Diamond |
| BiF | Bismuth Film |
| BMIMPF6 | 1-butyl-3-methylimidazolium hexafluorophosphate |
| BRET | Bioluminescence Resonance Energy Transfer |
| cDNA | Complimentary DNA |
| CGE | Coated Graphite Electrode |
| CMOS | Complementary Metal Oxide Semiconductor |
| COF | Covalent Organic Framework |
| CPE | Carbon Paste Electrode |
| CS | Core/Shell |
| Cy3 | Cyanine 3 |
| DAO | Diamine Oxidase |
| DLDH | D-Lactic Acid Dehydrogenase |
| DMF | N,N-dimethylformamide |
| DNA | Deoxyribonucleic Acid |
| DP | Diaphorase |
| EC50 | Half Maximal Effective Concentration |
| EDM | Eukaryotic Double Mediator |
| E-nose | Electronic Nose |
| E-tongue | Electronic Tongue |
| Exo | Exonuclease |
| FAM | Carboxyfluorescein |
| FIA | Flow Injection Analysis |
| FN | Fumonisin |
| FRET | Fluorescence Resonance Energy Transfer |
| GA3 | Gibberellin A3 |
| GC | Gas Chromatography |
| GCE | Glassy Carbon Electrode |
| GQDs | Graphene Quantum Dots |
| H2O2 | Hydrogen Peroxide |
| IMERs | Integrated Immobilized Enzyme Microreactors |
| KMS | Potassium Metabisulphite |
| LOD | Limit of Detection |
| LRET | Luminescence Resonance Energy Transfer |
| MEMS | Micro Electromechanical Systems |
| MIP | Molecularly Imprinted Polymer |
| MIP-ECL | Molecularly Imprinted Electrochemical Luminescence |
| MOF | Metal-Organic Framework |
| MoS2 | Molybdenum Disulfide |
| MWCNT | Multi-Walled Carbon Nanotube |
| NADH | Nicotinamide Adenine Dinucleotide |
| NPs | Nanoparticles |
| OT | Ochratoxin |
| OTA | Ochratoxin A |
| OTB | Ochratoxin B |
| OTC | Ochratoxin C |
| PDTPA | Poly Diethylenetriaminepentaacetic Acid |
| PEI | Polyethyleneimine |
| PET | Photoinduced Electron Transfer |
| PLNR | Persistent Luminescence Nanorod |
| PME | Polymeric Membrane Electrode |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PtNPs | Platinum Nanoparticles |
| PVA | Polyvinyl Alcohol |
| PVC | Polyvinyl Chloride |
| Q-DNA | DNA Single Strand |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SPCE | Screen-Printed Carbon Electrodes |
| SPE | Screen-Printed Electrode |
| SWCNT | Single-Walled Carbon Nanotube |
| SWV | Square Wave Voltammetry |
| T2 | T-2 Toxin |
| TAMRA | Tetramethyl-6-carboxyrhodamine |
| TPE | Tetraphenylethene |
| TTF | Tetrathiafulvalene |
| UCL | Upconversion Luminescence |
| UCNPs | Upconverting Nanoparticles |
| ZEN | Zearalenone |
| ZIF-8 | Zeolitic Imidazolate Framework-8 |
References
- Baigts-Allende, D.K.; Pérez-Alva, A.; Ramírez-Rodrigues, M.A.; Palacios, A.; Ramírez-Rodrigues, M.M. A Comparative Study of Polyphenolic and Amino Acid Profiles of Commercial Fruit Beers. J. Food Compost. Anal. 2021, 100, 103921. [Google Scholar] [CrossRef]
- Hughes, S.S.; Nielsen, M.M.K.; Jonsbo, R.V.; Nielsen, C.U.; Lauritsen, F.R.; Prabhala, B.K. BeerMIMS: Exploring the Use of Membrane-Inlet Mass Spectrometry (MIMS) Coupled to KNIME for the Characterization of Danish Beers. Eur. J. Mass Spectrom. 2021, 27, 266–271. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Kamleh, M.A.; Hernández-Cassou, S.; Saurina, J.; Checa, A. Ultra-High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry Based Metabolomics as a Strategy for Beer Characterization. J. Inst. Brew. 2016, 122, 430–436. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Saurina, J. Liquid Chromatographic Fingerprints and Profiles of Polyphenolic Compounds Applied to the Chemometric Characterization and Classification of Beers. Anal. Methods 2015, 7, 8733–8739. [Google Scholar] [CrossRef]
- Mignani, A.G.; Ciaccheri, L.; Mencaglia, A.A.; Ottevaere, H.; Báca, E.E.S.; Thienpont, H. Optical Measurements and Pattern-Recognition Techniques for Identifying the Characteristics of Beer and Distinguishing Belgian Beers. Sens. Actuators B Chem. 2013, 179, 140–149. [Google Scholar] [CrossRef]
- Wang, L.; Hong, K.; Agbaka, J.I.; Song, Y.; Lv, C.; Ma, C. Characterization of Bitter-Tasting and Antioxidant Activity of Dry-Hopped Beers. J. Sci. Food Agric. 2022, 102, 4843–4853. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Breda, C.; Barros, A.I. Characterization and Discrimination of Commercial Portuguese Beers Based on Phenolic Composition and Antioxidant Capacity. Foods 2021, 10, 1144. [Google Scholar] [CrossRef]
- Rodrigues, J.E.; Gil, A.M. NMR Methods for Beer Characterization and Quality Control. Magn. Reson. Chem. 2011, 49 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and Weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, Y.; Mao, Y.; Peng, R.; Chen, J.; Soteyome, T.; Bai, C.; Chen, L.; Liang, Y.; Su, J.; et al. Spoilage Lactic Acid Bacteria in the Brewing Industry. J. Microbiol. Biotechnol. 2020, 30, 955–961. [Google Scholar] [CrossRef]
- Mastanjević, K.; Lukinac, J.; Jukić, M.; Šarkanj, B.; Krstanović, V.; Mastanjević, K. Multi-(myco)toxins in Malting and Brewing By-Products. Toxins 2019, 11, 30. [Google Scholar] [CrossRef]
