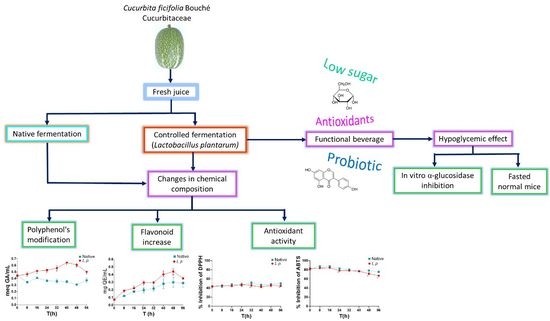
Fermentation of the Cucurbita ficifolia Fruit Juice: Its Antioxidant Activity and Effects on the Glycemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical Characterization of C. ficifolia Fruits and Juice Obtention
2.2. Fermentations of the C. ficifolia Juice: Native and Controlled
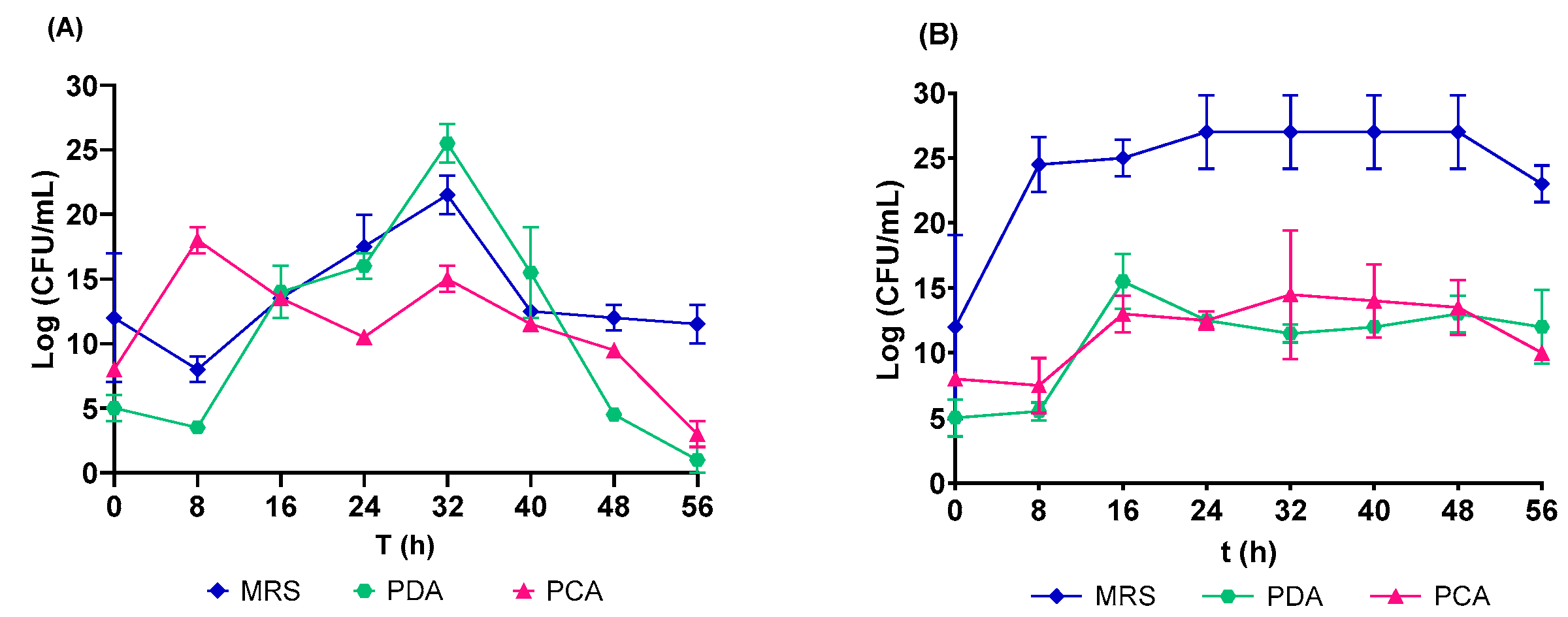
2.3. Microbial Growth Determination
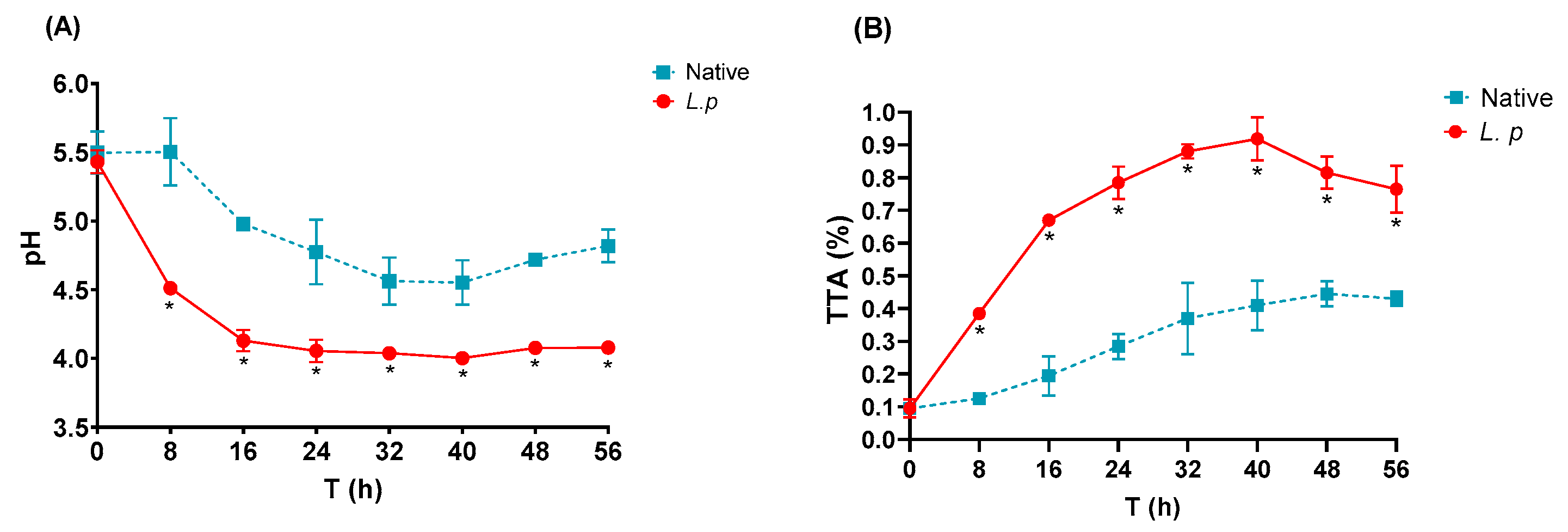
2.4. Determination of pH, Total Titratable Acidity (TTA), and °Brix
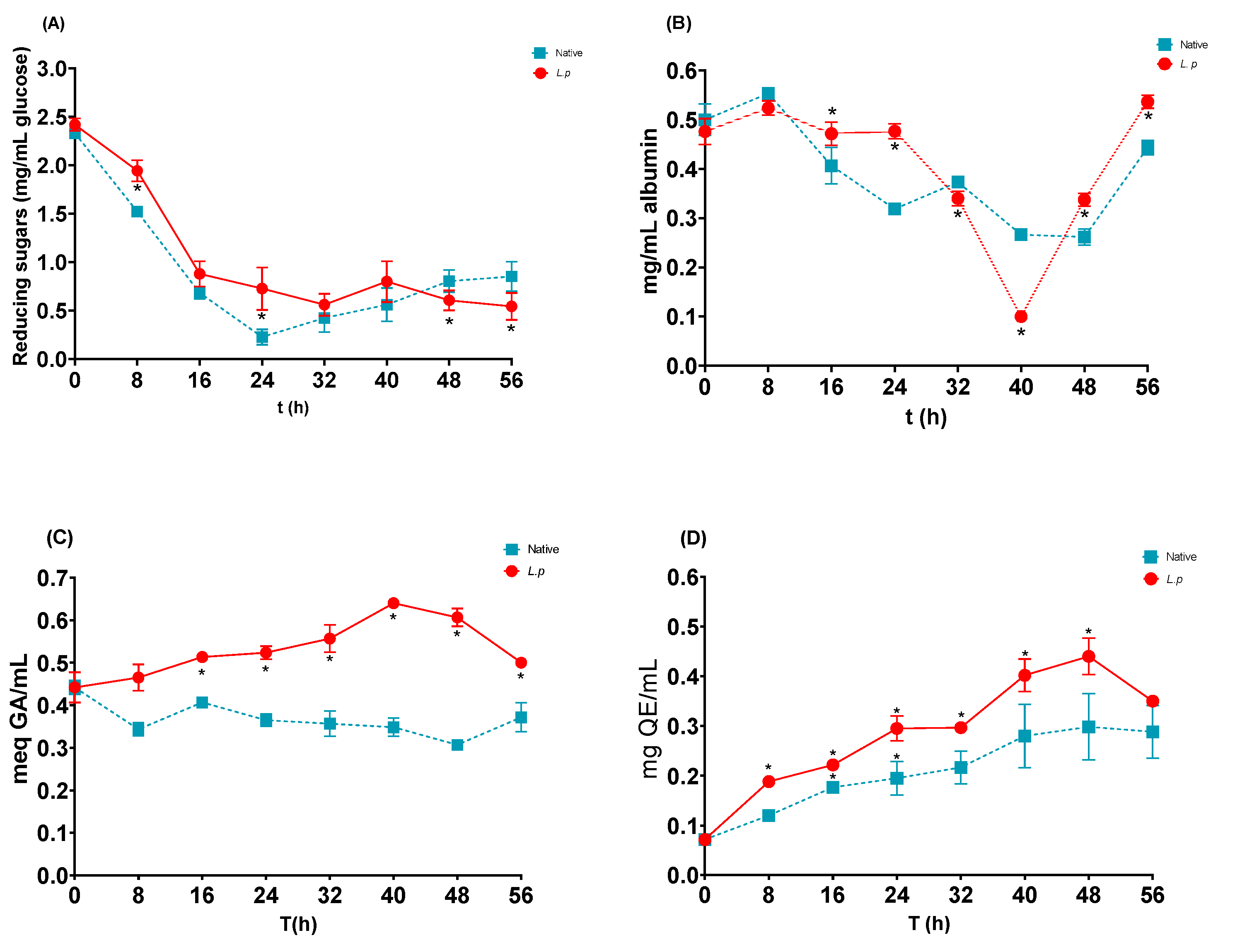
2.5. Determination of Soluble Protein and Reducing Sugars
2.6. Determination of Total Polyphenols and Flavonoids
2.7. Determination of Antioxidant Activity by ABTS and DPPH Radical Scavenging Activities
2.8. Alpha-Glucosidase Inhibitory In Vitro Assay
2.9. Effects on the Glycemia of the Juice and Samples at the 48 h of Native and Controlled Fermentation
2.10. Statistical Analysis
3. Results
3.1. Physical Characteristics of C. ficifolia Fruits
3.2. Microbial Growth in Native and Controlled Fermentation of the C. ficifolia Juice
3.3. pH and TTA Profile
3.4. Profile of Reducing Sugars and Soluble Protein
3.5. Polyphenols and Flavonoids
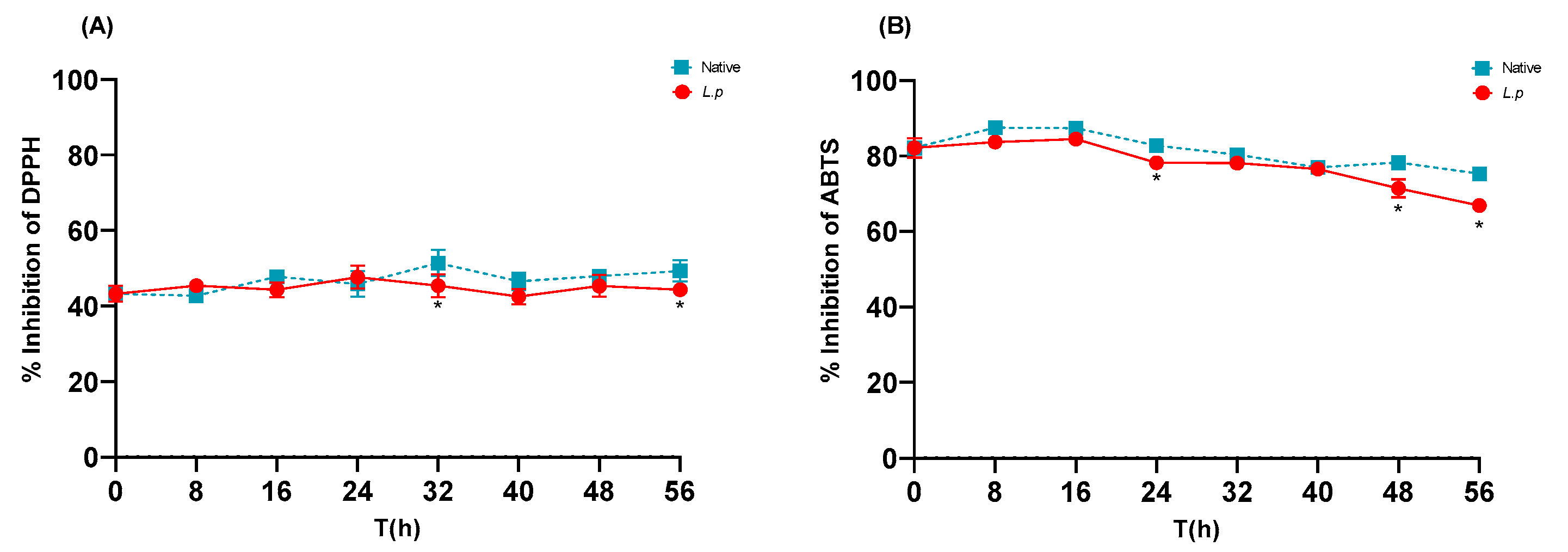
3.6. In Vitro Antioxidant Activity of Native and Controlled Fermentations
3.7. Comparative Analysis at 48 h of the Native and Controlled Fermentations against the C. ficifolia Juice (t = 0)
3.8. Alpha-Glucosidase Inhibitory Effect and on the Glycemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alarcon-Aguilar, F.J.; Hernandez-Galicia, E.; Campos-Sepulveda, A.E.; Xolalpa-Molina, S.; Rivas-Vilchis, J.F.; Vazquez-Carrillo, L.I.; Roman-Ramos, R. Evaluation of the hypoglycemic effect of Cucurbita ficifolia Bouche (Cucurbitaceae) in different experimental models. J. Ethnopharmacol. 2002, 82, 185–189. [Google Scholar] [CrossRef]
