Detection of Red Wine Faults over Time with Flash Profiling and the Electronic Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Yeast Strains and Starter Cultures
2.3. Bacterial Strains and Starter Cultures
2.4. Wines
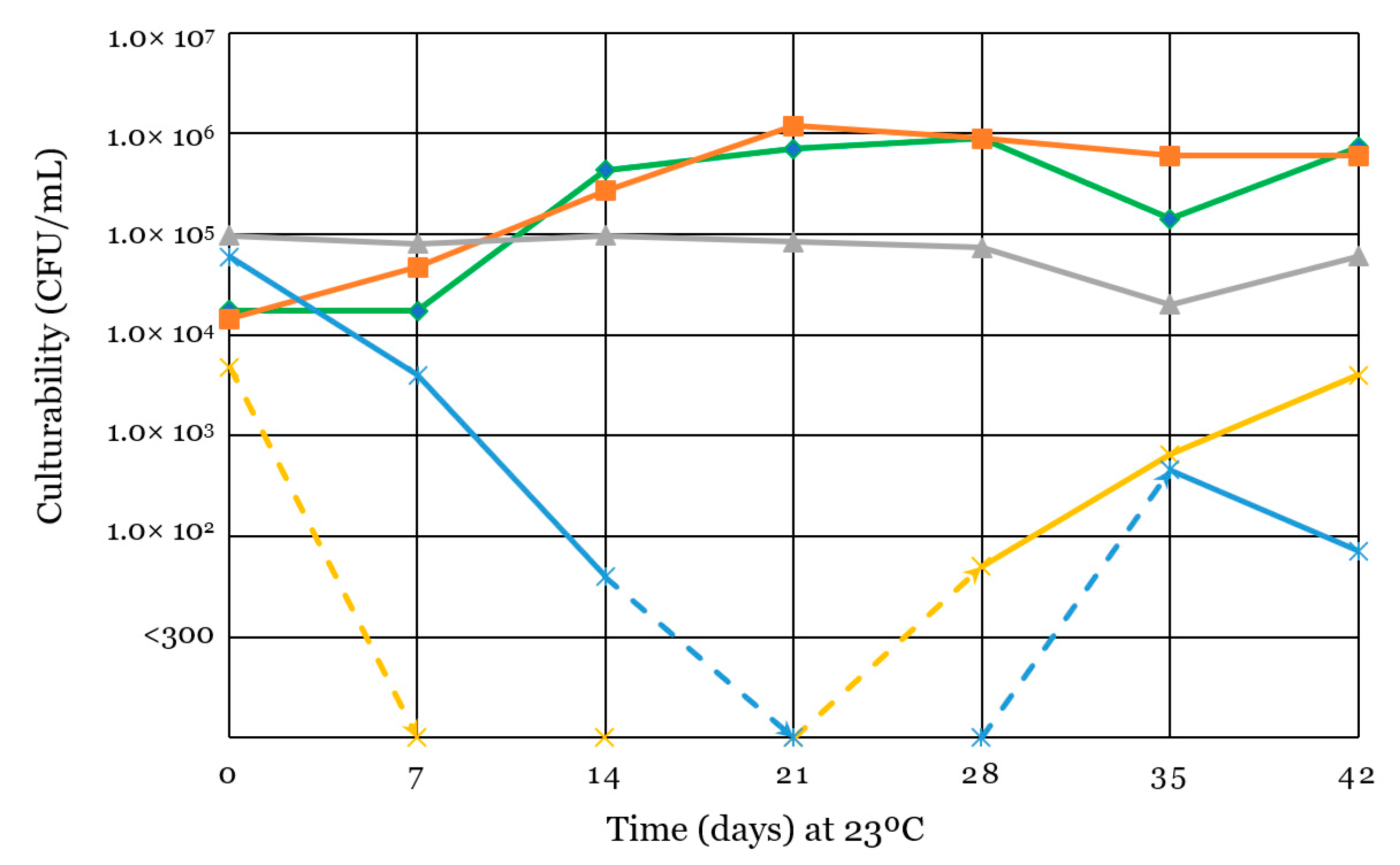
2.5. Spoilage Microorganism Culturability