- Guo, Z.; Ren, J.; Wang, J.; Wang, E. Single-Walled Carbon Nanotubes Based Quenching of Free FAM-Aptamer for Selective Determination of Ochratoxin A. Talanta 2011, 85, 2517–2521. [Google Scholar] [CrossRef]
- Wu, H.; Liu, R.; Kang, X.; Liang, C.; Lv, L.; Guo, Z. Fluorometric Aptamer Assay for Ochratoxin A Based on the Use of Single Walled Carbon Nanohorns and Exonuclease III-Aided Amplification. Mikrochim. Acta 2017, 185, 27. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly Imprinted Electrochemical Sensor for Ochratoxin A Detection in Food Samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Huang, H.; Wang, D.; Zhou, Y.; Wu, D.; Liao, X.; Xiong, W.; Du, J.; Hong, Y. Multiwalled Carbon Nanotubes Modified Two Dimensional MXene with High Antifouling Property for Sensitive Detection of Ochratoxin A. Nanotechnology 2021, 32, 455501. [Google Scholar] [CrossRef]
- Ma, L.; Bai, L.; Zhao, M.; Zhou, J.; Chen, Y.; Mu, Z. An Electrochemical Aptasensor for Highly Sensitive Detection of Zearalenone Based on PEI-MoS2-MWCNTs Nanocomposite for Signal Enhancement. Anal. Chim. Acta 2019, 1060, 71–78. [Google Scholar] [CrossRef]
- Yin, N.; Yuan, S.; Zhang, M.; Wang, J.; Li, Y.; Peng, Y.; Bai, J.; Ning, B.; Liang, J.; Gao, Z. An Aptamer-Based Fluorometric Zearalenone Assay Using a Lighting-up Silver Nanocluster Probe and Catalyzed by a Hairpin Assembly. Mikrochim. Acta 2019, 186, 765. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Y.; Ma, Y.; Jia, S.; Gao, M.; Li, J.; Zhang, H.; Xu, D.; Wu, M.; Chen, Y.; et al. Design and Synthesis of Target-Responsive Aptamer-Cross-Linked Hydrogel for Visual Quantitative Detection of Ochratoxin A. ACS Appl. Mater. Interfaces 2015, 7, 6982–6990. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Long, H.; Wang, Z. Nanosheet-Assembled Hierarchical SnO2 Nanostructures for Efficient Gas-Sensing Applications. Sens. Actuators B Chem. 2016, 231, 120–128. [Google Scholar] [CrossRef]
- Hao, L.; Wang, W.; Shen, X.; Wang, S.; Li, Q.; An, F.; Wu, S. A Fluorescent DNA Hydrogel Aptasensor Based on the Self-Assembly of Rolling Circle Amplification Products for Sensitive Detection of Ochratoxin A. J. Agric. Food Chem. 2020, 68, 369–375. [Google Scholar] [CrossRef]
- Dai, S.; Wu, S.; Duan, N.; Wang, Z. A Luminescence Resonance Energy Transfer Based Aptasensor for the Mycotoxin Ochratoxin A Using Upconversion Nanoparticles and Gold Nanorods. Mikrochim. Acta 2016, 183, 1909–1916. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Sitdikov, R.; Evtugyn, V.; Stoikov, I.; Antipin, I.; Hianik, T. Electrochemical Aptasensor for the Determination of Ochratoxin A at the Au Electrode Modified with Ag Nanoparticles Decorated with Macrocyclic Ligand. Electroanalysis 2013, 25, 1847–1854. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Zhao, X.; Chen, L.-J.; Yang, C.; Yin, X.-B.; Yan, X.-P. Persistent Luminescence Nanorod Based Luminescence Resonance Energy Transfer Aptasensor for Autofluorescence-Free Detection of Mycotoxin. Talanta 2020, 218, 121101. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Chen, D. Fluorescence Method for Quickly Detecting Ochratoxin A in Flour and Beer Using Nitrogen Doped Carbon Dots and Silver Nanoparticles. Talanta 2018, 182, 363–370. [Google Scholar] [CrossRef]
- Afzali, D.; Fathirad, F.; Ghaseminezhad, S. Determination of Trace Amounts of Ochratoxin A in Different Food Samples Based on Gold Nanoparticles Modified Carbon Paste Electrode. J. Food Sci. Technol. 2016, 53, 909–914. [Google Scholar] [CrossRef]
- Chen, R.; Sun, Y.; Huo, B.; Mao, Z.; Wang, X.; Li, S.; Lu, R.; Li, S.; Liang, J.; Gao, Z. Development of Fe3O4@Au Nanoparticles Coupled to Au@Ag Core-Shell Nanoparticles for the Sensitive Detection of Zearalenone. Anal. Chim. Acta 2021, 1180, 338888. [Google Scholar] [CrossRef]
- Ma, X.; Shao, B.; Wang, Z. Gold@silver Nanodumbbell Based Inter-Nanogap Aptasensor for the Surface Enhanced Raman Spectroscopy Determination of Ochratoxin A. Anal. Chim. Acta 2021, 1188, 339189. [Google Scholar] [CrossRef]
- Yu, X.; Lin, Y.; Wang, X.; Xu, L.; Wang, Z.; Fu, F. Exonuclease-Assisted Multicolor Aptasensor for Visual Detection of Ochratoxin A Based on G-Quadruplex-Hemin DNAzyme-Mediated Etching of Gold Nanorod. Mikrochim. Acta 2018, 185, 259. [Google Scholar] [CrossRef]
- Wang, L.; Jin, H.; Wei, M.; Ren, W.; Zhang, Y.; Jiang, L.; Wei, T.; He, B. A DNAzyme-Assisted Triple-Amplified Electrochemical Aptasensor for Ultra-Sensitive Detection of T-2 Toxin. Sens. Actuators B Chem. 2021, 328, 129063. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Jodra, A.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Magnetic Reduced Graphene Oxide/nickel/platinum Nanoparticles Micromotors for Mycotoxin Analysis. Chemistry 2018, 24, 7172–7176. [Google Scholar] [CrossRef]