- Fortis-Barrera, A.; Alarcon-Aguilar, F.J.; Banderas-Dorantes, T.; Díaz-Flores, M.; Roman-Ramos, R.; Cruz, M.; García-Macedo, R. Cucurbita ficifolia Bouché (Cucurbitaceae) and D-chiro-inositol modulate the redox state and inflammation in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2013, 65, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Wang, Q. D-chiro-inositol found in Cucurbita ficifolia (Cucurbitaceae) fruit extracts play the hypoglycaemic role in streptozocin-diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1527–1532. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Garcia-Lorenzana, M.; Zamilpa, A.; Almanza-Perez, J.C.; Jasso-Villagomez, E.I.; Roman-Ramos, R.; Alarcon-Aguilar, F.J. Chemical characterization of a hypoglycemic extract from Cucurbita ficifolia Bouche that induces liver glycogen accumulation in diabetic mice. Afr. J. Trad. Comp. Altern. Med. 2017, 14, 218–230. [Google Scholar]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented Portulaca oleracea L. juice: A novel functional beverage with potential ameliorating effects on the intestinal inflammation and epithelial injury. Nutrients 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Ankolekar, C.; Johnson, K.; Pinto, M.; Johnson, D.; Labbe, R.G.; Greene, D.; Shetty, K. Fermentation of whole apple juice using Lactobacillus acidophilus for the potential dietary management of hyperglycemia, hypertension, and modulation of beneficial bacterial responses. J. Food Biochem. 2012, 36, 718–738. [Google Scholar] [CrossRef]
- Du, X.; Myracle, A.D. Fermentation alters the bioaccessible phenolic compounds and increases the alpha-glucosidase inhibitory effects of aronioa juice in a dairy matrix following in vitro digestion. R. Soc. Chem. 2018, 9, 2998–3007. [Google Scholar]