2.6. Flash Profiling
2.7. Electronic Tongue
2.8. Statistical Analysis
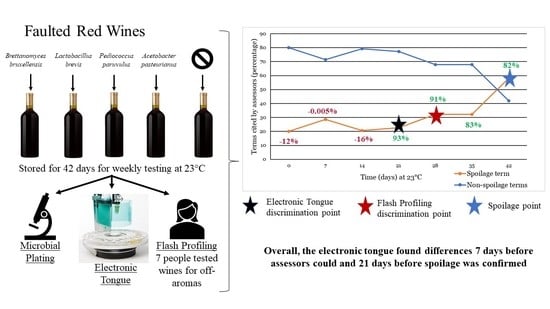
3. Results
3.1. Culturability of Inoculated Microorganisms
3.2. Electronic Tongue
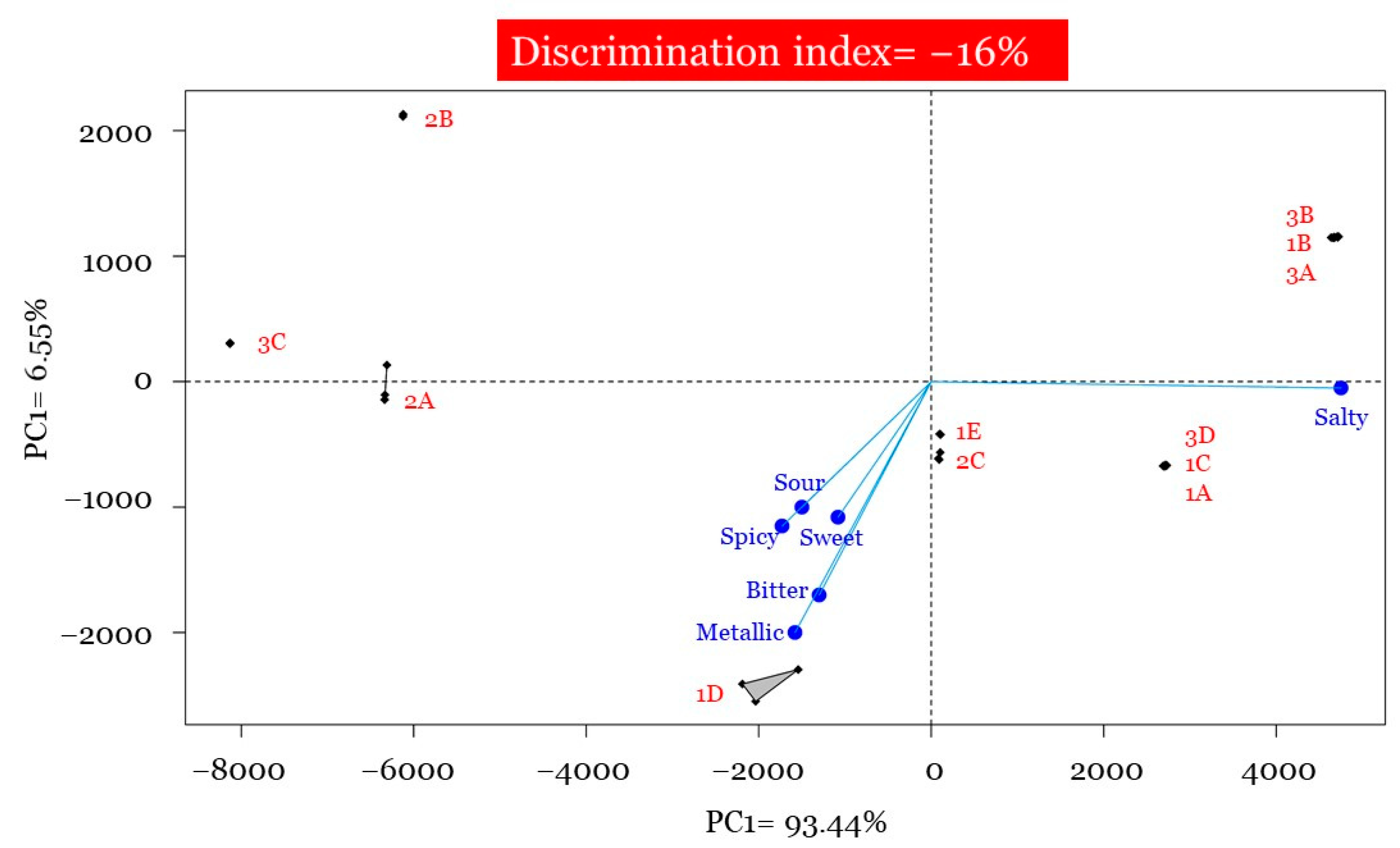
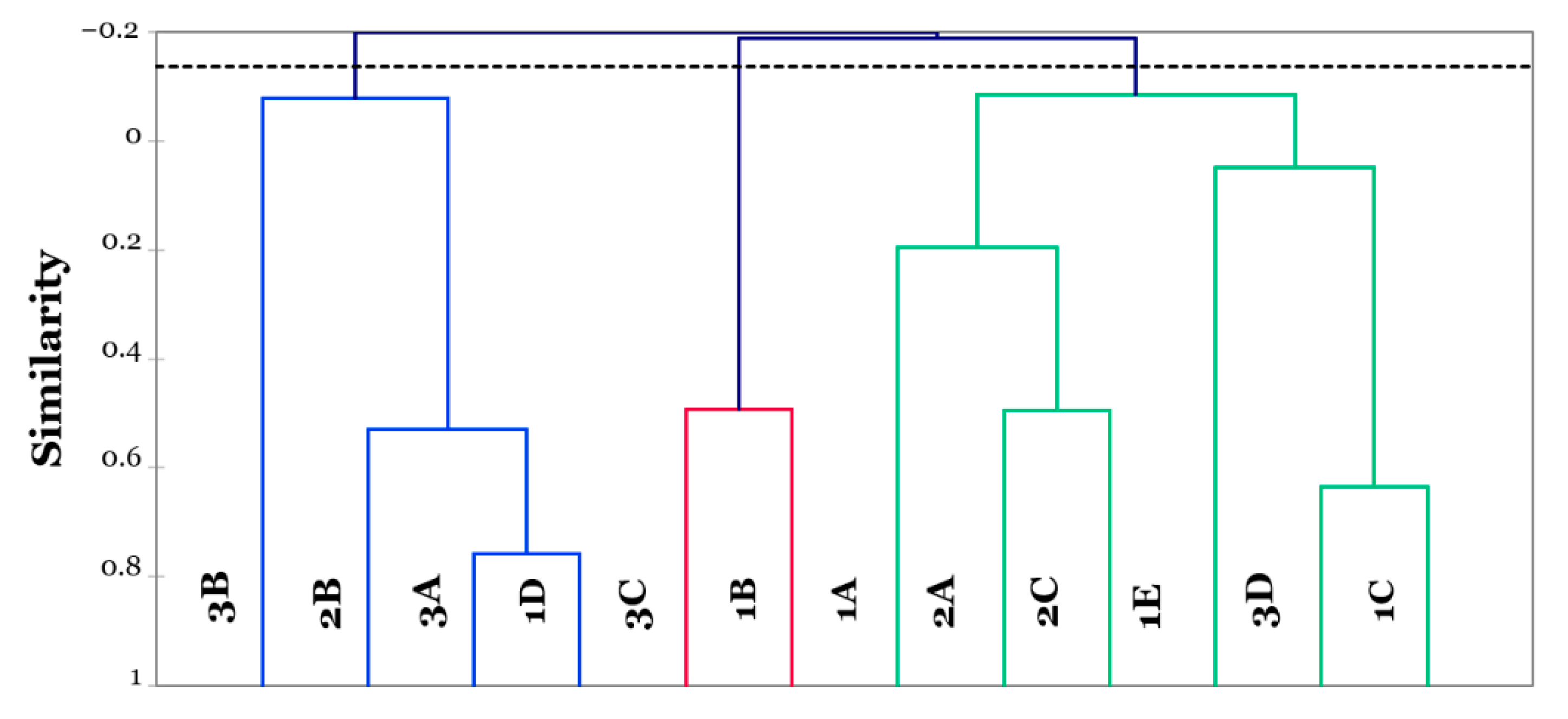
3.3. Flash Profiling
3.4. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AWRI. Wine Faults, Taints and Flavours. Australian Wine Research Institute. Available online: https://www.awri.com.au/industry_support/winemaking_resources/sensory_assessment/recognition-of-wine-faults-and-taints/ (accessed on 8 July 2020).
