Pulsed Electric Field Processing of Red Wine: Effect on Wine Quality and Microbial Inactivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Red Wine Samples
2.2. Test Microorganisms and Their Inoculation into Wine Samples
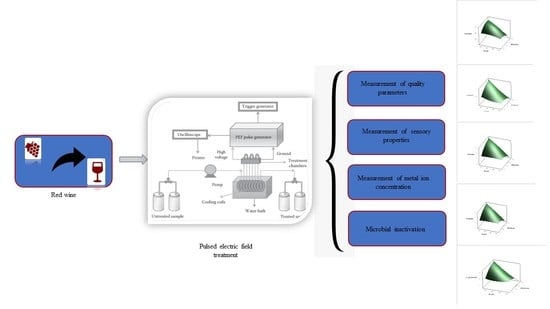
2.3. PEF Treatment
2.4. Red Wine Physicochemical Analyses
2.5. Microbiological Analyses
2.6. Sensory Analyses
2.7. Data Analyses
3. Results and Discussions
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, F.V.M.; van Wyk, S. Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine. Foods 2021, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- Sainz-García, E.; López-Alfaro, I.; Múgica-Vidal, R.; López, R.; Escribano-Viana, R.; Portu, J.; Alba-Elías, F.; González-Arenzana, L. Effect of the Atmospheric Pressure Cold Plasma Treatment on Tempranillo Red Wine Quality in Batch and Flow Systems. Beverages 2019, 5, 50. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of Non Thermal Processing Technologies on the Anthocyanin Content of Fruit Juices. Trends Food Sci. Technol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Hernández-Orte, P.; Álvarez, I.; Raso, J. Effect of a Pulsed Electric Field Treatment on the Anthocyanins Composition and Other Quality Parameters of Cabernet Sauvignon Freshly Fermented Model Wines Obtained after Different Maceration Times. LWT-Food Sci. Technol. 2009, 42, 1225–1231. [Google Scholar] [CrossRef]
- González-Neves, G.; Gil, G.; Barreiro, L. Influence of Grape Variety on the Extraction of Anthocyanins during the Fermentation on Skins. Eur. Food Res. Technol. 2008, 226, 1349–1355. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Boussetta, N.; Lebovka, N.; Vorobiev, E.; Adenier, H.; Bedel-Cloutour, C.; Lanoisellé, J.-L. Electrically Assisted Extraction of Soluble Matter from Chardonnay Grape Skins for Polyphenol Recovery. J. Agric. Food Chem. 2009, 57, 1491–1497. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldaña, G.; Condón, S.; Álvarez, I.; Raso, J. Evolution of Polyphenolic Compounds in Red Wine from Cabernet Sauvignon Grapes Processed by Pulsed Electric Fields during Aging in Bottle. Food Chem. 2010, 119, 1063–1070. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed Electric Fields Inactivation of Wine Spoilage Yeast and Bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef]
