Beer and Allergens
Abstract
:1. Introduction
| Allergen | Common Descriptor | Prevalence in EU Population (%) [6] |
|---|---|---|
| Celery | Includes celery stalks, leaves, seeds and the root (celeriac) | 5.50 |
| Milk | Secretion from mammary glands intended to be the nutritional input of mammalian neonates | 3.84 |
| Mustard | Member of the brassica family. Limited cross-reactivity from other brassica | 3.00 |
| Eggs | Typically refers to hen eggs but does not exclude eggs from other birds | 2.85 |
| Peanuts | Also known as a groundnut. The peanut is not a nut; instead, it is a legume | 2.59 |
| Fish | Typically, a limbless cold-blooded vertebrate animal with gills and fins, living in water | 2.53 |
| Crustaceans | Includes crabs, lobster, shrimp, prawns, and scampi | 1.80 |
| Tree nuts | Includes a wide range of nuts. Fruit composed of a hard shell and an indehiscent seed | 1.45 |
| Cereals/Gluten | Cereals containing gluten or gluten-like proteins, such as wheat, rye, barley, and oats | 1.13 |
| Molluscs | Typically includes mussels, oysters, abalone, squid, and snails | 1.00 |
| Sesame seeds | Sesame is a flowering plant whose seeds yields very large amounts of oil | 0.70 |
| Soy | A high oil- and protein-yielding legume. Can show cross-reactive to other legumes | 0.53 |
| Lupin | A legume belonging to the same family as peanuts. Hence, cross-reactivity to peanuts is common | NR 1 |
| Sulphites | Chemical preservative and antioxidant, typically reported as SO2 | NA 2 |
| Country/Region | Reference | Milk | Eggs | Fish | Crustaceans | Molluscs | Cereals with Gluten | Wheat | Buckwheat | Tree Nuts | Peanuts | Soy | Sulphite | Sesame Seeds | Lupin | Mustard | Celery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EU | [11] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| UK | [12] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Turkey | [13] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Australia | [14] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ |
| New Zealand | [14] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ |
| USA | [9] | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ✓ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ 1 | ∅ | ∅ | ∅ |
| Canada | [15] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ∅ |
| Taiwan | [16] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ |
| FAO-WHO | [17] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| China | [18] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Mexico | [19] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Russia | [20] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Argentina | [21] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Brazil | [22] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Chile | [23] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| India | [24] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Vietnam | [24] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Thailand | [24] | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| Japan | [10] | ✓ | ✓ | ☑ | ✓ | ☑ | ∅ | ✓ | ✓ | ☑ | ✓ | ☑ | ∅ | ☑ | ∅ | ∅ | ∅ |
| Korea | [24] | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ |
| South Africa | [25] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⊗ | ∅ | ✓ | ✓ | ✓ | ∅ | ∅ | ∅ | ∅ | ∅ |
2. Allergenic Reactions and Beer
2.1. Case Studies—Beer Causing Allergic Reactions
2.2. Beer-Related Allergic Reactions in the Workplace
3. Allergens Found in Beer
3.1. Gluten (Wheat and Other Cereals with Gluten-Like Proteins) as Allergens in Beer
3.2. Exemptions
3.3. Fish as Allergens in Beer
3.4. Molluscs as Allergens in Beer
3.5. Crustaceans as Allergens in Beer
3.6. Milk as an Allergen in Beer
3.7. Eggs as an Allergen in Beer
3.8. Celery as an Allergen in Beer
3.9. Tree Nuts as Allergens in Beer
3.10. Peanuts (Groundnuts) as Allergens in Beer
3.11. Lupin as an Allergen in Beer
3.12. Soy as an Allergen in Beer
3.13. Mustard as an Allergen in Beer
3.14. Sesame as an Allergen in Beer
3.15. Sulfur Dioxide as an Allergen in Beer
3.16. Miscellaneous Allergens in Beer
4. Cross-Contamination Risks When Brewing with Allergens
4.1. Cross-Contamination with Unconventional Allergens
4.2. Cross-Contamination When Brewing Gluten-Free Beers
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Cerevisiography
| Code, “name of beer”, name and place of brewery [allergen in beer] | |
| C01 | “Mussels in Brussels”, Siphon Brewery, Oostkerke, Belgium [mussels] |
| C02 | “Kansom range of premier abalone beers”, Red Duck Brewery, Ballarat, Australia [abalone] |
| C03 | “Renown Cockle Stout”, Leigh on Sea Brewery, Leigh on Sea, UK [cockles] |
| C04 | “Squid ink XXPA”, Shelter Brewery, Busselton, Australia [squid] |
| C05 | “Squid Ink Gose”, Time & Tide Brewery, Deal, UK [squid] |
| C06 | “Black Magic Salt & Pepper Squid Ink Gose”, Dainton, Carrum Downs, Australia [squid] |
| C07 | “Saison dell’Aragosta”, Oxbow Brewing Company, Portland, Maine, USA [lobster] |
| C08 | “Neapolitan Ice Cream IPA”, Northern Monk, Leeds, UK [lactose] |
| C09 | “Mango Milkshake IPA”, Stone and Wood Brewing Co, Byron Bay, Australia [lactose] |
| C10 | “Blue Brew”, Belvoir Brewery, Melton Mowbray, UK [whey] |