- Alcohol and Tobacco Tax and Trade Bureau (TTB). TTB: TAD: Wine-Common Audit Issues. TTBGov-Wine-Common Audit Issues. Available online: https://www.ttb.gov/wine/wine-common-audit-issues (accessed on 19 May 2015).
- Bartowsky, E.J. Bacterial spoilage of wine and approaches to minimize it. Lett. Appl. Microbiol. 2009, 48, 149–156. [Google Scholar] [CrossRef]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology: Practical Applications and Procedures; Springer: New York, NY, USA, 2006. [Google Scholar]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M.; Barata, A.; Loureiro, V. Wine spoilage by fungal metabolites. In Wine Chemistry and Biochemistry; Polo, C., Moreno-Arribas, M.V., Eds.; Springer: New York, NY, USA, 2009; pp. 615–645. [Google Scholar]
- Schumaker, M.R.; Chandr, M.; Malfeito-Ferreira, M.; Ross, C.F. Influence of Brettanomyces ethylphenols on red wine aroma evaluated by consumers in the United States and Portugal. Food Res. Int. 2017, 100, 161–167. [Google Scholar] [CrossRef]
- Davis, E.R.; Wibowo, D.; Fleet, G.H.; Lee, T.H. Properties of wine lactic acid bacteria: Their potential enological significance. AJEV 1988, 39, 137–142. [Google Scholar]
- Lonvaud-Funel, A.; Guilloux, Y.; Joyeux, A. Isolation of a DNA probe for identification of glucan-producing Pediococcus damnosus in wines. J. Appl. Bacteriol. 1993, 74, 41–47. [Google Scholar] [CrossRef]
- Sponholz, W.R. Wine spoilage by microorganisms. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: New York, NY, USA, 1993; pp. 395–420. [Google Scholar]
- Liu, S.Q.; Pilone, G.J. A review: Arginine metabolism in wine lactic acid bacteria and its practical significance. J. Appl. Microbiol. 1998, 84, 315–327. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Xia, D.; Gibson, R.L.; Feet, G.H.; Henschke, P.A. Spoilage of bottled red wine by acetic acid bacteria. Lett. Appl. Microbiol. 2003, 36, 307–314. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.; Pons, M. The origins of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine- diacetyl- desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef]
- Liu, J.; Gronbeck, M.S.; Di Monaco, R.; Giacalone, D.; Bredie, W.L.P. Performance of flash profile and napping with and without training for describing small sensory differences in a model wine. Food Qual. Prefer. 2016, 48, 41–49. [Google Scholar] [CrossRef]
- Sieffermann, J.M. Le profil Flash: Un Outil Rapide et Innovant d’e’ Valuation Sensorielle Descriptive [Conference Paper Presentation]; The L’innovation: De l’ide’eau succe’s-Douzie’mes rencontres AGORAL: Paris, France, 2000. [Google Scholar]
- Williams, A.A.; Langron, S.P. The use of free-choice profiling for the evaluation of commercial ports. J. Sci. Food Agric. 1984, 35, 558–568. [Google Scholar] [CrossRef]
- Dairo, V.; Sieffermann, J.M. A comparison of 14 jams characterized by conventional profile and a quick original method, the flash profile. J. Food Sci. 2002, 67, 826–834. [Google Scholar] [CrossRef]
- Delarue, J. Flash profile, its evolution and uses in sensory and consumer science. In Rapid Sensory Profiling Techniques: Applications in New Product Development and Consumer Research; Delarue, J., Lawlor, J.B., Rogeaux, M., Eds.; Elsevier: New York, NY, USA, 2015; pp. 119–148. [Google Scholar]
- Liu, J.; Bredie, W.L.O.; Sherman, E.; Harbertson, J.F.; Heymann, H. Comparison of rapid descriptive sensory methodologies: Free-choice profiling, flash profile and modified flash profile. Food Res. Int. 2018, 106, 892–900. [Google Scholar] [CrossRef]