- Wei, M.; Xin, L.; Feng, S.; Liu, Y. Simultaneous Electrochemical Determination of Ochratoxin A and Fumonisin B1 with an Aptasensor Based on the Use of a Y-Shaped DNA Structure on Gold Nanorods. Microchim. Acta 2020, 187, 102. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Jiang, X.; Zhou, B. Electrochemical Determination of Aflatoxin B1 (AFB1) Using a Copper-Based Metal-Organic Framework (Cu-MOF) and Gold Nanoparticles (AuNPs) with Exonuclease III (Exo III) Assisted Recycling by Differential Pulse Voltammetry (DPV). Anal. Lett. 2019, 52, 2439–2453. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Chen, D.; Tong, Q.-X. Carbon Dots/α-Fe2O3-Fe3O4 Nanocomposite: Efficient Synthesis and Application as a Novel Electrochemical Aptasensor for the Ultrasensitive Determination of Aflatoxin B1. Food Chem. 2022, 373, 131415. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, M.; Jian, Y.; Luo, J.; Xue, X.; Chen, X.; Zheng, X.; Ai, F. Simultaneous Aptasensor Assay of Ochratoxin A and Adenosine Triphosphate in Beer Based on Fe3O4 and SiO2 Nanoparticle as Carriers. Anal. Methods 2020, 12, 2253–2259. [Google Scholar] [CrossRef]
- Dai, S.; Wu, S.; Duan, N.; Chen, J.; Zheng, Z.; Wang, Z. An Ultrasensitive Aptasensor for Ochratoxin A Using Hexagonal Core/shell Upconversion Nanoparticles as Luminophores. Biosens. Bioelectron. 2017, 91, 538–544. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Wang, W.; Yu, Q.; Wang, Z. A Test Strip for Ochratoxin A Based on the Use of Aptamer-Modified Fluorescence Upconversion Nanoparticles. Mikrochim. Acta 2018, 185, 497. [Google Scholar] [CrossRef]
- Jodra, A.; López, M.Á.; Escarpa, A. Disposable and Reliable Electrochemical Magnetoimmunosensor for Fumonisins Simplified Determination in Maize-Based Foodstuffs. Biosens. Bioelectron. 2015, 64, 633–638. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Huang, H.; Bai, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; et al. A Fluorescence Aptasensor for the Sensitive Detection of T-2 Toxin Based on FRET by Adjusting the Surface Electric Potentials of UCNPs and MIL-101. Anal. Chim. Acta 2021, 1160, 338450. [Google Scholar] [CrossRef]
- Dai, S.; Wu, S.; Duan, N.; Wang, Z. A near-Infrared Magnetic Aptasensor for Ochratoxin A Based on near-Infrared Upconversion Nanoparticles and Magnetic Nanoparticles. Talanta 2016, 158, 246–253. [Google Scholar] [CrossRef]
- Goud, K.Y.; Yugender Goud, K.; Sunil Kumar, V.; Hayat, A.; Vengatajalabathy Gobi, K.; Song, H.; Kim, K.-H.; Marty, J.L. A Highly Sensitive Electrochemical Immunosensor for Zearalenone Using Screen-Printed Disposable Electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Lin, X.; Li, C.; He, C.; Zhou, Y.; Wang, Z.; Duan, N.; Wu, S. Upconversion Nanoparticles Assembled with Gold Nanourchins as Luminescence and Surface-Enhanced Raman Scattering Dual-Mode Aptasensors for Detection of Ochratoxin A. ACS Appl. Nano Mater. 2021, 4, 8231–8240. [Google Scholar] [CrossRef]
- Goud, K.Y.; Catanante, G.; Hayat, A.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. Disposable and Portable Electrochemical Aptasensor for Label Free Detection of Aflatoxin B1 in Alcoholic Beverages. Sens. Actuators B Chem. 2016, 235, 466–473. [Google Scholar] [CrossRef]
- Hayat, A.; Sassolas, A.; Marty, J.-L.; Radi, A.-E. Highly Sensitive Ochratoxin A Impedimetric Aptasensor Based on the Immobilization of Azido-Aptamer onto Electrografted Binary Film via Click Chemistry. Talanta 2013, 103, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lomillo, M.A.; Asunción Alonso-Lomillo, M.; Domínguez-Renedo, O.; del Torno-de Román, L.; Julia Arcos-Martínez, M. Horseradish Peroxidase-Screen Printed Biosensors for Determination of Ochratoxin A. Anal. Chim. Acta 2011, 688, 49–53. [Google Scholar] [CrossRef]
- Rhouati, A.; Hayat, A.; Hernandez, D.B.; Meraihi, Z.; Munoz, R.; Marty, J.-L. Development of an Automated Flow-Based Electrochemical Aptasensor for on-Line Detection of Ochratoxin A. Sens. Actuators B Chem. 2013, 176, 1160–1166. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Ferreira-Gonçalves, L.; Arcos-Martínez, M.J. Sensitive Enzyme-Biosensor Based on Screen-Printed Electrodes for Ochratoxin A. Biosens. Bioelectron. 2010, 25, 1333–1337. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Duan, N.; Wu, S.; Xia, Y.; Ma, X.; Zhu, C.; Jiang, Y.; Wang, Z. Screening and Identification of DNA Aptamers against T-2 Toxin Assisted by Graphene Oxide. J. Agric. Food Chem. 2014, 62, 10368–10374. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Qin, Y.; Zhang, J.; Chen, M.; Peng, Y.; Bai, J.; Li, S.; et al. A Fluorescence Aptasensor Based on Controlled Zirconium–based MOFs for the Highly Sensitive Detection of T–2 Toxin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 259, 119893. [Google Scholar] [CrossRef]