| C11 | “Flamingo”, Bass Brewing Company, Burton upon Trent, UK [hydrolysed egg albumin] |
| C12 | “Sellray Celery Ale”, Pipeworks Brewing Company, Chicago, Illinois, USA [celery] |
| C13 | “Celery Gose”, Black Shirt Brewing Company, Denver, Colorado, USA [celery] |
| C14 | “Celery Salt Hippy”, 8-Wired Brewery, Warkworth, New Zealand [celery] |
| C15 | “De Molen Hel & Verdoemenis Hazelnoot” Brewery ‘de molen’, Bodegraven, Netherlands [hazelnut] |
| C16 | “Oil of Aphrodite”, Jackie O’s Brewhouse, Athens, Ohio, United States [walnut] |
| C17 | “Imperial Biscotti Break”, Evil Twin Brewery, Ridgewood, NY, USA [almonds] |
| C18 | “Pistachio Cream Ale”, Short’s Brewing Company, Bellaire, MI, USA [pistachio] |
| C19 | “Peanut Butter Milk Stout”, Tailgate Brewery, Nashville, TN, USA [peanuts] |
| C20 | “Crunch, Peanut Butter Milk Stout”, Hammerton Brewery, London, UK [peanuts] |
| C21 | “Peanut Butter Jelly Time” Catawba Brewing Co., Morgaton, NC, USA [peanuts] |
| C22 | “Lupini Bean Gose”, Mean Sardine Brewery, Ericeira, Portugal [lupini beans] |
| C23 | “Nodogogi Nama”, Kirin Brewery, Japan [soy] |
| C24 | “New Life”, Asahi Brewery, Japan [soy] |
| C25 | “Akita Edamame”, Tazawako Brewery, Semoku, Japan [soy] |
| C26 | “French’s Mustard Beer”, Oskar Blues Brewery, Lyons, Colorado, USA [mustard] |
| C27 | “Torhouts Mustard Ale”, Smisje Brewery, Oudenaarde, Belgium [mustard] |
| C28 | “Perfectly Imperfect”, Kabinet Brewery, Sopot, Serbia [sesame] |
| C29 | “Zentus Fat & Jolly Tinsel Holly”, Zentus craft beer, Szentes, Hungary [sesame] |
| C30 | “Hoegaarden witbier”, AB Inbev, Hoegaarden, Belgium [coriander] |
| C31 | “Hoppy Table Beer”, Allagash Brewing Co., Portland, ME, USA [coriander] |
| C32 | “Settle Down Country Wit”, Suarez Family Brewery, Hudson, NY, USA [coriander] |
| C33 | “Pepper Johnson”, a collaboration beer from Lervig Brewery, Stavanger, Norway & Basqueland Brewing, Gipuzkoa, Spain [cracked pepper] |
| C34 | “Taco hands”, a collaboration beer from Cellarmaker Brewing Co., San Francisco, CA, USA & Tired Hands, Ardmore, PA, USA [cumin] |
| C35 | “Dragonwort Stout”, Nøgne Ø, Grimstad, Norway [anise] |
| C36 | “Marathos”, Solo Brewery (Σόλο), Iraklio, Greece [fennel] |
| C37 | “Slow Down Brown” Denali Brewery, Talkeetna, AK, USA [coriander, cumin, fennel, fenugreek] |
| C38 | “Kriek”, Boon Brewery, Lembeek, Belgium [cherries, raspberries] |
| C39 | “Banana Bread”, The Bruery, Placenta, CA, USA [banana] |
| C40 | “Imperial Banoffee Pie Stout”, The Garden Brewery, Zagreb, Croatia [banana] |
| C41 | “Mango Milkshake IPA” Eight Stacks Brewery, Traralgon, Vic, Australia [mango] |
| C42 | “Mangolorian”, Brew York, York, UK [mango] |
| C43 | “Sour Tomato Ale”, Breaker Brewing Company, Wilkes-Barre, PA, USA [tomato] |
| C44 | “Pizza IPA”, Epic Brewing Co., Utah, CO, USA [tomato] |
| C45 | “Sol Clamato”, Sol Brewery, Nuevo León, Mexico [tomato] [clams] [MSG] |
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Versluis, A.; Knulst, A.C.; Kruizinga, A.G.; Michelsen, A.; Houben, G.F.; Baumert, J.L.; van Os-Medendorp, H. Frequency, severity and causes of unexpected allergic reactions to food: A systematic literature review. Clin. Exp. Allergy 2015, 45, 347–367. [Google Scholar] [CrossRef] [Green Version]
- Jin, J. Treatments for Food Allergies. JAMA 2017, 318, 1945. [Google Scholar] [CrossRef] [PubMed]
- Popping, B. Challenges in Detecting Food Allergens—Analytical Methods in the Legal Context. In The Science of Gluten-Free Foods and Beverages; Arendt, E.K., Bello, F.D., Eds.; AACC International Press: St. Paul, MN, USA, 2009; pp. 35–40. [Google Scholar]
- Loh, W.; Tang, M.L. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [Green Version]
- Anon. Literature Searches and Reviews Related to The Prevalence of Food Allergy in Europe; EFSA Supporting Publications: Portsmouth, UK, 2013; Volume 10, EN-506. [Google Scholar]
- Dostálek, P.; Dvorak, J.; Hulin, P. Allergens in beer. Kvas. Prum. 2010, 56, 105–108. [Google Scholar] [CrossRef]
- Vriesekoop, F.; Wright, E.; Swinyard, S.; de Koning, W. Gluten-free products in the UK retail environment. Availability, pricing, consumer opinions in a longitudinal study. Int. J. Celiac Dis. 2020, 8, 95–103. [Google Scholar]
- Public Law 108–282, an Act to Amend the Federal Food, Drug, and Cosmetic Act with Regard to New Animal Drugs, and for other Purposes. 2004. Available online: https://www.govinfo.gov/app/details/PLAW-108publ282 (accessed on 7 December 2021).
- CAA No. 322, Japanese Government Allergen Labelling. 2019. Available online: https://www.caa.go.jp/policies/policy/food_labeling/food_sanitation/allergy/pdf/allergy_190925_0002.pdf (accessed on 7 December 2021).
- EU Food Allergen Labelling. 2021. Available online: https://europa.eu/youreurope/business/product-requirements/food-labelling/general-rules/index_en.htm#shortcut-4 (accessed on 7 December 2021).
- UK Food Allergen Labelling. 2021. Available online: https://www.food.gov.uk/business-guidance/allergen-guidance-for-food-businesses (accessed on 7 December 2021).
- Turkey Food Allergen Labelling. 2021. Available online: https://www.tarimorman.gov.tr/Konu/2023/Toplu_Tuketim_Yerlerinde_Alerjen_Bildirimi (accessed on 7 December 2021).