- Albert, A.; Varela, P.; Salvador, A.; Hough, G.; Fiszman, S. Overcoming the issues in the sensory description of hot served foods with a complex texture. Application of QDA, flash profiling and projective mapping using panels with different degrees of training. Food Qual. Prefer. 2011, 22, 463–473. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Clapham, D.; Seleznev, B.; Lord, K.; Vlasov, Y. Electronic tongue for pharmaceutical analytics: Quantification of tastes and masking effects. Anal. Bioanal. Chem. 2004, 380, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rudinitskaya, A.; Rocha, S.M.; Legin, A.; Pereira, V.; Marques, J.C. Evaluation of the feasibility of the electronic tongue as a rapid analytical tool for wine age prediction and quantification of the organic acids and phenolic compounds. The case-study of Madeira wine. Anal. Chim. Acta 2010, 662, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.; Bataller, R.; Armero, R.; Gandia, J.M.; Soto, J.; Martinez-Manez, R.; Gil-Sanchez, L. Monitoring grape ripeness using a voltametric electronic tongue. Food Res. Int. 2013, 54, 1369–1375. [Google Scholar] [CrossRef]
- Diako, C.; McMahon, K.; Mattinson, S.; Evans, M.; Ross, C.F. Alcohol, tannins, and mannoprotein and their interactions influence the sensory properties of selected commercial merlot wines: A preliminary study. J. Food Sci. 2016, 81, S2039–S2048. [Google Scholar] [CrossRef]
- Diako, C.; Vixie, B.; Weller, K.M.; Dycus, D.A.; Ross, C.F. Determination of 4-ethylcatechol in a merlot wine using sensory evaluation and the electronic tongue. Int. J. Food Sci. Technol. 2017, 52, 2489–2496. [Google Scholar] [CrossRef]
- Newman, J.; O’Riordan, D.; Jacquier, J.C.; O’Sullivan, M. Masking of bitterness in dairy protein hydrolysates: Comparison of an electronic tongue and a trained sensory panel as means of directing the masking strategy. LWT 2015, 63, 751–757. [Google Scholar] [CrossRef]
- Lipkowitz, J.B.; Ross, C.F.; Diako, C.; Smith, D.M. Discriminating aging and protein-to-fat ration in cheddar cheese using sensory analysis and a potentiometric electronic tongue. J. Dairy Sci. 2018, 101, 1990–2004. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.A.; Diako, C.; Smith, D.M.; Ross, C.F. Influence of storage time and elevated ripening temperature on the chemical and sensory properties of white cheddar cheese. J. Food Sci. 2020, 85, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Dyminski, D.S.; Paterno, L.G.; Takeda, H.H.; Bolini, H.; Mattoso, L.H.C.; Candido, L.M.B. Correlation between human and electronic tongues responses on the analysis of commercial sweeteners. Sens. Lett. 2006, 4, 403–408. [Google Scholar] [CrossRef]
- Waldrop, M.E.; Ross, C.F. Sweetener blend optimization using mixture design methodology and the electronic tongue. J. Food Sci. 2014, 79, S1782–S1794. [Google Scholar] [CrossRef] [PubMed]
- Paup, V.D.; Barnett, S.M.; Diako, C.; Ross, C.F. Detection of spicy compounds using the electronic tongue. J. Food Sci. 2019, 84, 2619–2627. [Google Scholar] [CrossRef]
- Schlossareck, C.; Ross, C.F. Electronic tongue and consumer sensory evaluation of spicy paneer cheese. J. Food Sci. 2019, 8, 1563–1569. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Integrated, paper-based potentiometric electronic tongue for the analysis of beer and wine. Anal. Chim. Acta 2016, 918, 60–68. [Google Scholar] [CrossRef]
- Lvova, L.; Yaroshenko, I.; Kirsanov, D.; Natale, C.D.; Paolesse, R.; Legin, A. Electronic tongue for brand uniformity control: A case study of Apulian red wines recognition and defects evaluation. Sensors 2018, 18, 2584. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.L.; Umiker, N.L.; Arneborg, N.; Edwards, C.G. Identification and characterization of Dekkera bruxellensis, Candida pararugosa, and Pichia guilliermondii isolated from commercial red wines. Food Microbiol. 2009, 26, 915–921. [Google Scholar] [CrossRef]
- Noble, A.C.; Arnold, R.A.; Buechsenstein, J.; Leach, E.J.; Schmidt, J.O.; Stern, P.M. Modification of a standardized system of wine aroma terminology. AJEV 1987, 38, 143–146. Available online: https://www.ajevonline.org/content/ajev/38/2/143.full.pdf (accessed on 12 February 2021).