- Goud, K.Y.; Sharma, A.; Hayat, A.; Catanante, G.; Gobi, K.V.; Gurban, A.M.; Marty, J.L. Tetramethyl-6-Carboxyrhodamine Quenching-Based Aptasensing Platform for Aflatoxin B1: Analytical Performance Comparison of Two Aptamers. Anal. Biochem. 2016, 508, 19–24. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zhao, Q. A Signal-on Electrochemical Aptasensor for Rapid Detection of Aflatoxin B1 Based on Competition with Complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.-H. Rapid and Sensitive Inhibition-Based Assay for the Electrochemical Detection of Ochratoxin A and Aflatoxin M1 in Red Wine and Milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- de Cesare, G.; Nascetti, A.; Scipinotti, R.; Fanelli, C.; Ricelli, A.; Caputo, D. Optoelectronic System for Mycotoxin Detection in Food Quality Control. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1195–1202. [Google Scholar] [CrossRef]
- Bueno Hernández, D.; Mishra, R.K.; Muñoz, R.; Marty, J.L. Low Cost Optical Device for Detection of Fluorescence from Ochratoxin A Using a CMOS Sensor. Sens. Actuators B Chem. 2017, 246, 606–614. [Google Scholar] [CrossRef]
- Novo, P.; Moulas, G.; França Prazeres, D.M.; Chu, V.; Conde, J.P. Detection of Ochratoxin A in Wine and Beer by Chemiluminescence-Based ELISA in Microfluidics with Integrated Photodiodes. Sens. Actuators B Chem. 2013, 176, 232–240. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Huang, J.-D.; Chiu, C.-C. Amperometric Ethanol Biosensor Based on Poly(vinyl Alcohol)-Multiwalled Carbon Nanotube-Alcohol Dehydrogenase Biocomposite. Biosens. Bioelectron. 2007, 22, 3051–3056. [Google Scholar] [CrossRef]
- Manso, J.; Mena, M.L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Alcohol Dehydrogenase Amperometric Biosensor Based on a Colloidal Gold–carbon Nanotubes Composite Electrode. Electrochim. Acta 2008, 53, 4007–4012. [Google Scholar] [CrossRef]
- Lee, C.-A.; Tsai, Y.-C. Preparation of Multiwalled Carbon Nanotube-Chitosan-Alcohol Dehydrogenase Nanobiocomposite for Amperometric Detection of Ethanol. Sens. Actuators B Chem. 2009, 138, 518–523. [Google Scholar] [CrossRef]
- Adhikari, B.-R.; Schraft, H.; Chen, A. A High-Performance Enzyme Entrapment Platform Facilitated by a Cationic Polymer for the Efficient Electrochemical Sensing of Ethanol. Analyst 2017, 142, 2595–2602. [Google Scholar] [CrossRef]
- Wang, S.; Yao, Z.; Yang, T.; Zhang, Q.; Gao, F. Editors’ Choice—An Enzymatic Electrode Integrated with Alcohol Dehydrogenase and Chloranil in Liquid-Crystalline Cubic Phases on Carbon Nanotubes for Sensitive Amperometric Detection of NADH and Ethanol. J. Electrochem. Soc. 2019, 166, G116–G121. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Zhao, F.; Zeng, B. Direct Electrochemistry of Glucose Oxidase Entrapped in Nano Gold Particles-Ionic Liquid-N,N-Dimethylformamide Composite Film on Glassy Carbon Electrode and Glucose Sensing. Anal. Chim. Acta 2007, 587, 33–40. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Wang, X.; Li, C. One-Step Construction of an Electrode Modified with Electrodeposited Au/SiO2 Nanoparticles, and Its Application to the Determination of NADH and Ethanol. Mikrochim. Acta 2010, 171, 399–405. [Google Scholar] [CrossRef]
- Scarano, S.; Pascale, E.; Palladino, P.; Fratini, E.; Minunni, M. Determination of Fermentable Sugars in Beer Wort by Gold Nanoparticles@polydopamine: A Layer-by-Layer Approach for Localized Surface Plasmon Resonance Measurements at Fixed Wavelength. Talanta 2018, 183, 24–32. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A Paper-Based Nanomodified Electrochemical Biosensor for Ethanol Detection in Beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Li, C. Sensitive Determination of Dihydronicotinamide Adenine Dinucleotide and Ethanol with a Nano-Porous Carbon Electrode. J. Serb. Chem. Soc. 2011, 76, 113–123. [Google Scholar] [CrossRef]
- Jashari, G.; Švancara, I.; Sýs, M. Determination of Ethanol in Alcoholic Drinks: Flow Injection Analysis with Amperometric Detection versus Portable Raman Spectrometer. Electroanalysis 2020, 32, 1949–1956. [Google Scholar] [CrossRef]
- Venkatesan, R.; Cindrella, L. Semiconductive poly[N1,N4-bis (thiophen-2-Ylmethylene)benzene-1,4-Diamine]-Nickel Oxide Nanocomposite Based Ethanol Sensor. J. Appl. Polym. Sci. 2018, 135, 45918. [Google Scholar] [CrossRef]
- Zuliani, I.; Fattori, A.; Svigelj, R.; Dossi, N.; Grazioli, C.; Bontempelli, G.; Toniolo, R. Amperometric Detection of Ethanol Vapors by Screen Printed Electrodes Modified by Paper Crowns Soaked with Room Temperature Ionic Liquids. Electroanalysis 2022. [Google Scholar] [CrossRef]
- Bucur, B.; Radu, G.L.; Toader, C.N. Analysis of Methanol–ethanol Mixtures from Falsified Beverages Using a Dual Biosensors Amperometric System Based on Alcohol Dehydrogenase and Alcohol Oxidase. Eur. Food Res. Technol. 2008, 226, 1335–1342. [Google Scholar] [CrossRef]