- Australia and New Zealand Food Allergen Labelling. 2021. Available online: https://www.foodstandards.gov.au/consumer/foodallergies/Pages/default.aspx (accessed on 7 December 2021).
- Canada Food Allergen Labelling. 2021. Available online: https://www.canada.ca/en/health-canada/services/food-allergies-intolerances/avoiding-allergens-food/allergen-labelling.html (accessed on 7 December 2021).
- Taiwan Food Allergen Labelling. 2021. Available online: https://www.mohw.gov.tw/dl-47353-14a49285-23d7-4c8c-ad7b-5cce709a6b09.html (accessed on 7 December 2021).
- World Health Organisation Food Allergen Labelling. 2006. Available online: https://www.who.int/foodsafety/fs_management/No_03_allergy_June06_en.pdf (accessed on 7 December 2021).
- China Food Allergen Labelling. 2009. Available online: https://www.chinesestandard.net/PDF.aspx/GBT23779-2009 (accessed on 7 December 2021).
- Mexico Food Allergen Labelling. NOM-051-SCFI/SSA1-2010. 2021. Available online: https://www.basham.com.mx/ministry-of-economy-publishes-modifications-to-mexican-official-standard-nom-051-scfi-ssa1-2010-which-sets-forth-the-commercial-and-sanitary-specifications-matters-of-labeling-for-pre-packaged-food-a/#:~:text=The%20modifications%20to%20NOM%2D051,health%20risks%20if%20consumed%20excessively (accessed on 7 December 2021).
- Russia Food Allergen Labelling. 2004. Available online: https://fsvps.gov.ru/fsvps-docs/ru/importExport/gonkong/files/gonkong_tu_markdate.pdf (accessed on 7 December 2021).
- Argentina Food Allergen Labelling. Acta No. 118. 2017. Available online: http://www.anmat.gov.ar/alimentos/directrices_rotulado_alergenos.pdf (accessed on 7 December 2021).
- Brazil Food Allergen Labelling. RDC No 26/15. 2017. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/alimentos/perguntas-e-respostas/rotulagem-de-alergenicos.pdf (accessed on 7 December 2021).
- Chile Food Allergen Labelling. Decreto 88, Modifica Decreto 977 (1996). 2013. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1021822 (accessed on 7 December 2021).
- International Regulatory Chart on Food Allergens. University of Nebraska-Lincoln. 2021. Available online: https://farrp.unl.edu/IRChart (accessed on 7 December 2021).
- South Africa Food Allergen Labelling. No. 146. Staatskoerant Maart. 2010. Available online: https://www.sabio.org.za/wp-content/uploads/2020/02/Foodstuffs-Cosmetics-and-Disenfectants-Act-Food-Labelling-Regs-R-146-of-1March-2010.pdf (accessed on 7 December 2021).
- Vriesekoop, F. Product Integrity. In Handbook of Brewing; Stewart, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 653–678. [Google Scholar]
- Drevets, C.C.; Seebohm, P.M. Dermatitis from alcohol. J. Allergy 1961, 32, 277–282. [Google Scholar] [CrossRef]
- Igelman, S.J.; Na, C.; Simpson, E.L. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020, 6, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Koelemij, I.; Van Zuuren, E.J. Contact urticaria from beer. Clin. Exp. Dermatol. 2014, 39, 407–409. [Google Scholar] [CrossRef]
- Gutgesell, C.; Fuchs, T. Contact urticaria from beer. Contact Derm. 1995, 33, 436–437. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, S.; Gupta, A. Beer induced angioedema–A case report. Our Dermatol. Online 2015, 6, 418–419. [Google Scholar] [CrossRef]
- Curioni, A.; Santucci, B.; Cristaudo, A.; Canistraci, C.; Pietravalle, M.; Simonato, B.; Giannattasio, M. Urticaria from beer: An immediate hypersensitivity reaction due to a 10-kDa protein derived from barley. Clin. Exp. Allergy 1999, 29, 407–413. [Google Scholar] [CrossRef]
- Pita, J.S.; Sousa, N.; Bartolome, B.; Loureiro, C.; Bom, A.T. Beer: An uncommon cause of anaphylaxis. BMJ Case Rep. 2019, 12, e227723. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, E.; Quirce, S.; Del Amo, A.; Cuesta, J.; Arrieta, I.; Lahoz, C.; Sastre, J. Beer-induced anaphylaxis: Identification of allergens. Allergy 1999, 54, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Anaya, S.; Crespo, J.F.; Rodríguez, J.R.; Daroca, P.; Carmona, E.; Herraez, L.; López-Rubio, A. Beer anaphylaxis. J. Allergy Clin. Immunol. 1999, 103, 959–960. [Google Scholar] [CrossRef]
- Bonadonna, P.; Crivellaro, M.; Dama, A.; Senna, G.E.; Mistrello, G.; Passalacqua, G. Beer-induced anaphylaxis due to barley sensitization: Two case reports. J. Investig. Allergol. Clin. Immunol. 1999, 9, 268–270. [Google Scholar]
- Asero, R.; Mistrello, G.; Roncarolo, D.; Amato, S.; van Ree, R. A case of allergy to beer showing cross-reactivity between lipid transfer proteins. Ann. Allergy Asthma Immunol. 2001, 87, 65–67. [Google Scholar] [CrossRef]
- Nusem, D.; Panasoff, J. Beer anaphylaxis. Isr. Med. Assoc. J. 2009, 11, 380–381. [Google Scholar] [PubMed]
- Herzinger, T.; Kick, G.; Ludolph-Hauser, D.; Przybilla, B. Anaphylaxis to wheat beer. Ann. Allergy Asthma Immunol. 2004, 92, 673–675. [Google Scholar] [CrossRef]
- Quercia, O.; Zoccatelli, G.; Stefanini, G.F.; Mistrello, G.; Amato, S.; Bolla, M.; Emiliani, F.; Asero, R. Allergy to beer in LTP-sensitized patients: Beers are not all the same. Allergy 2012, 67, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, W.; Huang, X.; Zhou, X.; Luo, J.; Wang, H.; Darsow, U.; Becker, T.; Qian, F.; Hao, F.; et al. Sensitization to beer ingredients in Chinese individuals with beer allergy: A clinical study of 20 cases. Int. Arch. Allergy Immunol. 2014, 163, 135–141. [Google Scholar] [CrossRef]