- Park, E.J.; Garcia, C.V.; Youn, S.J.; Park, C.D.; Lee, S.P. Fortification of γ-aminobutyric acid and bioactive compounds in Cucurbita moschata by novel two-step fermentation using Bacillus subtilis and Lactobacillus plantarum. LWT-Food Sci. Technol. 2019, 102, 22–29. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Nie, S.P.; Xiong, T.; Xie, M.Y. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019, 29, 62–72. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Inter. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nut. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Moya-Hernández, A.; Bosquez-Molina, E.; Verde-Calvo, J.R.; Blancas-Flores, G.; Trejo-Aguilar, G.M. Hypoglycemic effect and bioactive compounds associated with the ripening stages of the Cucurbita ficifolia Bouché fruit. J. Sci. Food Agric. 2020, 100, 5171–5181. [Google Scholar] [CrossRef]
- Valbuena, E.; Barreiro, J.; Sánchez, E.; Castro, G.; Briñez, W.; Tovar, A. Modelos cinéticos aplicados al crecimiento de Lactococcus lactis subsp. lactis en leche. Rev. Cient. 2005, 15, 464–475. [Google Scholar]
- Ojokoh, A.; Orekoya, E. Effect of fermentation on the proximate composition of the epicarp of watermelon (Citrullus lanatus). Int. J. Swarm Intel. Evol. Comp. 2017, 5, 1–5. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology: Oxidants and Antioxidant Part A, 1st ed.; Abelson, J., Simon, M., Sidney, S., Kaplan, P., Eds.; Academic Press: Cambrige, MA, USA, 1998; Volume 299, pp. 152–178. [Google Scholar]
- Escudero-López, B.; Cerrillo, I.; Herrero-Martín, G.; Hornero-Méndez, D.; Gil-Izquierdo, A.; Medina, S.; Ferreres, F.; Berná, G.; Martin, F.; Fernández-Pachón, M.S. Fermented orange juice: Source of higher carotenoid and flavanone contents. J. Agric. Food Chem. 2013, 61, 8773–8782. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Tibiru, M.; Wu, M.; Sackle, A.; Xiao, L.; Elrasheid, H. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Saade, R.L. Catálogo de la Familia Cucurbitaceae de México; Final Inform SNIB-CONABIO Proyecto DS002; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2006. [Google Scholar]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nut. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: Correlation with physicochemical, antioxidant properties and classification using SPME-GC–MS and E-nose methods. J. Food Sci. Technol. 2017, 54, 3118–3131. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple juice as a medium for fermentation by the probiotic Lactobacillus plantarum PCS 26 strain. Ann. Microbiol. 2015, 65, 2161–2170. [Google Scholar] [CrossRef]
- Saarela, M. Probiotics. Lactobacilli. In Probiotics and Probiotics: Ingredients Handbook, 2nd ed.; Jardine, S., Ed.; Wiley-Blackwell: New York, NY, USA, 2009; pp. 101–113. [Google Scholar]
- Koh, W.Y.; Uthumporn, U.; Rosma, A.; Irfan, A.R.; Park, Y.H. Optimization of a fermented pumpkin-based beverage to improve Lactobacillus mali survival and α-glucosidase inhibitory activity: A response surface methodology approach. Food Sci. Hum. Well. 2018, 7, 57–70. [Google Scholar] [CrossRef]
- Koh, W.Y.; Utra, U.; Rosma, A.; Effarizah, M.E.M.; Rosli, W.I.W.; Park, Y.H. Development of a novel fermented pumpkin-based beverage inoculated with water kefir grains: A response surface methodology approach. Food Sci. Biotechnol. 2018, 27, 525–535. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, M. The effect of fermentation by Lactobacillus plantarum on the physicochemical and functional properties of liquorice root extract. LWT-Food Sci. Technol. 2019, 105, 164–168. [Google Scholar] [CrossRef]
- Mazlan, F.A.; Annuar, M.S.M.; Sharifuddin, Y. Biotransformation of Momordica charantia fresh juice by Lactobacillus plantarum BET003 and its putative anti-diabetic potential. PeerJ 2015, 3, e1376. [Google Scholar] [CrossRef]