- Tomassetti, M.; Angeloni, R.; Merola, G.; Castrucci, M.; Campanella, L. Catalytic Fuel Cell Used as an Analytical Tool for Methanol and Ethanol Determination. Application to Ethanol Determination in Alcoholic Beverages. Electrochim. Acta 2016, 191, 1001–1009. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Negulescu, G.P. Ethanol Determination by an Amperometric Bienzyme Sensor Based on a Clark-Type Transducer. J. Electroanal. Chem. 2012, 671, 85–91. [Google Scholar] [CrossRef]
- Nakamura, H.; Tanaka, R.; Suzuki, K.; Yataka, M.; Mogi, Y. A Direct Determination Method for Ethanol Concentrations in Alcoholic Beverages Employing a Eukaryote Double-Mediator System. Food Chem. 2009, 117, 509–513. [Google Scholar] [CrossRef]
- Wen, G.M.; Shuang, S.M.; Dong, C.; Choi, M.M.F. An Ethanol Biosensor Based on a Bacterial Cell-Immobilized Eggshell Membrane. Chin. Chem. Lett. 2012, 23, 481–483. [Google Scholar] [CrossRef]
- Curbani, L.; Gelinski, J.M.L.N.; Borges, E.M. Determination of Ethanol in Beers Using a Flatbed Scanner and Automated Digital Image Analysis. Food Anal. Methods 2020, 13, 249–259. [Google Scholar] [CrossRef]
- Farina, D.; Zinellu, M.; Fanari, M.; Porcu, M.C.; Scognamillo, S.; Puggioni, G.M.G.; Rocchitta, G.; Serra, P.A.; Pretti, L. Development of a Biosensor Telemetry System for Monitoring Fermentation in Craft Breweries. Food Chem. 2017, 218, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Zór, K.; Gáspár, S.; Hashimoto, M.; Suzuki, H.; Csöregi, E. High Temporal Resolution Monitoring of Fermentations Using an on-Line Amperometric Flow-through Microdetector. Electroanalysis 2007, 19, 43–48. [Google Scholar] [CrossRef]
- Hoche, S.; Krause, D.; Hussein, M.A.; Becker, T. Ultrasound-Based, in-Line Monitoring of Anaerobe Yeast Fermentation: Model, Sensor Design and Process Application. Int. J. Food Sci. Technol. 2016, 51, 710–719. [Google Scholar] [CrossRef]
- Magdalena Pisoschi, A. Improvement of Alcohol Dehydrogenase and Horseradish Peroxidase Loadings in Ethanol Determination by a Bienzyme Sensor. Lett. Org. Chem. 2013, 10, 611–616. [Google Scholar] [CrossRef]
- Odaci, D.; Telefoncu, A.; Timur, S. Maltose Biosensing Based on Co-Immobilization of Alpha-Glucosidase and Pyranose Oxidase. Bioelectrochemistry 2010, 79, 108–113. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Feng, B.; Chen, H.; You, L.; Pei, H. Monitoring Glucose in Fermented Beer by an Electrochemical Sensor Based on Graphene Oxide Decorated by Silver Nanoparticles. Int. J. Electrochem. Sci. 2021, 16, 210812. [Google Scholar] [CrossRef]
- Kornii, A.; Saska, V.; Lisnyak, V.V.; Tananaiko, O. Carbon Nanostructured Screen-printed Electrodes Modified with CuO/Glucose Oxidase/Maltase/SiO2 Composite Film for Maltose Determination. Electroanalysis 2020, 32, 1468–1479. [Google Scholar] [CrossRef]
- Dacres, H.; Michie, M.; Anderson, A.; Trowell, S.C. Advantages of Substituting Bioluminescence for Fluorescence in a Resonance Energy Transfer-Based Periplasmic Binding Protein Biosensor. Biosens. Bioelectron. 2013, 41, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Le, N.C.H.; Gel, M.; Zhu, Y.; Dacres, H.; Anderson, A.; Trowell, S.C. Real-Time, Continuous Detection of Maltose Using Bioluminescence Resonance Energy Transfer (BRET) on a Microfluidic System. Biosens. Bioelectron. 2014, 62, 177–181. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Koller, H.; Perkins, L.B. Brewing and the Chemical Composition of Amine-Containing Compounds in Beer: A Review. Foods 2022, 11, 257. [Google Scholar] [CrossRef]
- Zotou, A.; Loukou, Z. 100—Methods for Determining Biogenic Amines in Beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 1031–1041. ISBN 9780123738912. [Google Scholar]
- Stojanović, Z.S.; Mehmeti, E.; Kalcher, K.; Guzsvány, V.; Stanković, D.M. SWCNT-Modified Carbon Paste Electrode as an Electrochemical Sensor for Histamine Determination in Alcoholic Beverages. Food Anal. Methods 2016, 9, 2701–2710. [Google Scholar] [CrossRef]
- Shumeiko, V.; Paltiel, Y.; Bisker, G.; Hayouka, Z.; Shoseyov, O. A Nanoscale Paper-Based near-Infrared Optical Nose (NIRON). Biosens. Bioelectron. 2021, 172, 112763. [Google Scholar] [CrossRef]
- Li, S.; Zhong, T.; Long, Q.; Huang, C.; Chen, L.; Lu, D.; Li, X.; Zhang, Z.; Shen, G.; Hou, X. A Gold Nanoparticles-Based Molecularly Imprinted Electrochemical Sensor for Histamine Specific-Recognition and Determination. Microchem. J. 2021, 171, 106844. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Devi, R.; Yadav, S.; Pundir, C.S. Fabrication of an Amperometric Tyramine Biosensor Based on Immobilization of Tyramine Oxidase on AgNPs/l-Cys-Modified Au Electrode. J. Solid State Electrochem. 2012, 16, 3869–3876. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, L.; Li, R.; Qu, Z.; Zou, X.; Jia, S. Electrochemical Non-Enzymatic Biosensor for Tyramine Detection in Food Based on Silver-Substituted ZnO Nano-Flower Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2021, 16, 210234. [Google Scholar] [CrossRef]