- Brussino, L.; Nicola, S.; Giorgis, V.; Rolla, G. Beer anaphylaxis due to coriander as hidden allergen. BMJ Case Rep. 2018, 2018, bcr-2018-225562. [Google Scholar] [CrossRef] [PubMed]
- García-Casado, G.; Crespo, J.F.; Rodríguez, J.; Salcedo, G. Isolation and characterization of barley lipid transfer protein and protein Z as beer allergens. J. Allergy Clin. Immunol. 2001, 108, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Yagami, A.; Shimojo, N.; Hara, K.; Nakamura, M.; Matsunaga, K. Case of immediate hypersensitivity to beer. J. Dermatol. 2016, 43, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Chan-Yeung, M.; Dimich-Ward, H.; Enarson, D.A.; Kennedy, S.M. Five cross-sectional studies of grain elevator workers. Am. J. Epidemiol. 1992, 136, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.W.; Blackadder, E.S.; Greenberg, M.; Blyth, W. Extrinsic allergic alveolitis in Scottish maltworkers. Br. Med. J. 1976, 1, 490–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miedinger, D.; Malo, J.L.; Cartier, A.; Labrecque, M. Malt can cause both occupational asthma and allergic alveolitis. Allergy 2009, 64, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- Spiewak, R.; Gora, A.; Dutkiewicz, J. Work-related skin symptoms and type I allergy among eastern-Polish farmers growing hops and other crops. Ann. Agric. Environ. Med. 2001, 8, 51–56. [Google Scholar]
- Reeb-Whitaker, C.K.; Bonauto, D.K. Respiratory disease associated with occupational inhalation to hop (Humulus lupulus) during harvest and processing. Ann. Allergy Asthma Immunol. 2014, 113, 534–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A. Occupational rhinoconjunctivitis due to hops exposure in a brewery worker. J. Allergy Clin. Immunol. 2004, 113, S62. [Google Scholar] [CrossRef]
- Godnic-Cvar, J.; Zuskin, E.; Mustajbegovic, J.; Schachter, E.N.; Kanceljak, B.; Macan, J.; Ilic, Z.; Ebling, Z. Respiratory and immunological findings in brewery workers. Am. J. Ind. Med. 1999, 35, 68–75. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Sanders, D.S.; Aeschlimann, D. The Neuroimmunology of Gluten Intolerance. In Neuro-Immuno-Gastroenterology; Constantinescu, C., Arsenescu, R., Arsenescu, V., Eds.; Springer: Cham, Switzerland, 2016; pp. 263–285. [Google Scholar]
- Cerf-Bensussan, N.; Meresse, B. Coeliac disease and gluten sensitivity: Epithelial stress enters the dance in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 491–492. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Hager, A.S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer–A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Halmos, E.P.; Di Bella, C.A.; Webster, R.; Deng, M.; Tye-Din, J.A. Gluten in “gluten-free” food from food outlets in Melbourne: A cross-sectional study. Med. J. Aust. 2018, 209, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, P.; Hochel, I.; Méndez, E.; Hernando, A.; Gabrovská, D. Immunochemical determination of gluten in malts and beers. Food Addit. Contam. 2006, 23, 1074–1078. [Google Scholar] [CrossRef] [Green Version]
- Guerdrum, L.J.; Bamforth, C.W. Levels of gliadin in commercial beers. Food Chem. 2011, 129, 1783–1784. [Google Scholar] [CrossRef]
- Koning, F.; Vader, W. Gluten peptides and celiac disease. Science 2003, 299, 513–515. [Google Scholar] [CrossRef]
- Smulders, M.J.; van de Wiel, C.C.; van den Broeck, H.C.; van der Meer, I.M.; Israel-Hoevelaken, T.P.M.; Timmer, R.D.; van Dinter, B.J.; Braun, S.; Gilissen, L.J. Oats in healthy gluten-free and regular diets: A perspective. Food Res. Int. 2018, 110, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Kucek, L.K.; Veenstra, L.D.; Amnuaycheewa, P.; Sorrells, M.E. A grounded guide to gluten: How modern genotypes and processing impact wheat sensitivity. Compr. Rev. Food Sci. Food Saf. 2015, 14, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.; Walker, C.; Booer, C. Of pseudocereals and roasted rice. The quest for gluten-free brewing materials. Brew. Distill. 2005, 1, 24–26. [Google Scholar]
- de Meo, B.; Freeman, G.; Marconi, O.; Booer, C.; Perretti, G.; Fantozzi, P. Behaviour of malted cereals and pseudo-cereals for gluten-free beer production. J. Inst. Brew. 2011, 117, 541–546. [Google Scholar] [CrossRef]
- Tanner, G.J.; Blundell, M.J.; Colgrave, M.L.; Howitt, C.A. Creation of the first ultra-low gluten barley (Hordeum vulgare L.) for coeliac and gluten-intolerant populations. Plant Biotechnol. J. 2016, 14, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Rybalka, O.; Katrii, V.; Polishchuk, S.; Morgun, B. Development of hull-less barley with ultra-low gluten content via target genes combination. I. Isolation of triple mutants and black grained genotypes. Agric. Sci. Pract. 2021, 8, 47–57. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, D.B.; Kim, C.W.; Ko, S.H.; Yum, H.Y.; Kim, K.E.; Hong, C.S.; Lee, K.Y. Identification and characterization of the major allergens of buckwheat. Allergy 2000, 55, 1035–1041. [Google Scholar] [CrossRef]