- Roh, H.J.; Kim, G.E. Fermentation of Cucurbita maxima extracts with microorganisms from Kimchi. J. KSBB 2009, 24, 149–155. [Google Scholar]
- Filannino, P.; Di Cagno, R.; Trani, A.; Cantatore, V. Lactic acid fermentation enriches the profile of biogenic compounds and enhances the functional features of common purslane (Portulaca oleracea L.). J. Funct. Foods 2017, 39, 175–185. [Google Scholar] [CrossRef]
- Linares-Morales, J.R.; Cuellar-Nevárez, G.E.; Rivera-Chavira, B.E.; Gutiérrez Méndez, N.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Selection of lactic acid bacteria isolated from fresh fruits and vegetables based on their antimicrobial and enzymatic activities. Foods 2020, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Proc. Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Garcia, C.; Guérin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Chiu, H.F.; Wang, C.K. Popular functional foods and herbs for the management of type-2-diabetes mellitus: A comprehensive review with special reference to clinical trials and its proposed mechanism. J. Funct. Foods 2019, 57, 425–438. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Banwo, K.; Asogwa, F.C.; Ogunremi, O.R.; Adesulu-Dahunsi, A.; Sanni, A. Nutritional profile and antioxidant capacities of fermented millet and sorghum gruels using lactic acid bacteria and yeasts. Food Biotech. 2021, 35, 199–220. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Fereidon, S.; Priyatharini, A. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Shashank, K.; Abhay, K. Review article chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 4, 32–48. [Google Scholar]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity, and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 1–9. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Roman-Ramos, R.; Almanza-Perez, J.C.; Fortis-Barrera, A.; Angeles-Mejia, S.; Banderas-Dorantes, T.R.; Zamilpa-Alvarez, A.; Díaz-Flores, M.; Jasso, I.; Blancas-Flores, G.; Cruz, J.; et al. Antioxidant and anti-inflammatory effects of a hypoglycemic fraction from Cucurbita ficifolia Bouché in streptozotocin-induced diabetes mice. Am. J. Chin. Med. 2012, 40, 97–110. [Google Scholar] [CrossRef]