- Li, F.; Wu, F.; Luan, X.; Yuan, Y.; Zhang, L.; Xu, G.; Niu, W. Highly Enantioselective Electrochemical Sensing Based on Helicoid Au Nanoparticles with Intrinsic Chirality. Sens. Actuators B Chem. 2022, 362, 131757. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.; Zhao, T.; Mao, Y.; Fang, G.; Wang, S. A Core-Shell Molecularly Imprinted Optical Sensor Based on the Upconversion Nanoparticles Decorated with Zinc-Based Metal-Organic Framework for Selective and Rapid Detection of Octopamine. Sens. Actuators B Chem. 2021, 326, 128838. [Google Scholar] [CrossRef]
- Ramon-Marquez, T.; Medina-Castillo, A.L.; Fernandez-Gutierrez, A.; Fernandez-Sanchez, J.F. Novel Optical Sensing Film Based on a Functional Nonwoven Nanofibre Mat for an Easy, Fast and Highly Selective and Sensitive Detection of Tryptamine in Beer. Biosens. Bioelectron. 2016, 79, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Federico, R.; Boffi, A.; Macone, A.; Favero, G.; Mazzei, F. Characterization and Application of a Diamine Oxidase from Lathyrus Sativus as Component of an Electrochemical Biosensor for the Determination of Biogenic Amines in Wine and Beer. Anal. Bioanal. Chem. 2011, 401, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gong, Y.; Fan, Z. Cysteine Detection Using a High-Fluorescence Sensor Based on a Nitrogen-Doped Graphene Quantum dot–mercury(II) System. J. Lumin. 2016, 175, 129–134. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, J.; He, Y.; Sheng, Q. A Sandwich-Type Phenolic Biosensor Based on Tyrosinase Embedding into Single-Wall Carbon Nanotubes and Polyaniline Nanocomposites. Sens. Actuators B Chem. 2013, 186, 417–422. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, K.; Li, Y.; Li, K.; Ye, B. Study on the Electrochemical Properties of Maltol at a Carbon Paste Electrode and Its Analytical Application. Anal. Methods 2012, 4, 3206–3211. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and Direct Amperometric Analysis of Polyphenols in Beers Using Tyrosinase-Modified Screen-Printed Gold Nanoparticles Biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Wang, Y.-Y.; Shen, X.-F.; Qiao, J.-Y. Covalent Organic Framework Modified Carbon Cloth for Ratiometric Electrochemical Sensing of Bisphenol A and S. Mikrochim. Acta 2022, 189, 189. [Google Scholar] [CrossRef]
- Yang, H.; He, L.; Pan, S.; Liu, H.; Hu, X. Nitrogen-Doped Fluorescent Carbon Dots for Highly Sensitive and Selective Detection of Tannic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 111–119. [Google Scholar] [CrossRef] [PubMed]
- de Paula, N.T.; Milani, R.; Lavorante, A.; Paim, A.P. Use of Carbon Dots Synthesized from Citrate as a Fluorescent Probe for Quercetin Determination in Tea and Beer Samples. J. Braz. Chem. Soc. 2019, 30, 2355–2366. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, J.; Liu, Y.; Wang, Y.; Xiao, Y.; Zhang, Y. One-Step Synthesis of N, S-Doped Carbon Dots with Orange Emission and Their Application in Tetracycline Antibiotics, Quercetin Sensing, and Cell Imaging. Microchim. Acta 2021, 188. [Google Scholar] [CrossRef] [PubMed]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; de la Vega, M.D.; de Cisneros, J.L.H.-H. Dual Laccase-Tyrosinase Based Sonogel-Carbon Biosensor for Monitoring Polyphenols in Beers. J. Agric. Food Chem. 2007, 55, 8011–8018. [Google Scholar] [CrossRef]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; Hernández-Artiga, M.P.; Bellido-Milla, D.; de Cisneros, J.L.H.H. A Comparison of Three Amperometric Phenoloxidase-Sonogel-Carbon Based Biosensors for Determination of Polyphenols in Beers. Food Chem. 2008, 110, 1019–1024. [Google Scholar] [CrossRef]
- Martinez-Periñan, E.; Hernández-Artiga, M.P.; Palacios-Santander, J.M.; ElKaoutit, M.; Naranjo-Rodriguez, I.; Bellido-Milla, D. Estimation of Beer Stability by Sulphur Dioxide and Polyphenol Determination. Evaluation of a Laccase-Sonogel-Carbon Biosensor. Food Chem. 2011, 127, 234–239. [Google Scholar] [CrossRef]
- Molina-García, L.; Ruiz-Medina, A.; Fernández-de Córdova, M.L. A Novel Multicommuted Fluorimetric Optosensor for Determination of Resveratrol in Beer. Talanta 2011, 83, 850–856. [Google Scholar] [CrossRef]
- El Kaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; Hidalgo-Hidalgo de Cisneros, J.L. The Sonogel–Carbon Materials as Basis for Development of Enzyme Biosensors for Phenols and Polyphenols Monitoring: A Detailed Comparative Study of Three Immobilization Matrixes. Biosens. Bioelectron. 2007, 22, 2958–2966. [Google Scholar] [CrossRef]
- Montanari, L.; Perretti, G.; Natella, F.; Guidi, A.; Fantozzi, P. Organic and Phenolic Acids in Beer. LWT-Food Sci. Technol. 1999, 32, 535–539. [Google Scholar] [CrossRef]