- Stewart, G. Adjuncts. In Handbook of Brewing; Stewart, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 129–143. [Google Scholar]
- Watson, H.G.; Vanderputten, D.; Van Landschoot, A.; Decloedt, A.I. Applicability of different brewhouse technologies and gluten-minimization treatments for the production of gluten-free (barley) malt beers: Pilot-to industrial-scale. J. Food Eng. 2019, 245, 33–42. [Google Scholar] [CrossRef]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-free brewing: Issues and perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- van Zandijke, S. Gluten-reduced beers made with barley. New Brew. 2013, Nov. Dec., 79–84. [Google Scholar]
- Walter, T.; Wieser, H.; Koehler, P. Degradation of gluten in wheat bran and bread drink by means of a proline-specific peptidase. J. Nutr. Food Sci. 2014, 4, 10–4172. [Google Scholar] [CrossRef] [Green Version]
- van Landschoot, A. Gluten-free barley malt beers. Cerevisia 2011, 36, 93–97. [Google Scholar] [CrossRef]
- Allred, L.K.; Lesko, K.; McKiernan, D.; Kupper, C.; Guandalini, S. The celiac patient antibody response to conventional and gluten-removed beer. J. AOAC Int. 2017, 100, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Real, A.; Comino, I.; Moreno, M.D.L.; Lopez-Casado, M.A.; Lorite, P.; Torres, M.I.; Cebolla, Á.; Sousa, C. Identification and in vitro reactivity of celiac immunoactive peptides in an apparent gluten-free beer. PLoS ONE 2014, 9, e100917. [Google Scholar] [CrossRef] [PubMed]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; Wiley-Blackwell: Oxford, UK, 2006; p. 660. [Google Scholar]
- Hucker, B.; Vriesekoop, F.; Vriesekoop-Beswick, A.; Wakeling, L.; Vriesekoop-Beswick, H.; Hucker, A. Vitamins in brewing: Effects of post-fermentation treatments and exposure and maturation on the thiamine and riboflavin vitamer content of beer. J. Inst. Brew. 2016, 122, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Ryder, D. Processing Aids in Brewing. In Handbook of Brewing; Stewart, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 287–327. [Google Scholar]
- Yildirim, H. Effects of fining agents on antioxidant capacity of red wines. J. Inst. Brew. 2011, 117, 55–60. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-da-Silva, J.M.; Laureano, O. Interactions between protein fining agents and proanthocyanidins in white wine. Food Chem. 2008, 106, 536–544. [Google Scholar] [CrossRef]
- Hashim, P.; Ridzwan, M.M.; Bakar, J.; Hashim, M.D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- André, F.; Cavagna, S.; André, C. Gelatine prepared from tuna skin: A risk factor for fish allergy or sensitization? Int. Arch. Allergy Immunol. 2003, 130, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.K.; Poulsen, L.K.; Skov, P.S.; Hefle, S.L.; Hlywka, J.J.; Taylor, S.L.; Bindslev-Jensen, U.; Bindslev-Jensen, C. A randomized, double-blinded, placebo-controlled oral challenge study to evaluate the allergenicity of commercial, food-grade fish gelatin. Food Chem. Toxicol. 2004, 42, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- EFSA–EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to a notification from Brewers of Europe and BFBi on isinglass used as a clarifying agent in brewing pursuant to Article 6 paragraph 11 of Directive 2000/13/EC—For permanent exemption from labelling. EFSA J. 2007, 536, 1–10. [Google Scholar]
- Walker, S.; Camarena, M.; Freeman, G. Alternatives to isinglass for beer clarification. J. Inst. Brew. 2007, 113, 347–354. [Google Scholar] [CrossRef]
- Evans, J.G. The Exploitation of Molluscs. In The Domestication and Exploitation of Plants and Animals, 2nd ed.; Ucko, P.J., Dimbleby, G.W., Eds.; Routledge: New York, NY, USA, 2017; pp. 479–484. [Google Scholar]
- Emoto, A.; Ishizaki, S.; Shiomi, K. Tropomyosins in gastropods and bivalves: Identification as major allergens and amino acid sequence features. Food Chem. 2009, 114, 634–641. [Google Scholar] [CrossRef]
- Kamath, S.D.; Rahman, A.M.A.; Komoda, T.; Lopata, A.L. Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies. Food Chem. 2013, 141, 4031–4039. [Google Scholar] [CrossRef]
- Brown, B.M. Oyster stout. J. Inst. Brew. 1939, 65, 77. [Google Scholar]
- VanHook, A.M.; Patel, N.H. Crustaceans. Curr. Biol. 2008, 18, R547–R550. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.J.; Costa, J.; Oliveira, M.B.P.; Mafra, I. A new real-time PCR quantitative approach for the detection of shrimp crustaceans as potential allergens. J. Food Comp. Anal. 2018, 72, 7–14. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lee, C.H.; Huang, E.S.; Sheu, S.C.; Yu, H.S. Quantification of crustacean tropomyosin, a major food allergen, in eight species of Taiwanese shrimp based on immunoassay. Food Anal. Methods 2018, 11, 2607–2613. [Google Scholar] [CrossRef]
- Cabral, J. Interview with Tim Adams, Oxbow Brewing Head Brewer. 2015. Available online: https://www.vice.com/en/article/nz9kvd/we-spoke-to-the-man-who-brewed-a-dozen-lobsters-into-his-beer (accessed on 7 December 2021).