- Abca, E.E.; Akdemir Evrendilek, G. Processing of Red Wine by Pulsed Electric Fields with Respect to Quality Parameters. J. Food Process. Preserv. 2015, 39, 758–767. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Marsellés-Fontanet, A.R.; Arias-Gil, M.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effect of Storage Conditions on the Volatile Composition of Wines Obtained from Must Stabilized by PEF during Ageing without SO2. Innov. Food Sci. Emerg. Technol. 2008, 9, 469–476. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Effects of Pulsed Electric Fields on the Extraction of Phenolic Compounds during the Fermentation of Must of Tempranillo Grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Álvarez, I.; Raso, J. Potential Applications of PEF to Improve Red Wine Quality. Trends Food Sci. Technol. 2010, 21, 247–255. [Google Scholar] [CrossRef]
- Ates, C.; Akdemir Evrendilek, G.; Uzuner, S. High-Pressure Processing of Shalgam with Respect to Quality Characteristics, Microbial Inactivation, and Shelflife Extension. J. Food Process. Preserv. 2021, 45, e15598. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Bakay, S.; Uzuner, S. High Pressure Processing of Licorice Drink with Respect to Quality Characteristics, Microbial Inactivation, and Shelf-Life Extension. J. Food Process. Preserv. 2021, 45, e15465. [Google Scholar] [CrossRef]
- Delsart, C.; Grimi, N.; Boussetta, N.; Miot Sertier, C.; Ghidossi, R.; Vorobiev, E.; Mietton Peuchot, M. Impact of Pulsed-Electric Field and High-Voltage Electrical Discharges on Red Wine Microbial Stabilization and Quality Characteristics. J. Appl. Microbiol. 2016, 120, 152–164. [Google Scholar] [CrossRef]
- Zulueta, A.; Barba, F.J.; Esteve, M.J.; Frígola, A. Changes in Quality and Nutritional Parameters During Refrigerated Storage of an Orange Juice–Milk Beverage Treated by Equivalent Thermal and Non-Thermal Processes for Mild Pasteurization. Food Bioprocess Technol. 2013, 6, 2018–2030. [Google Scholar] [CrossRef]
- van Wyk, S.; Farid, M.M.; Silva, F.V.M. SO2, High Pressure Processing and Pulsed Electric Field Treatments of Red Wine: Effect on Sensory, Brettanomyces Inactivation and Other Quality Parameters during One Year Storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 204–211. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Application of Pulsed Electric Fields for Improving the Maceration Process During Vinification of Red Wine: Influence of Grape Variety. Eur. Food Res. Technol. 2008, 227, 1099–1107. [Google Scholar] [CrossRef]
- Morren, J.; Roodenburg, B.; de Haan, S.W.H. Electrochemical Reactions and Electrode Corrosion in Pulsed Electric Field (PEF) Treatment Chambers. Innov. Food Sci. Emerg. Technol. 2003, 4, 285–295. [Google Scholar] [CrossRef]
- Rein, M.J.; Heinonen, M. Stability and Enhancement of Berry Juice Color. J. Agric. Food Chem. 2004, 52, 3106–3114. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage Yeasts in the Wine Industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Pretorius, I.S. Microbial Spoilage and Preservation of Wine: Using Weapons for Nature’s Own Arsenal. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar] [CrossRef][Green Version]