- Pai, T.V.; Sawant, S.Y.; Ghatak, A.A.; Chaturvedi, P.A.; Gupte, A.M.; Desai, N.S. Characterization of Indian Beers: Chemical Composition and Antioxidant Potential. J. Food Sci. Technol. 2015, 52, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.E.; Santos, I.C.; Hildenbrand, Z.L.; Schug, K.A. A Review of the Analytical Methods Used for Beer Ingredient and Finished Product Analysis and Quality Control. Anal. Chim. Acta 2019, 1085, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Fàbregas, E. Amperometric Bienzymatic Biosensor for L-Lactate Analysis in Wine and Beer Samples. Analyst 2012, 137, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- Paz Zanini, V.I.; Tulli, F.; Martino, D.M.; López de Mishima, B.; Borsarelli, C.D. Improvement of the Amperometric Response to L-Lactate by Using a Cationic Bioinspired Thymine Polycation in a Bioelectrode with Immobilized Lactate Oxidase. Sens. Actuators B Chem. 2013, 181, 251–258. [Google Scholar] [CrossRef]
- Batra, B.; Narwal, V.; Pundir, C.S. An Amperometric Lactate Biosensor Based on Lactate Dehydrogenase Immobilized onto Graphene Oxide Nanoparticles-Modified Pencil Graphite Electrode. Eng. Life Sci. 2016, 16, 786–794. [Google Scholar] [CrossRef]
- Vargas, E.; Ruiz, M.A.; Campuzano, S.; González de Rivera, G.; López-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. Implementation of a New Integrated D-Lactic Acid Biosensor in a Semiautomatic FIA System for the Simultaneous Determination of Lactic Acid Enantiomers. Application to the Analysis of Beer Samples. Talanta 2016, 152, 147–154. [Google Scholar] [CrossRef]
- Lowinsohn, D.; Bertotti, M. A Biosensor Based on Immobilization of Lactate Oxidase in a PB-CTAB Film for FIA Determination of Lactate in Beer Samples. J. Braz. Chem. Soc. 2008, 19, 637–642. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chen, A. Voltammetric Detection of the α-Dicarbonyl Compound: Methylglyoxal as a Flavoring Agent in Wine and Beer. Anal. Chim. Acta 2012, 751, 66–70. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Y.; Hu, X.; Chen, Z.; Li, J.; Zhang, M. A “two-Step” Assay Based on Electro-Activation for Rapid Determination of Methylglyoxal in Honey and Beer. Anal. Chim. Acta 2022, 1203, 339688. [Google Scholar] [CrossRef]
- Li, X.; Yin, Z.; Cui, X.; Yang, L. Capillary Electrophoresis-integrated Immobilized Enzyme Microreactor with Graphene Oxide as Support: Immobilization of Negatively Charged L-lactate Dehydrogenase via Hydrophobic Interactions. Electrophoresis 2020, 41, 175–182. [Google Scholar] [CrossRef]
- Wooldridge, H.B. The Use of Hydrogen Peroxide in the Brewery. J. Inst. Brew. 1916, 22, 436–448. [Google Scholar] [CrossRef]
- Bleoanca, I.; Silva, A.R.C.; Pimentel, C.; Rodrigues-Pousada, C.; de Andrade Menezes, R. Relationship between Ethanol and Oxidative Stress in Laboratory and Brewing Yeast Strains. J. Biosci. Bioeng. 2013, 116, 697–705. [Google Scholar] [CrossRef]
- Wang, C.; Huang, S.; Luo, L.; Zhou, Y.; Lu, X.; Zhang, G.; Ye, H.; Gu, J.; Cao, F. Ultrathin Two-Dimension Metal-Organic Framework Nanosheets/multi-Walled Carbon Nanotube Composite Films for the Electrochemical Detection of H2O2. J. Electroanal. Chem. 2019, 835, 178–185. [Google Scholar] [CrossRef]
- Wang, N.; Hei, Y.; Liu, J.; Sun, M.; Sha, T.; Hassan, M.; Bo, X.; Guo, Y.; Zhou, M. Low-Cost and Environment-Friendly Synthesis of Carbon Nanorods Assembled Hierarchical Meso-Macroporous Carbons Networks Aerogels from Natural Apples for the Electrochemical Determination of Ascorbic Acid and Hydrogen Peroxide. Anal. Chim. Acta 2019, 1047, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, H.; Liu, C.; Su, C.; Liu, W.; Wang, K.; Jiang, J. Ultrathin Phthalocyanine-Conjugated Polymer Nanosheet-Based Electrochemical Platform for Accurately Detecting H2O2 in Real Time. ACS Appl. Mater. Interfaces 2019, 11, 11466–11473. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on Metal–Organic Framework Engenders High Sensitivity for Enzymatic Electrochemical Detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to Endocrine Disruptors during Adulthood: Consequences for Female Fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef]
- Rahm, J.V.; Malkusch, S.; Endesfelder, U.; Dietz, M.S.; Heilemann, M. Diffusion State Transitions in Single-Particle Trajectories of MET Receptor Tyrosine Kinase Measured in Live Cells. Front. Comput. Sci. 2021, 3, 757653. [Google Scholar] [CrossRef]
- Izah, S.C.; Inyang, I.R.; Angaye, T.C.N.; Okowa, I.P. A Review of Heavy Metal Concentration and Potential Health Implications of Beverages Consumed in Nigeria. Toxics 2016, 5, 1. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of Exposure of Heavy Metals and Their Impact on Health Consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Loganathan, C.; Narayanamoorthi, E.; John, S.A. Leaching of AuNPs from the Surface of GO: Sensitive Turn on Fluorescence Detection of Toxic Preservative. Food Chem. 2020, 309, 125751. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Martins, A.; Lobato, A.; Tasić, N.; Perez-Sanz, F.J.; Vidinha, P.; Paixão, T.R.L.C.; Moreira Gonçalves, L. Laser-Pyrolyzed Electrochemical Paper-Based Analytical Sensor for Sulphite Analysis. Electrochem. Commun. 2019, 107, 106541. [Google Scholar] [CrossRef]