- Villa, C.; Costa, J.; Oliveira, M.B.P.; Mafra, I. Bovine milk allergens: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokosinski, E. Improvement in the Manufacture of Beer. U.S. Patent 222,507, 2 October 1879. [Google Scholar]
- Holsinger, V.H.; Posati, L.P.; DeVilbiss, E.D. Whey beverages: A review. J. Dairy Sci. 1974, 57, 849–859. [Google Scholar] [CrossRef]
- Dietrich, K.R. Whey-containing malt wort as a raw material for the preparation of a malt-whey beer. Brauwissenschaft 1949, 2, 26. [Google Scholar]
- Brunner, R.; Vogl, A. The production of beer with the use of whey. Gambrinus 1944, 5, 181. [Google Scholar]
- Hesse, A. Molkegetränke (whey beverages). Z. Lebensm. Unters. Forsch. 1948, 88, 499–506. [Google Scholar] [CrossRef]
- Pavsler, A.; Buiatti, S. Non-Lager Beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2009; pp. 17–31. [Google Scholar]
- Harding, L. Whey. Int. J. Dairy Technol. 1963, 16, 53–61. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, N.; Gao, D.; Ma, M. Comparative proteomic analysis of chicken, duck, and quail egg yolks. Int. J. Food Prop. 2018, 21, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Anet, J.; Back, J.F.; Baker, R.S.; Barnett, D.; Burley, R.W.; Howden, M.E.H. Allergens in the white and yolk of hen’s egg. Int. Arch. Allergy Immunol. 1985, 77, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Rupa, P. Immunological and biochemical properties of egg allergens. Worlds Poult. Sci. J. 2004, 60, 321–330. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Cope, R. Egg albumen as a source of foam polypeptide in beer. J. Am. Soc. Brew. Chem. 1987, 45, 27–32. [Google Scholar] [CrossRef]
- Bamforth, C.W. Enzymes, Egg White, and Eccentrics: Memories from 37 Years of Research in the Brewing Industry. J. Am. Soc. Brew. Chem. 2016, 74, 1–15. [Google Scholar] [CrossRef]
- Dölle, S.; Welter, S.; Ruppel, E.; Lehmann, K.; Schwarz, D.; Jensen-Jarolim, E.; Zieglmayer, P.; Franken, P.; Worm, M. Clinical reactivity of celery cultivars in allergic patients: Role of Api g 1. Clin. Exp. Allergy 2018, 48, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, D.J.; Key, M.M.; Tubich, G.E.; Perone, V.B. Phototoxic bullae among celery harvesters. Arch. Dermatol. 1961, 83, 73–87. [Google Scholar] [CrossRef]
- Poncet, P.; Sénéchal, H.; Charpin, D. Update on pollen-food allergy syndrome. Expert Rev. Clin. Immunol. 2020, 16, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, J.M.; Bagley, K.; Kulis, M. Tree nut allergies: Allergen homology, cross-reactivity, and implications for therapy. Clin. Exp. Allergy 2018, 48, 762–772. [Google Scholar] [CrossRef] [PubMed]
- de Leon, M.P.; Glaspole, I.N.; Drew, A.C.; Rolland, J.M.; O’Hehir, R.E.; Suphioglu, C. Immunological analysis of allergenic cross-reactivity between peanut and tree nuts. Clin. Exp. Allergy 2003, 33, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z. Food Allergy. J. Immunol. Infect. Dis. 2017, 4, 202. [Google Scholar] [CrossRef]
- Bublin, M.; Breiteneder, H. Cross-reactivity of peanut allergens. Curr. Allergy Asthma Rep. 2014, 14, 426. [Google Scholar] [CrossRef] [Green Version]