- Usseglio-Tomasset, L. Properties and Use of Sulphur Dioxide. Food Addit. Contam. 1992, 9, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The Production of Ethylphenols in Wine by Yeasts of the Genera Brettanomyces and Dekkera: A Review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Couto, J.A.; Neves, F.; Campos, F.; Hogg, T. Thermal Inactivation of the Wine Spoilage Yeasts Dekkera/Brettanomyces. Int. J. Food Microbiol. 2005, 104, 337–344. [Google Scholar] [CrossRef]
- Puértolas, E.; Hernández-Orte, P.; Sladaña, G.; Álvarez, I.; Raso, J. Improvement of Winemaking Process Using Pulsed Electric Fields at Pilot-Plant Scale. Evolution of Chromatic Parameters and Phenolic Content of Cabernet Sauvignon Red Wines. Food Res. Int. 2010, 43, 761–766. [Google Scholar] [CrossRef]
| Energy (kJ) | pH | TA (g of Tartaric Acid/L) | Conductivity (mS/cm) | L* | a* | b* | TPSC (mg GAE/L) | TAC (%) | TMAC (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 2.94 ± 0.04 c | 1.98 ±0.04 a | 2.27 ± 0.02 d | 13.92 ± 0.60 ab | 39.85 ± 2.17 abc | 22.21 ± 0.95 f | 2212.5 ± 23.4 c | 72.68 ± 0.45 b | 37.19 ± 0.46 e |
| 2.4 | 2.96 ± 0.04 abc | 1.20 ± 0.01 a | 2.27 ± 0.01 cd | 12.03 ± 0.64 cd | 38.73 ± 0.05 abcd | 21.10 ± 0.07 f | 2225 ± 18.5 bc | 72.65 ± 0.08 b | 37.09 ±0.15 de |
| 3.4 | 2.97 ± 0.03 abc | 2.00 ± 0.00 a | 2.26 ± 0.02 d | 10.66 ± 0.56 d | 34.47 ± 0.10 d | 21.43 ± 0.06 e | 2339 ± 35.2 abc | 72.83 ± 0.63 ab | 37.12 ± 0.04 de |
| 4.4 | 3.00 ±0.01 abc | 1.98 ± 0.01 a | 2.30 ± 0.01 bcd | 10.82 ± 0.59 d | 37.93 ± 0.05 bcd | 21.64 ± 0.06 d | 2424 ± 1.82 ab | 72.86 ± 0.63 ab | 38.01 ± 0.23 bcd |
| 4.9 | 2.93 ± 0.01 bc | 1.99 ± 0.01 a | 2.30 ± 0.01 bcd | 13.13 ± 0.03 abc | 39.70 ± 0.06 abc | 21.80 ± 0.07 c | 2295 ± 6.26 abc | 73.34 ±0.77 ab | 37.67 ± 0.24 cde |
| 6.8 | 2.96 ± 0.02 abc | 2.00 ± 0.00 a | 2.31 ± 0.01 abc | 13.58 ± 1.50 abc | 41.04 ± 0.09 a | 22.43 ± 0.06 b | 2366.3 ± 38.5 abc | 73.18 ±0.86 ab | 37.51 ± 0.43 cde |
| 7.3 | 2.98 ± 0.01 abc | 1.99 ±0.01 a | 2.31 ± 0.01 abc | 14.76 ± 0.05 a | 41.85 ± 0.09 ab | 23.94 ± 0.17 a | 2382 ± 6.44 abc | 73.58 ± 0.23 ab | 37.99 ± 0.08 bcd |
| 8.8 | 3.03 ± 0.01 ab | 1.99 ± 0.01 a | 2.32 ± 0.02 ab | 12.01 ± 0.96 cd | 37.08 ± 1.05 cd | 17.64 ± 0.06 c | 2477 ± 6.85 a | 73.49 ± 0.35 ab | 38.70 ± 0.32 ab |
| 10.2 | 3.03 ± 0.06 b | 1.20 ± 0.01 a | 2.33 ± 0.01 ab | 12.71 ± 0.34 bc | 36.83 ± 0.53 cd | 14.82 ± 0.62 d | 2373.3 ± 29.1 abc | 73.58 ± 0.42 ab | 38.24 ± 0.12 abc |
| 13.2 | 3.05 ± 0.02 a | 1.97 ±0.01 a | 2.34 ± 0.01 a | 13.06 ± 0.09 abc | 40.22 ± 0.05 abc | 21.20 ± 0.10 b | 2494. 59 ± 2.12 a | 74.04 ± 0.18 a | 39.25 ± 0.37 a |
| Energy (kJ) | C. lipoliytica | S. cerevisiae | H. anemola | L. delbrueckii ssp. bulgaricus | E. coli O157:H7 |