- Anhê, F.F.; Desjardins, Y.; Pilon, G.; Dudonné, S.; Genovese, M.I.; Lajolo, F.M.; Marette, A. Polyphenols and type 2 diabetes: A prospective review. Pharma. Nutr. 2013, 1, 105–114. [Google Scholar] [CrossRef]
- Becerra, S.M.; Miranda, P.E.; Gomez, V.J.C.; Fortis, B.M.A.; Perez, R.J.; Alarcon, A.F.J. Potential of the chlorogenic acid as multitarget agent: Insulin-secretagogue and PPAR α/γ dual agonist. Biomed. Pharmacother. 2017, 94, 169–175. [Google Scholar]
- Filannino, P.; Azzi, L.; Cavoski, I.; Vincentini, O.; Rizzello, C.G.; Gobbetti, M.; Di Cagno, R. Exploitation of the health-promoting and sensory properties of organic pomegranate (Punica granatum L.) juice through lactic acid fermentation. Int. J. Food Microbiol. 2013, 163, 184–192. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Fruits | Seeds |
|---|---|---|
| Longitudinal perimeter (LP, cm) | 68.4 ± 9.7 | 1.4 ± 0.1 |
| Equatorial perimeter (EP, cm) | 56.4 ± 3.7 | 0.9 ± 0.1 |
| Sphericity coefficient (ε) | 0.8 ± 0.1 | - |
| Weight (g) | 4478.3 ± 527.7 | 136.7 ± 35.1 |
| Juice (mL) | 2025 ± 217 | - |
| Yield juice (%) | 45.2 ± 1.3 | - |
| Fiber (g) | 361.7 ± 114.5 | - |
| Yield fiber (%) | 12.2 ± 2.2 | - |
| °Brix | 4.1 ± 0.2 | - |
| Parameter Value | |||||
|---|---|---|---|---|---|
| Controlled | Native | ||||
| Medium | Microorganisms | µ | M | µ | M |
| MRS | L. plantarum | 0.46 ± 0.01 | 0.30 ± 0.30 | 0.26 ± 0.12 | 32.94 ± 0.23 |
| PDA | Yeast and fungi | 0.25 ± 0.17 | 1.77 ± 0.23 | 0.30 ± 0.02 | 1.24 ± 0.55 |
| PCA | Mesophilic | 0.33 ± 0.18 | 0.10 ± 0.03 | 0.21 ± 0.1 | 2.80 ± 1.26 |
| Native | Controlled | |||||
|---|---|---|---|---|---|---|