- Joseph, L.; Albino, E.; Bisson, L.F. Creation and use of a Brettanomyces aroma wheel. Catal. Discov. Pract. 2017, 1, 12–20. [Google Scholar] [CrossRef][Green Version]
- Zuehlke, J.M.; Edwards, C.G. Impact of sulfur dioxide and temperature on Culturability and viability of Brettanomyces bruxellensis in wine. J. Food Prot. 2013, 76, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- du Toit, M.; Pretorius, I.S. Microbial spoilage and preservation of wine: Using weapons for nature’s own arsenal. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Dekkera/Brettanomyces spp. In Food Spoilage Microorganisms; Blackburn, C.D.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 354–388. [Google Scholar]
- Wedral, D.; Shewfelt, R.; Frank, J. The challenge of Brettanomyces in wine. LWT 2010, 43, 1474–1479. [Google Scholar] [CrossRef]
- Jia, K.; Zhang, Y.; Li, Y. Systematic engineering of microorganisms to improve alcohol tolerance. Eng. Life Sci. 2010, 10, 422–429. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; Albarracin, E.S.; Silva, A.J.; Lima, L.L.A.; Ferreira, T.A. Wine quality rapid detection using a compact electronic nose system: Application focused on spoilage thresholds by acetic acid. LWT 2019, 108, 377–384. [Google Scholar] [CrossRef]