|---|---|---|---|---|---|
| 0.0 | 6.25 ± 0.76 a | 7.86 ± 1.12 a | 6.30 ± 1.34 a | 6.11 ± 0.23 a | 6.40 ± 0.55 a |
| 2.4 | 3.54 ± 0.11 b | 5.13 ± 0.11 b | 5.03 ± 0.02 ab | 5.52 ± 0.16 b | 4.70 ± 0.33 b |
| 3.4 | 3.17 ± 0.27 b | 3.96 ± 0.47 bc | 4.77 ± 0.09 b | 5.30 ± 0.26 b | 4.67 ± 0.12 b |
| 4.4 | 3.02 ± 0.14 b | 3.41 ± 0.24 bcd | 4.34 ± 0.32 b | 5.19 ± 0.03 b | 3.48 ± 0.35 c |
| 4.9 | 2.85 ± 0.14 b | 3.16 ±± 0.53 bcde | 4.05 ± 0.20 bc | 4.29 ± 0.23 c | 3.23 ± 0.08 abcd |
| 6.8 | 2.39 ± 0.51 bc | 2.63 ± 0.52 cdef | 3.74 ± 0.08 bcd | 4.04 ± 0.02 c | 2.15 ± 0.36 cd |
| 7.3 | 1.05 ± 0.08 cd | 2.43 ± 0.09 cdef | 3.08 ± 0.12 bcd | 3.30 ± 0.33 d | 1.48 ± 0.09 ef |
| 8.8 | 1.02 ± 0.03 cd | 1.63 ± 0.42 def | 1.96 ± 0.07 cde | 3.00 ± 0.08 d | 1.20 ± 0.18 ef |
| 10.2 | 0.95 ± 0.07 cd | 0.89 ± 0.26 ef | 1.64 ± 0.06 de | 2.28 ± 0.34 e | 1.01 ± 0.17 ef |
| 13.2 | 0.60 ± 0.07 d | 0.50 ± 0.16 f | 0.33 ± 0.12 e | 1.09 ± 0.36 f | 0.49 ± 0.14 f |
| Energy (kJ) | Na (μg/mL) | Mg (μg/mL) | K (μg/mL) | Mn (μg/mL) |
|---|---|---|---|---|
| 0.0 | 80.00 ± 5.77 a | 339.62 ± 26.43 a | 2246.20 ± 136.00 a | 103.6 ± 3.67 a |
| 2.4 | 73.30 ± 4.46 a | 310.00 ± 31.40 a | 2301.11 ± 264.08 a | 11.67 ± 3.87 a |
| 3.4 | 76.56 ± 2.28 a | 350.40 ± 39.30 a | 2224.54 ± 186.89 a | 7.18 ± 2.55 a |
| 4.4 | 77.30 ± 2.93 a | 366.73 ± 12.34 a | 2121.70 ± 120.10 a | 6.82 ± 0.82 a |
| 4.9 | 72.51 ± 5.45 a | 362.20 ± 20.20 a | 2134.00 ± 152.50 a | 9.92 ± 1.19 a |
| 6.8 | 75.78 ± 3.00 a | 350.65 ± 28.10 a | 2303.90 ± 33.40 a | 7.35 ± 0.28 a |
| 7.3 | 75.75 ± 6.07 a | 347.30 ± 30.30 a | 2366.00 ± 103.33 a | 8.89 ± 1.44 a |
| 8.8 | 77.38 ± 2.76 a | 340.80 ± 54.32 a | 2307.70 ± 105.00 a | 6.78 ± 0.38 a |
| 10.2 | 73.75 ± 6.07 a | 351.65 ± 28.98 a | 2266.50 ± 155.80 a | 7.13 ± 0.68 a |
| 13.2 | 77.38 ± 5.55 a | 350.29 ± 39.58 a | 2273.60 ± 72.50 a | 6.64 ± 0.24 a |
| Energy (kJ) | Cloudiness/ Clearness | Dullness/ Brightness | Red Color Intensity | Density | Particle Status | Odor/Flavor | Bitterness | Sour Taste | After Taste |
|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 8.19 ± 0.93 a | 7.67 ± 1.11 a | 8.00 ± 0.89 a | 7.48 ± 0.68 a | 2.48 ± 0.68 a | 7.62 ± 0.74 a | 1.91 ± 0.62 a | 3.04 ± 0.74 a | 4.09 ± 0.80 a |
| 2.4 | 8.67 ± 0.52 a | 7.00 ± 1.00 a | 8.00 ± 1.00 a | 7.34 ± 0.58 a | 3.00 ± 1.00 a | 7.67 ± 0.58 a | 2.67 ± 0.58 a | 3.34 ± 0.58 ab | 4.00 ± 1.00 a |
| 3.4 | 7.00 ± 0.00 a | 7.00 ± 2.00 a | 7.68 ± 0.60 a | 8.34 ± 0.60 a | 1.68 ± 0.58 ab | 7.60 ± 1.16 a | 2.33 ± 0.52 a | 2.67 ± 0.58 abc | 3.68 ± 0.58 a |
| 4.4 | 7.33 ± 0.58 a | 7.33 ± 0.58 a | 8.00 ± 1.00 a | 7.70 ± 0.80 a | 1.68 ± 0.60 ab | 7.33 ± 0.58 a | 1.68 ± 0.52 a | 2.00 ± 0.00 abc | 3.72 ± 0.68 a |