| Parameter | L. plantarum | Yeast-Fungi | Mesophilic | L. plantarum | Yeast-Fungi | Mesophilic |
| A | 14.19 ± 1.3 | 12.80 ± 1.5 | 11.57 ± 0.4 | 26.07 ± 2.4 | 12.69 ± 0.5 | 13.15 ± 0.6 |
| B | 29.08 ± 2.0 | 9.55 ± 1.3 | −1.00 ± 0.1 | 0.47 ± 0.5 | 13.49 ± 1.9 | −0.47 ± 0.04 |
| M | 32.94 ± 0.2 | 1.24 ± 0.6 | 2.80 ± 1.3 | 0.30 ± 0.3 | 1.77 ± 0.2 | 0.10 ± 0.03 |
| Assay | Fresh Juice (t = 0) | Native Ferm. (48 h) | % Variation | Lactic Ferm. (48-h) | % Variation |
|---|---|---|---|---|---|
| pH | 5.4 ± 0.1 | 4.7 ± 0.018 # | −13 | 4.1 ± 0.04 * | −24.1 |
| TTA (%) | 0.09 ± 0.01 | 0.44 ± 0.03 # | +388.9 | 0.81 ± 0.07 * | +800 |
| Reducing sugars (mg/mL) | 2.4 ± 0.19 | 0.80 ± 0.28 # | −66.6 | 0.6 ± 0.41 * | −75 |
| Protein (mg/mL) | 0.47 ± 0.05 | 0.26 ± 0.04 # | −44.7 | 0.33 ± 0.02 * | −29.8 |
| Polyphenols (mg EqGA/mL) | 0.44 ± 0.01 | 0.30 ± 0.01 # | −31.8 | 0.61 ± 0.01 * | +38.6 |
| Total flavonoids (mg EqQ/mL) | 0.07 ± 0.003 | 0.29 ± 0.16 # | +314 | 0.44 ± 0.02 * | +528 |
| Inhibition DPPH (%) | 43.3 ± 1.4 | 47.91 ± 1.31 # | +10.6 | 45.29 ± 1.8 | +4.6 |
| Inhibition ABTS (%) | 86.5 ± 1.2 | 78.25 ± 0.71 # | −9.5 | 71.43 ± 1.3 * | −17.4 |
| Treatment | Inhibition (%) |
|---|---|
| Juice | 37.4 ± 1.0 |
| Native fermentation | 30.1 ± 2.9 |
| Controlled fermentation | 42.0 ± 1.0 * |
| Fasted normal mice | |||||
| Glycemia (mg/dL) | |||||
| Treatment/Doses | 0 min | 120 min | 240 min | 360 min | |
| ISS | 101.9 ± 22.6 | 100.9 ± 12.2 | 101.6 ± 20.3 | 90.6 ± 20.9 | |
| Glibenclamide | 10 mg/kg | 102.6 ± 15.4 | 76.9 ± 12.4 | 56.6 ± 15.2 + | 62.3 ± 18.7 + |
| Juice | 25 mg/kg | 95.5 ± 10.9 | 115.0 ± 9.9 | 92.5 ± 2.2 | 86.0 ± 15.6 |
| 50 mg/kg | 98.0 ± 21.0 | 106.7 ± 3.1 | 82.0 ± 9.8 | 77.0 ± 12.5 + | |
| Native | 25 mg/kg | 97.0 ± 5.3 | 126.0 ± 18.6 | 82.7 ± 15.0 | 80.7 ± 13.3 |
| 50 mg/kg | 90.0 ± 9.9 | 95.5 ± 23.2 | 79.0 ± 18.0 | 74.3 ± 9.4 | |