- Sirtori, E.; Resta, D.; Arnoldi, A.; Savelkoul, H.F.; Wichers, H.J. Cross-reactivity between peanut and lupin proteins. Food Chem. 2011, 126, 902–910. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Hussain, L. Essential amino acids profile of differentially processed soy products and their efficiency in meeting daily requirement. Nutr. Food Sci. 2016, 46, 237–245. [Google Scholar] [CrossRef]
- Ogawa, T.; Samoto, M.; Takahashi, K. Soybean allergens and hypoallergenic soybean products. J. Nutr. Sci. Vitaminol. 2000, 46, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Verma, A.K.; Gupta, R.K.; Dwivedi, P.D. A comprehensive review on mustard-induced allergy and implications for human health. Clin. Rev. Allergy Immunol. 2019, 57, 39–54. [Google Scholar] [CrossRef]
- Oñaderra, M.; Monsalve, R.I.; Mancheño, J.M.; Villalba, M.; Del Pozo, A.M.; Gavilanes, J.G.; Rodriguez, R. Food mustard allergen interaction with phospholipid vesicles. Eur. J. Biochem. 1994, 225, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Adatia, A.; Clarke, A.E.; Yanishevsky, Y.; Ben-Shoshan, M. Sesame allergy: Current perspectives. J. Asthma Allergy 2017, 10, 41–151. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, A.M.; Rossnagel, M.; Brix, B.; Blankestijn, M.A.; Le, T.M.; Suer, W.; Otten, H.G.; Knulst, A.C. Sesame oleosins are minor allergens. Clin. Transl. Allergy 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Nerlich, U. Evaluation of sulphite in beer and spirits after the new allergen labelling rules. Brew. Sci. 2006, 59, 114–117. [Google Scholar]
- Ilett, D.R. Aspects of the analysis, role, and fate of sulphur dioxide in beer—A review. Tech. Q. Master Brew. Assoc. Am. 1995, 32, 213–221. [Google Scholar]
- Guido, L.F. Sulfites in beer: Reviewing regulation, analysis and role. Sci. Agric. 2016, 73, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Bahna, S.L. Spice allergy. Ann. Allergy Asthma Immunol. 2011, 107, 191–199. [Google Scholar] [CrossRef]
- Scheurer, S.; Pastorello, E.A.; Wangorsch, A.; Kästner, M.; Haustein, D.; Vieths, S. Recombinant allergens Pru av 1 and Pru av 4 and a newly identified lipid transfer protein in the in vitro diagnosis of cherry allergy. J. Allergy Clin. Immunol. 2001, 107, 724–731. [Google Scholar] [CrossRef]
- Hassan, A.K.; Venkatesh, Y.P. An overview of fruit allergy and the causative allergens. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 180–187. [Google Scholar] [PubMed]
- Kurze, E.; Lo Scalzo, R.; Campanelli, G.; Schwab, W. Effect of tomato variety, cultivation, climate and processing on Sola l 4, an allergen from Solanum lycopersicum. PLoS ONE 2018, 13, e0197971. [Google Scholar] [CrossRef] [PubMed]
- Vriesekoop, F.; Krahl, M.; Hucker, B.; Menz, G. 125th Anniversary Review: Bacteria in brewing: The good, the bad and the ugly. J. Inst. Brew. 2012, 118, 335–345. [Google Scholar] [CrossRef]
- Baigts-Allende, D.K.; Pérez-Alva, A.; Ramírez-Rodrigues, M.A.; Palacios, A.; Ramírez-Rodrigues, M.M. A comparative study of polyphenolic and amino acid profiles of commercial fruit beers. J. Food Comp. Anal. 2021, 100, 103921. [Google Scholar] [CrossRef]