| 4.9 | 7.67 ± 0.88 a | 8.00 ± 1.00 a | 7.60 ± 1.00 a | 7.60 ± 0.60 a | 2.68 ± 0.60 ab | 7.00 ± 1.00 a | 2.34 ± 0.60 a | 3.34 ± 0.58 ab | 4.00 ± 1.00 a |
| 6.8 | 7.00 ± 0.00 a | 7.68 ± 0.60 a | 8.00 ± 1.00 a | 7.40 ±0.80 a | 2.34 ± 0.70 ab | 7.70 ± 1.53 a | 1.34 ± 0.58 a | 2.34 ± 0.52 abc | 3.34 ± 0.62 a |
| 7.3 | 8.00 ± 1.00 a | 8.00 ± 1.00 a | 8.60 ± 0.70 a | 8.30 ± 0.70 a | 2.24 ± 0.60 ab | 7.34 ± 0.58 a | 2.68 ± 0.58 a | 3.00 ± 0.00 ab | 4.00 ± 1.20 a |
| 8.8 | 7.00 ± 0.00 a | 7.33 ± 0.60 a | 7.70 ± 0.80 a | 7.40 ± 0.60 a | 1.70 ± 0.70 ab | 7.20 ± 0.54 a | 2.34 ± 0.50 a | 1.68 ± 0.58 bc | 3.68 ± 0.48 a |
| 10.2 | 7.00 ± 1.00 a | 7.80 ± 0.80 a | 8.33 ± 0.60 a | 8.00 ± 1.00 a | 2.70 ± 0.56 ab | 7.00 ± 1.00 a | 2.34 ± 0.50 a | 2.68 ± 0.60 abc | 3.66 ± 0.48 ab |
| 13.2 | 7.67 ± 0.58 a | 7.60 ± 0.60 a | 9.70 ± 0.70 a | 8.34 ± 0.58 a | 1.00 ± 0.00 b | 7.34 ± 0.60 a | 2.00 ± 0.00 a | 1.00 ± 0.00 c | 3.62 ± 0.54 a |
| Measured Properties | Regression Equation | R2 (%) | (%) | (%) | SE |
|---|---|---|---|---|---|
| pH | =−0.000888E + 0.1174EFS + 0.01962Trt − 0.000028Trt*Trt + 0.000001E*Trt | 92.78 | 91.95 | 92.43 | 0.84 |
| Conductivity (mS/cm) | =2.25885 − 0.000003E + 0.000202EFS + 0.000107Trt + 0.000000E*EFS | 88.65 | 87.59 | 86.84 | 0.009 |
| TA (g of tartaric acid/L) | =1.9785 − 0.000615EFS + 0.000197Trt − 0.000000Trt*Trt | 20.12 | 14.68 | 2.70 | 0.27 |
| Lightness (L*) | =14.025 − 0.0493EFS | 19.43 | 17.67 | 13.69 | 1.81 |
| b* | =22.267 − 0.002033E − 0.00117Trt + 0.000004E*Trt | 54.44 | 51.34 | 45.65 | 2.38 |
| TPSC (mg GAE/L) | =2017.6 + 4.22EFS + 0.6271Trt + 0.2584EFS*EFS − 0.01372EFS*Tr | 95.50 | 95.08 | 94.51 | 28.90 |
| TAC (%) | =2.862EFS + 0.4908Trt − 0.000714Trt*Trt−0.01930EFS*Trt + 0.000028EFS*Trt*Trt | 58.15 | 56.29 | 53.26 | 0.41 |
| TMAC (mg/L) | =36.733 + 0.02469E − 0.0326EFS + 0.002216Trt + 0.001734EFS*EFS + 0.000025E*EFS − 0.02213EFS*Trt | 88.43 | 86.73 | 83.55 | 0.268 |
| C. lipolytica inactivation | =6.366 − 0.00521Trt − 0.0188E − 0.1437EFS − 0.000001Trt*Trt + 0.000000E*E + 0.00149EFS*EFS − 0.000001Trt*E + 0.0172Trt*EFS − 0.000030E*EFS | 92.99 | 91.33 | 88.64 | 0.49 |
| S. cerevisiae inactivation | =8.375 − 0.00452Trt + 0.0224E − 0.0106EFS − 0.000016Trt*Trt − 0.000000E*E − 0.00205EFS*EFS + 0.000003Trt*E − 0.0212Trt*EFS + 0.000048E*EFS | 95.11 | 93.95 | 91.86 | 0.61 |
| H. anemola inactivation | =7.005 − 0.00637Trt + 0.0003E − 0.0511EFS − 0.000004Trt*Trt − 0.000000E*E − 0.00022EFS*EFS + 0.000000Trt*E − 0.0002Trt*EFS − 0.000004E*EFS | 96.30 | 95.43 | 93.38 | 0.37 |
| L. delbrueckii ssp. bulgaricus inactivation | =6.151 − 0.00275Trt − 0.01141E + 0.0072EFS + 0.000006Trt*Trt + 0.000000E*E − 0.000087EFS*EFS − 0.000001Trt*E + 0.01016Trt*EFS − 0.000013E*EFS | 98.41 | 98.03 | 97.32 | 0.22 |