| Controlled | 25 mg/kg | 93.3 ± 2.1 | 116.0 ± 11.4 | 87.5 ± 19.1 | 83.0 ± 21.6 |
| 50 mg/kg | 95.7 ± 9.6 | 91.0 ± 12.2 | 76.0 ± 6.6 + | 72.5 ± 8.3 + | |
| Tolerance test in mice | |||||
| Glycemia (mg/dL) | |||||
| Treatment | 0 min | 30 min | 60 min | 90 min | 120 min |
| ISS | 76.3 ± 11.7 | 254.7 ± 27.2 | 199.3 ± 42.8 | 140.17 ± 29.9 | 128.1 ± 24.6 |
| Metformin 150 mg/kg | 82.0 ± 13.6 | 128.4 ± 39.9 * | 92.0 ± 19.5 * | 90.2 ± 19.3 * | 87.4 ± 20.9 * |
| Juice 50 mg/kg | 83.6 ± 18.7 | 175.0 ± 25.5 * | 104.5 ± 19.1 * | 109.3 ± 24.0 | 115.5 ± 21.7 |
| Native 50 mg/kg | 73.7 ± 24.5 | 201.5 ± 37.5 | 134.0 ± 12.7 * | 118.0 ± 10.4 | 112.3 ± 3.5 |
| Controlled 50 mg/kg | 79.0 ± 18.5 | 157.0 ± 45.3 * | 108.0 ± 12.7 * | 108.5 ± 33.2 | 106.0 ± 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrón-Álvarez, N.; Prado-Barragán, L.A.; Fortis-Barrera, M.d.l.Á.; Alarcon-Aguilar, F.J. Fermentation of the Cucurbita ficifolia Fruit Juice: Its Antioxidant Activity and Effects on the Glycemia. Beverages 2022, 8, 55. https://doi.org/10.3390/beverages8030055
Barrón-Álvarez N, Prado-Barragán LA, Fortis-Barrera MdlÁ, Alarcon-Aguilar FJ. Fermentation of the Cucurbita ficifolia Fruit Juice: Its Antioxidant Activity and Effects on the Glycemia. Beverages. 2022; 8(3):55. https://doi.org/10.3390/beverages8030055
Chicago/Turabian StyleBarrón-Álvarez, Nayeli, Lilia Arely Prado-Barragán, María de los Ángeles Fortis-Barrera, and Francisco Javier Alarcon-Aguilar. 2022. "Fermentation of the Cucurbita ficifolia Fruit Juice: Its Antioxidant Activity and Effects on the Glycemia" Beverages 8, no. 3: 55. https://doi.org/10.3390/beverages8030055
APA StyleBarrón-Álvarez, N., Prado-Barragán, L. A., Fortis-Barrera, M. d. l. Á., & Alarcon-Aguilar, F. J. (2022). Fermentation of the Cucurbita ficifolia Fruit Juice: Its Antioxidant Activity and Effects on the Glycemia. Beverages, 8(3), 55. https://doi.org/10.3390/beverages8030055