- Trinh, L.; Willey, A.R.; Martin, P.J.; Ashley, J.; Tothill, I.E.; Rodgers, T.L. Rate-based approach to cleaning-in-place. Ind. Eng. Chem. Res. 2017, 56, 6695–6702. [Google Scholar] [CrossRef] [Green Version]
- Fryer, P.J.; Robbins, P.T.; Asteriadou, K. Current knowledge in hygienic design: Can we minimize fouling and speed cleaning? Procedia Food Sci. 2011, 1, 1753–1760. [Google Scholar] [CrossRef] [Green Version]
- Stephan, O.; Weisz, N.; Vieths, S.; Weiser, T.; Rabe, B.; Vatterott, W. Protein quantification, sandwich ELISA, and real-time PCR used to monitor industrial cleaning procedures for contamination with peanut and celery allergens. J. AOAC Int. 2004, 87, 1448–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, E.; Montague, G.; Robbins, P. A quality by design approach to process plant cleaning. Chem. Eng. Res. Des. 2013, 91, 1095–1105. [Google Scholar] [CrossRef]
- Barnes, Z. Cleaning in Place (CIP). In Handbook of Brewing; Stewart, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 415–431. [Google Scholar]
- Taylor, S.; Vriesekoop, F. Assessing the Factors behind the Increase in Gluten Free Beer. Honours Dissertation, Harper Adams University, Newport, UK, 2017. [Google Scholar]
- Fuciños, C.; Estévez, N.; Míguez, M.; Fajardo, P.; Chapela, M.J.; Gondar, D.; Rúa, M.L. Effectiveness of proteolytic enzymes to remove gluten residues and feasibility of incorporating them into cleaning products for industrial purposes. Food Res. Int. 2019, 120, 167–177. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Arora, A.; Patti, A.F. 2021. Green approach towards hydrolysing wheat gluten using waste ingredients from pineapple processing industries. Int. J. Food Sci. Technol. 2021, 56, 1724–1733. [Google Scholar] [CrossRef]
- Sarver, R.W.; Almy, D.J.; Bergeron, E.R.; Strong, B.F.; Steiner, B.A.; Donofrio, R.; Lupo, A.J.; Gray, R.L.; Sperry, A.K. Overview of Portable Assays for the Detection of Mycotoxins, Allergens, and Sanitation Monitoring. J. AOAC Int. 2021, 104, 39–48. [Google Scholar] [CrossRef]
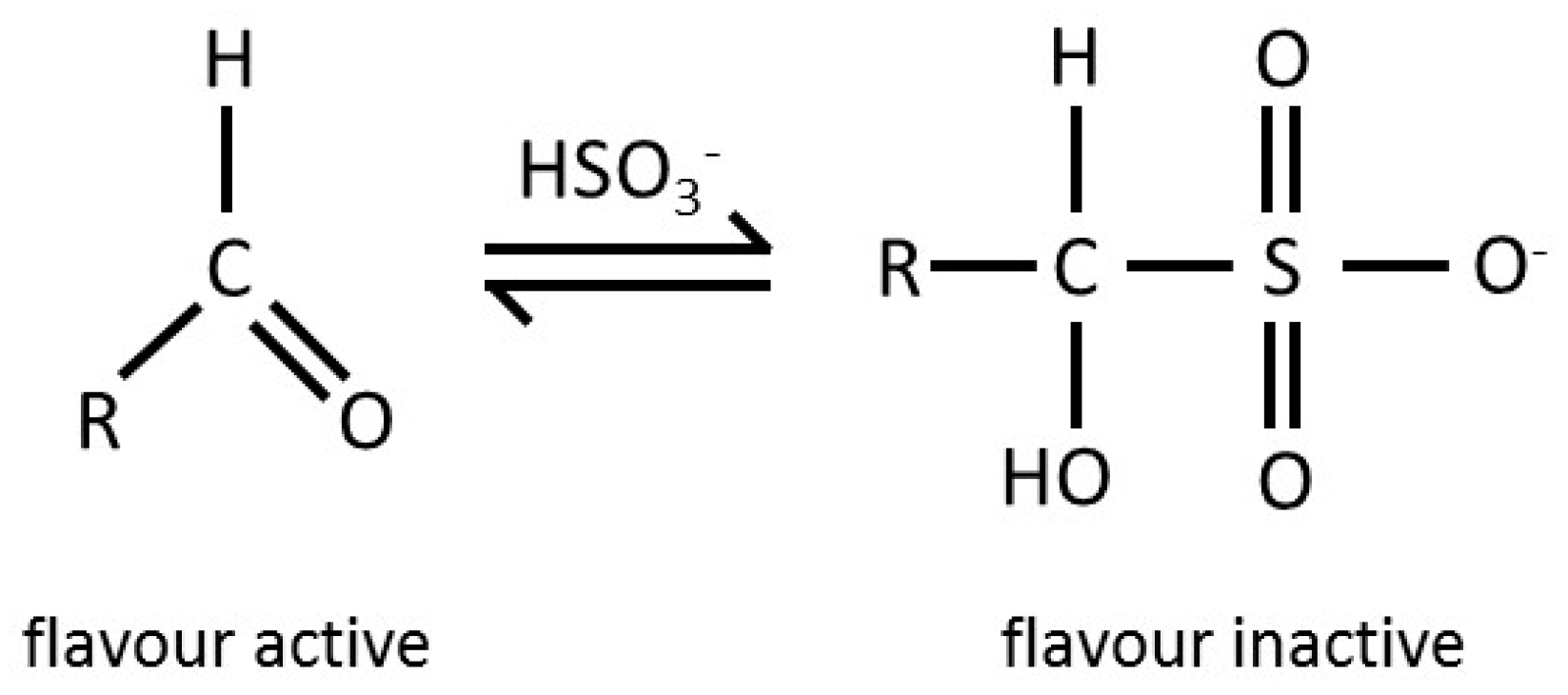
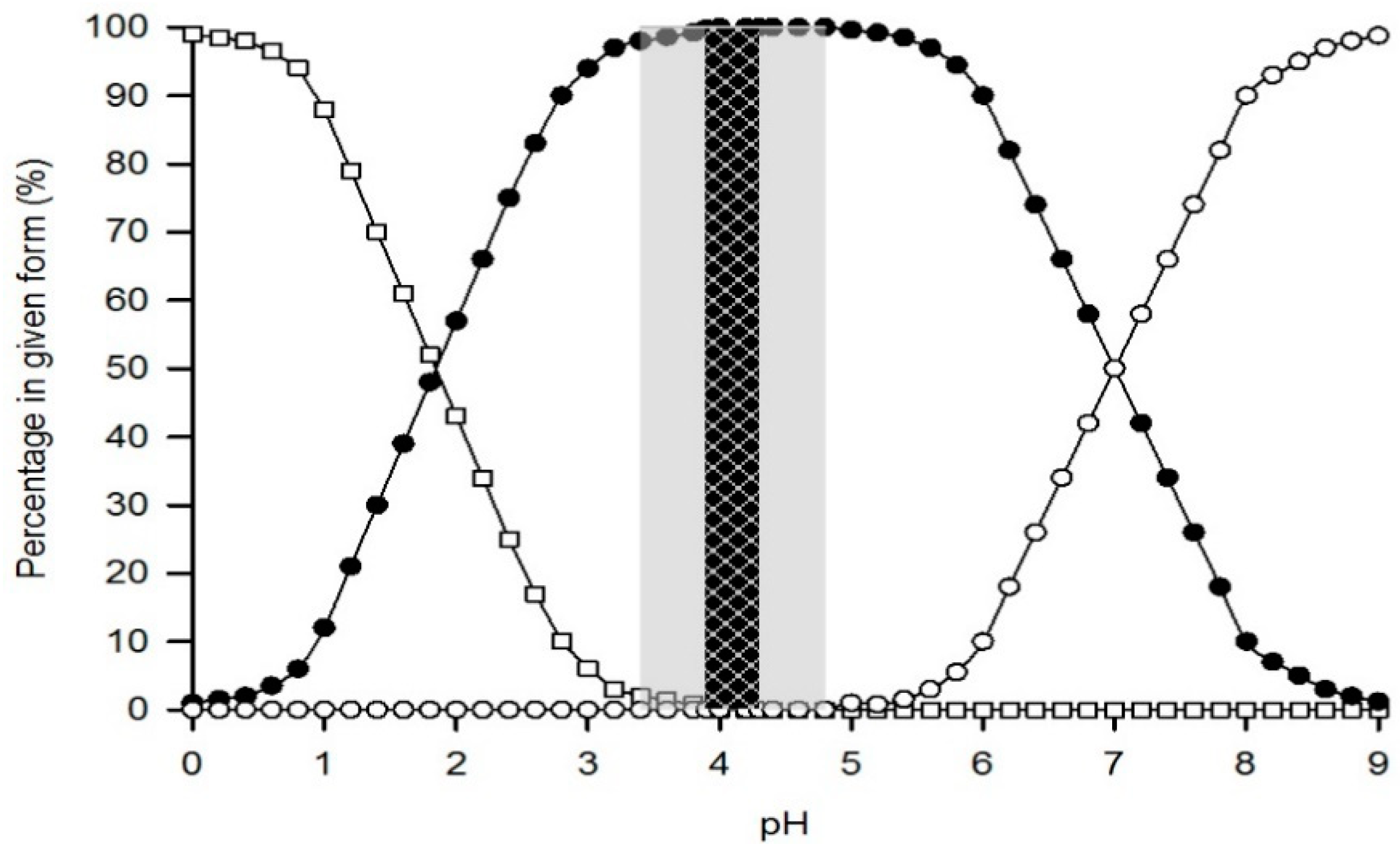
| Country/Region | Lower Limit Requiring Mention on Label (ppm) | Maximum Legal Limit (ppm) in Beer |
|---|---|---|
| EU | 10 | 20 (50 with secondary fermentation in cask/barrel) |
| USA | 10 | 25 |
| Canada | 10 | 15 |
| Mexico | 10 | 15 |
| UK | 10 | 20 (50 with secondary fermentation in cask/barrel) |
| Russia | 10 | 20 (50 with secondary fermentation in cask/barrel) |
| Turkey | 10 | 20 |
| Australia | 10 | 25 |
| New Zealand | 10 | 25 |
| Singapore | 10 | 25 |
| Vietnam | 10 | unknown |
| Thailand | 10 | 30 |
| India | unknown | 50 |
| Brazil | unknown | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vriesekoop, F. Beer and Allergens. Beverages 2021, 7, 79. https://doi.org/10.3390/beverages7040079
Vriesekoop F. Beer and Allergens. Beverages. 2021; 7(4):79. https://doi.org/10.3390/beverages7040079
Chicago/Turabian StyleVriesekoop, Frank. 2021. "Beer and Allergens" Beverages 7, no. 4: 79. https://doi.org/10.3390/beverages7040079
APA StyleVriesekoop, F. (2021). Beer and Allergens. Beverages, 7(4), 79. https://doi.org/10.3390/beverages7040079