| E. coli O157:H7 inactivaton | =6.456 − 0.00261Trt−0.0018E + 0.0149EFS − 0.000012Trt*Trt + 0.000000E*E − 0.00145EFS*EFS + 0.000000Trt*E + 0.0013Trt*EFS − 0.000009E*EFS | 97.06 | 96.37 | 95.54 | 0.38 |
| Responses | Solution 1 | Solution 2 | Solution 3 |
|---|---|---|---|
| pH | 2.95 | 2.95 | 2.95 |
| L* | 14.02 | 14.02 | 14.02 |
| a* | 40.14 | 40.14 | 40.14 |
| b* | 21.67 | 19.45 | 21.69 |
| TAC (%) | 73.32 | 73.32 | 73.32 |
| TPSC (mg GAE/L) | 2332.62 | 2332.62 | 2332.62 |
| TMAC (mg/L) | 41.10 | 36.31 | 37.81 |
| Composite desirability | 0.79 | 0.75 | 0.70 |
| Optimal value | |||
| Trt (s) | 488 | 488 | 488 |
| E (kJ) | 0.13 | 0.32 | 3.88 |
| EFS (kV) | 0.22 | 1.46 | 2.34 |
| H. anemola (log10 unit) | 2.511 | 3.442 | 4.511 |
| S. cerevisiae (log10 unit) | 2.342 | 3.442 | 4.511 |
| C. lipolytica (log10 unit) | 2.118 | 2.692 | 3.271 |
| L. delbrueckii ssp. bulgaricus (log10 unit) | 3.371 | 3.964 | 4.932 |
| E. coli O157:H7 (log10 unit) | 2.189 | 3.307 | 3.739 |
| Composite desirability | 0.69 | 0.65 | 0.62 |
| Optimal value | |||
| Trt (s) | 488 | 488 | 488 |
| E (kJ) | 13.2 | 10.2 | 8.80 |
| EFS (kV) | 32 | 24 | 17 |
| Density | 6.19 | 6.77 | 6.77 |
| Red color intensity | 8.71 | 8.86 | 8.86 |
| Dullness/brightness | 7.09 | 6.98 | 6.98 |
| Cloudiness/clearness | 6.55 | 7.60 | 7.60 |
| Bitterness | 1.28 | 1.00 | 1.00 |
| Sour taste | 1.14 | 2.71 | 2.71 |
| Odor/flavor | 7.92 | 7.93 | 7.93 |
| Aftertaste | 2.44 | 3.62 | 3.62 |
| Composite desirability | 0.72 | 0.64 | 0.64 |
| Optimal value | |||
| Trt (s) | 348 | 488 | 488 |
| E (kJ) | 9.39 | 2.04 | 2.04 |
| EFS (kV) | 31 | 2.31 | 2.31 |
| Mn (μg/L) | −11,076.50 | 6.2 | 6.2 |
| Ca (μg/L) | −189,357 | 190 | 190 |
| K (μg/L) | −1,854,423 | 2206 | 2162 |
| P (μg/L) | −49,117.0 | 138.8 | 134.5 |
| Composite desirability | 1.00 | 0.68 | 0.64 |
| Optimal value | |||
| Trt (s) | 488 | 488 | 93.01 |
| E (kJ) | 13.2 | 13.2 | 2.54 |
| EFS (kV) | 0 | 30.99 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akdemir Evrendilek, G. Pulsed Electric Field Processing of Red Wine: Effect on Wine Quality and Microbial Inactivation. Beverages 2022, 8, 78. https://doi.org/10.3390/beverages8040078
Akdemir Evrendilek G. Pulsed Electric Field Processing of Red Wine: Effect on Wine Quality and Microbial Inactivation. Beverages. 2022; 8(4):78. https://doi.org/10.3390/beverages8040078
Chicago/Turabian StyleAkdemir Evrendilek, Gulsun. 2022. "Pulsed Electric Field Processing of Red Wine: Effect on Wine Quality and Microbial Inactivation" Beverages 8, no. 4: 78. https://doi.org/10.3390/beverages8040078
APA StyleAkdemir Evrendilek, G. (2022). Pulsed Electric Field Processing of Red Wine: Effect on Wine Quality and Microbial Inactivation. Beverages, 8(4), 78. https://doi.org/10.3390/beverages8040078