- Ramos, R.M.; Brandão, P.F.; Gonçalves, L.M.; Vyskočil, V.; Rodrigues, J.A. Electrochemical Sensing of Total Sulphites in Beer Using Non-Modified Screen-Printed Carbon Electrodes. J. Inst. Brew. 2017, 123, 45–48. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Zhang, J.; Yun, W.; Xiong, Z.; Yang, L. Simultaneous and Ultra-Sensitive Detection of Cu2+ and Mg2+ in Wine and Beer Based on Dual DNA Tweezers and Entropy-Driven Three-Dimensional DNA Nanomachine. Food Chem. 2021, 358, 129835. [Google Scholar] [CrossRef]
- Silva, L.R.G.; Mutz, Y.S.; Stefano, J.S.; Conte-Junior, C.A.; de Ferreira, R.Q. A Simple and Reliable Electroanalytical Method Employing a Disposable Commercial Electrode for Simultaneous Determination of lead(II) and mercury(II) in Beer. J. Food Compost. Anal. 2022, 110, 104564. [Google Scholar] [CrossRef]
- Bandi, K.R.; Singh, A.K.; Kamaluddin; Jain, A.K.; Gupta, V.K. Electroanalytical Studies on cobalt(II) Ion-Selective Sensor of Polymeric Membrane Electrode and Coated Graphite Electrode Based on N2O2 Salen Ligands. Electroanalysis 2011, 23, 2839–2850. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Khayat, M.A.; Bhargava, S.K.; Raisoni, J.R. Electroanalytical Studies on cobalt(II) Selective Potentiometric Sensor Based on Bridge Modified Calixarene in Poly(vinyl Chloride). Electrochim. Acta 2008, 53, 5409–5414. [Google Scholar] [CrossRef]
- Eren, H.; Uzun, H.; Andac, M.; Bilir, S. Potentiometric Monitoring of Cobalt in Beer Sample by Solid Contact Ion Selective Electrode. J. Food Drug Anal. 2014, 22, 413–417. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Luo, X.; Kong, X.; Sun, Z.; You, J. A FRET-Based Ratiometric Fluorescent Probe for Sulfide Detection in Actual Samples and Imaging in Daphnia Magna. Talanta 2020, 209, 120517. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, X.; Zeng, F.; Yu, C.; Wu, S. A Novel Ratiometric Fluorescent Probe through Aggregation-Induced Emission and Analyte-Induced Excimer Dissociation. Sens. Actuators B Chem. 2014, 203, 504–510. [Google Scholar] [CrossRef]
- Xie, H.; Zeng, F.; Yu, C.; Wu, S. A Polylysine-Based Fluorescent Probe for Sulfite Anion Detection in Aqueous Media via Analyte-Induced Charge Generation and Complexation. Polym. Chem. 2013, 4, 5416–5424. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yang, S.; Tian, H.; Sun, B.; Liu, Y. A Reaction-Based Novel Fluorescent Probe for Detection of Hydrogen Sulfide and Its Application in Wine. J. Food Sci. 2018, 83, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. Highly Selective and Rapidly Responsive Fluorescent Probe for Hydrogen Sulfide Detection in Wine. Food Chem. 2018, 257, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-M.; Lee, C.-Y.; Liao, M.-H.; Lin, C.-H. Fabrication and Testing of High-Performance Detection Sensor for Capillary Electrophoresis Microchips. Biomed. Microdevices 2008, 10, 73–80. [Google Scholar] [CrossRef]
- Broncová, G.; Shishkanova, T.V.; Dendisová, M.; Člupek, M.; Kubáč, D.; Matějka, P. Poly(4-Amino-2,1,3-Benzothiadiazole) Films: Preparation, Characterization and Applications. Chem. Pap. 2017, 71, 359–366. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Performance of Selective and Partially Selective Sensors in the Recognition of Beverages. Talanta 2007, 71, 738–746. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Duan, H.; Niu, C.; Wang, J.; Zheng, F.; Li, Y.; Li, Q. Isomerization of Gibberellic Acid During the Brewing Process. J. Food Sci. 2019, 84, 1353–1361. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P.; et al. Heavy Metal Induced Regulation of Plant Biology: Recent Insights. Physiol. Plant. 2022, 174, e13688. [Google Scholar] [CrossRef]
- Xie, H.-Z.; Yang, B.; Li, J.-P. A Molecularly Imprinted Electrochemical Luminescence Sensor for Detection of Gibberellin Based on Energy Transfer. Chin. J. Anal. Chem. 2020, 48, 1633–1641. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Wei, X.-P.; Wei, Y.-X.; Li, J.-P.; Zeng, Y. Determination of Trace Gibberellin A3 by Magnetic Self-Assembly Molecularly Imprinted Electrochemical Sensor. Chin. J. Anal. Chem. 2014, 42, 1580–1585. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wei, X.; Tao, H.; Pan, H. Molecularly Imprinted Electrochemical Luminescence Sensor Based on Signal Amplification for Selective Determination of Trace Gibberellin A3. Anal. Chem. 2012, 84, 9951–9955. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, J.-P. A Molecular-Imprinted Sensor for Trace Detection of Gibberellin Based on Ferrocenecarboxylic Acid Multiply Marked Dendrimer. Chin. J. Anal. Chem. 2014, 42, 315–319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).