- Gil-Sanchez, L.; Soto, J.; Martinez-Manez, R.; Garcia-Breijo, E.; Ibanez, I.; Llobet, E. A novel humid electronic nose combined with an electronic tongue for assessing deterioration of wine. Sens. Actuators A Phys. 2011, 171, 152–158. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Reducing the negative sensory impact of volatile phenols in red wine with different chitosans: Effect of structure on efficiency. Food Chem. 2018, 242, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Tempere, S.; Schaaper, M.H.; Cuzange, E.; de Lescar, R.; de Revel, G.; Sicardd, G. The olfactory masking effect of ethylphenols: Characterization and elucidation of its origin. Food Qual. Prefer. 2016, 50, 135–144. [Google Scholar] [CrossRef]


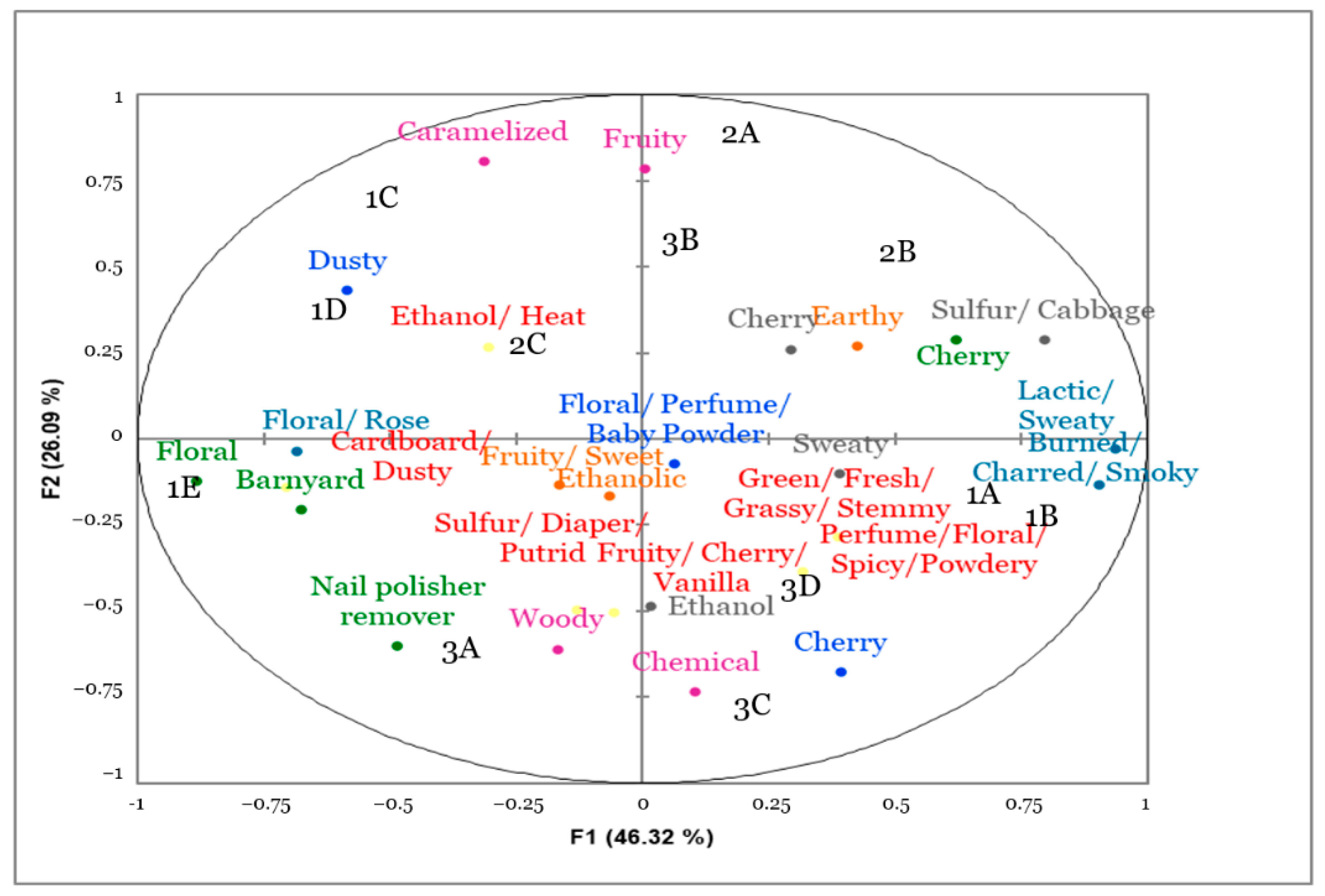
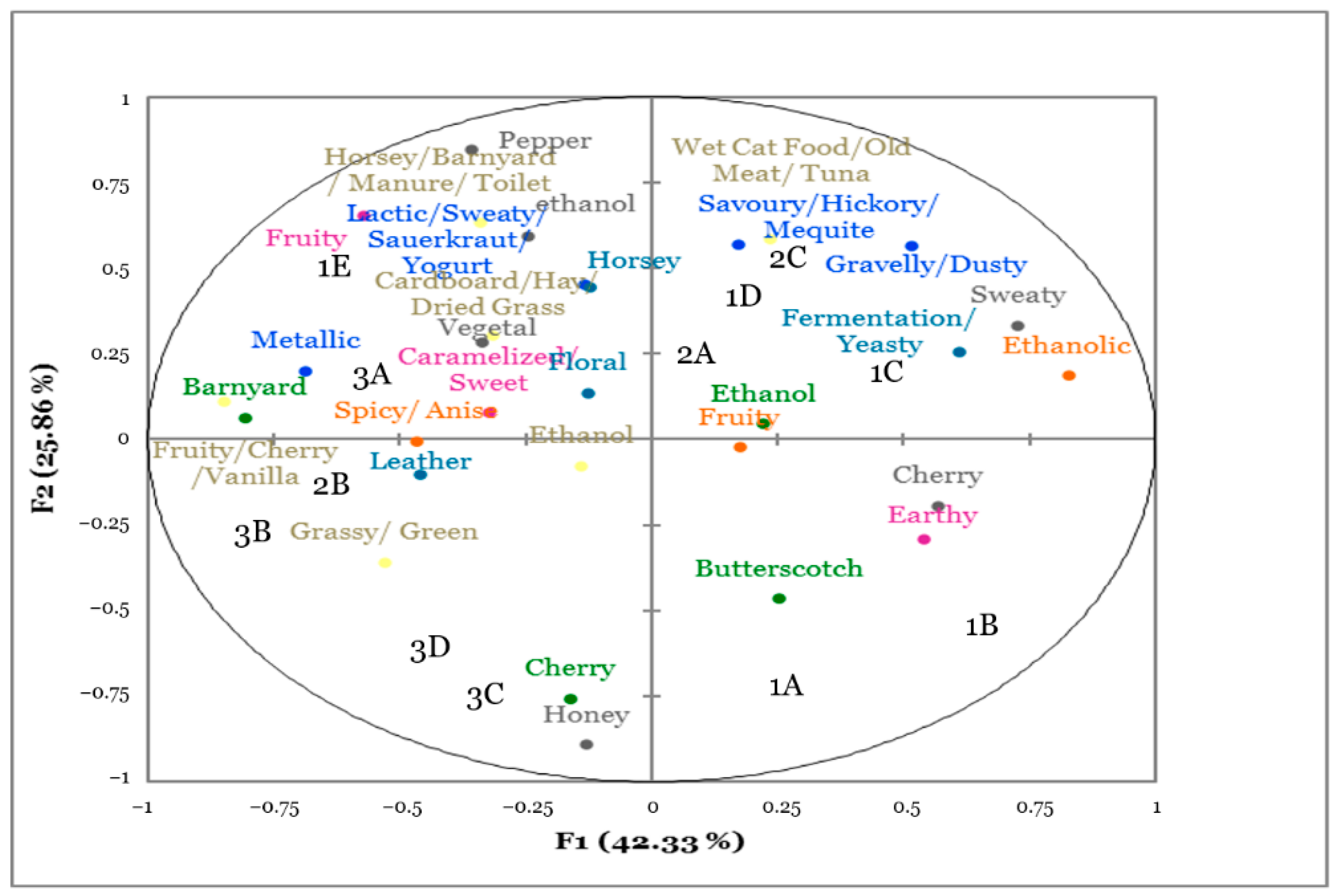

| Code | Inoculated Microorganism | Closure | Bottle Replicate |
|---|---|---|---|
| 1A | Brettanomyces I1A | Sealed | 2 |
| 1B | Brettanomyces F3 | Sealed | 1 |
| 1C | None | Sealed | 1 |
| 1D | None | Partially unscrewed | 1 |
| 1E | Acetobacter pasteurianus | Partially unscrewed | 1 |
| 2A | Pediococcus parvulus | Sealed | 1 |
| 2B | Lactobacillus brevis | Sealed | 1 |
| 2C | Acetobacter pasteurianus | Partially unscrewed | 2 |
| 3A | Pediococcus parvulus | Sealed | 2 |
| 3B | Lactobacillus brevis | Sealed | 2 |
| 3C | Brettanomyces I1A | Sealed | 1 |
| 3D | Brettanomyces F3 | Sealed | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paup, V.D.; Cook-Barton, T.; Diako, C.; Edwards, C.G.; Ross, C.F. Detection of Red Wine Faults over Time with Flash Profiling and the Electronic Tongue. Beverages 2021, 7, 52. https://doi.org/10.3390/beverages7030052
Paup VD, Cook-Barton T, Diako C, Edwards CG, Ross CF. Detection of Red Wine Faults over Time with Flash Profiling and the Electronic Tongue. Beverages. 2021; 7(3):52. https://doi.org/10.3390/beverages7030052
Chicago/Turabian StylePaup, Victoria D., Tara Cook-Barton, Charles Diako, Charles G. Edwards, and Carolyn F. Ross. 2021. "Detection of Red Wine Faults over Time with Flash Profiling and the Electronic Tongue" Beverages 7, no. 3: 52. https://doi.org/10.3390/beverages7030052
APA StylePaup, V. D., Cook-Barton, T., Diako, C., Edwards, C. G., & Ross, C. F. (2021). Detection of Red Wine Faults over Time with Flash Profiling and the Electronic Tongue. Beverages, 7(3), 52. https://doi.org/10.3390/beverages7030052