Perception of Aqueous Ethanol Binary Mixtures Containing Alcohol-Relevant Taste and Chemesthetic Stimuli
Abstract
1. Introduction
1.1. Ethanol
1.2. Orosensory Interactions
1.3. Ethanol and Taste/Chemesthetic Stimuli
1.4. Other Considerations: Thermal Taste
1.5. Study Aims
2. Materials and Methods
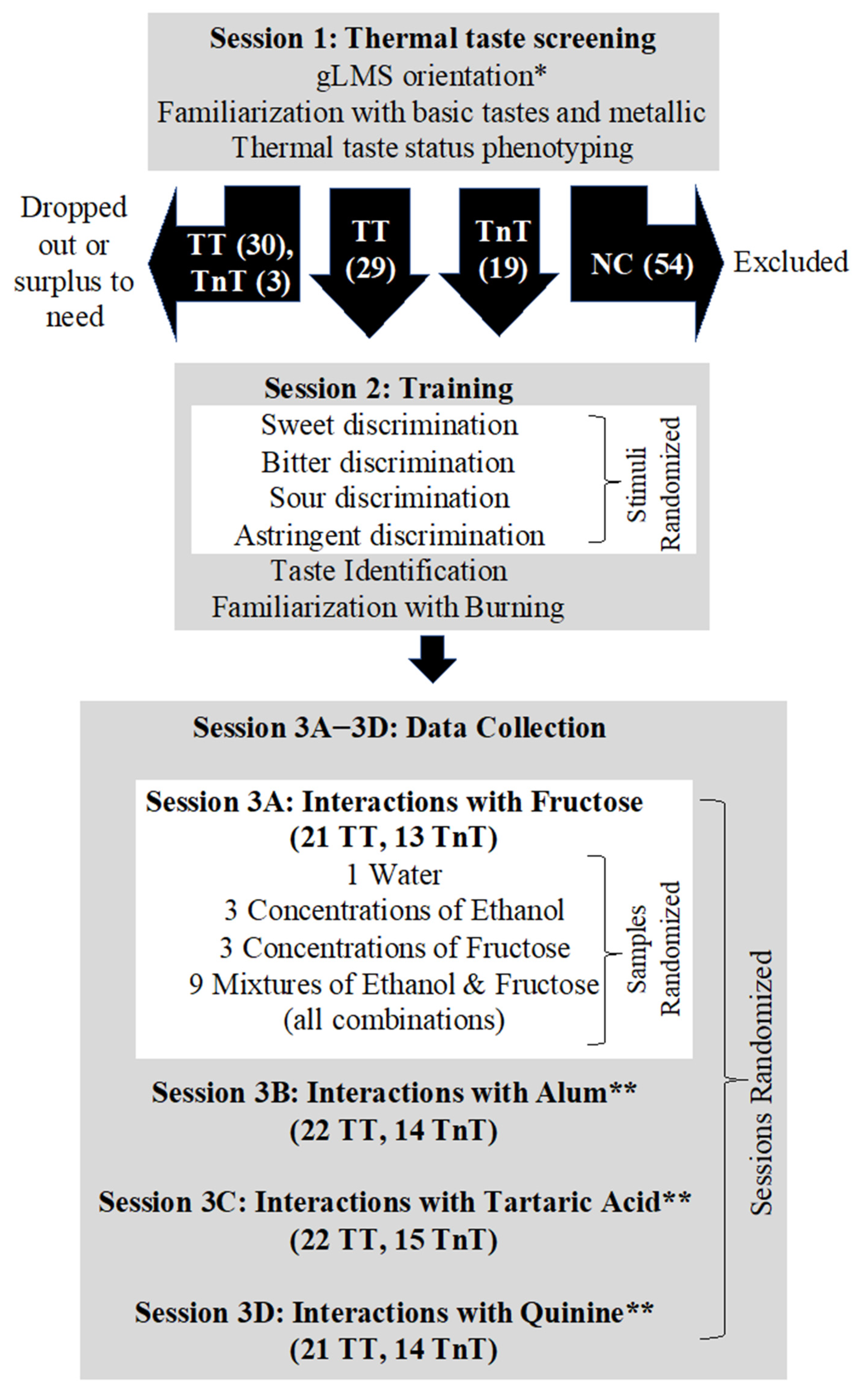
2.1. Thermal Taste Screening (Session 1)
2.2. Training (Session 2) and Data Collection (Sessions 3A–3D)
2.2.1. Orosensory Stimuli
2.2.2. Session 2
2.2.3. Session 3A–3D
2.3. Data Analysis
2.3.1. Data Treatment
2.3.2. Orosensory Training
2.3.3. Unary Solutions
2.3.4. Binary Mixtures
2.3.5. Other Considerations
3. Results
3.1. Orosensory Training
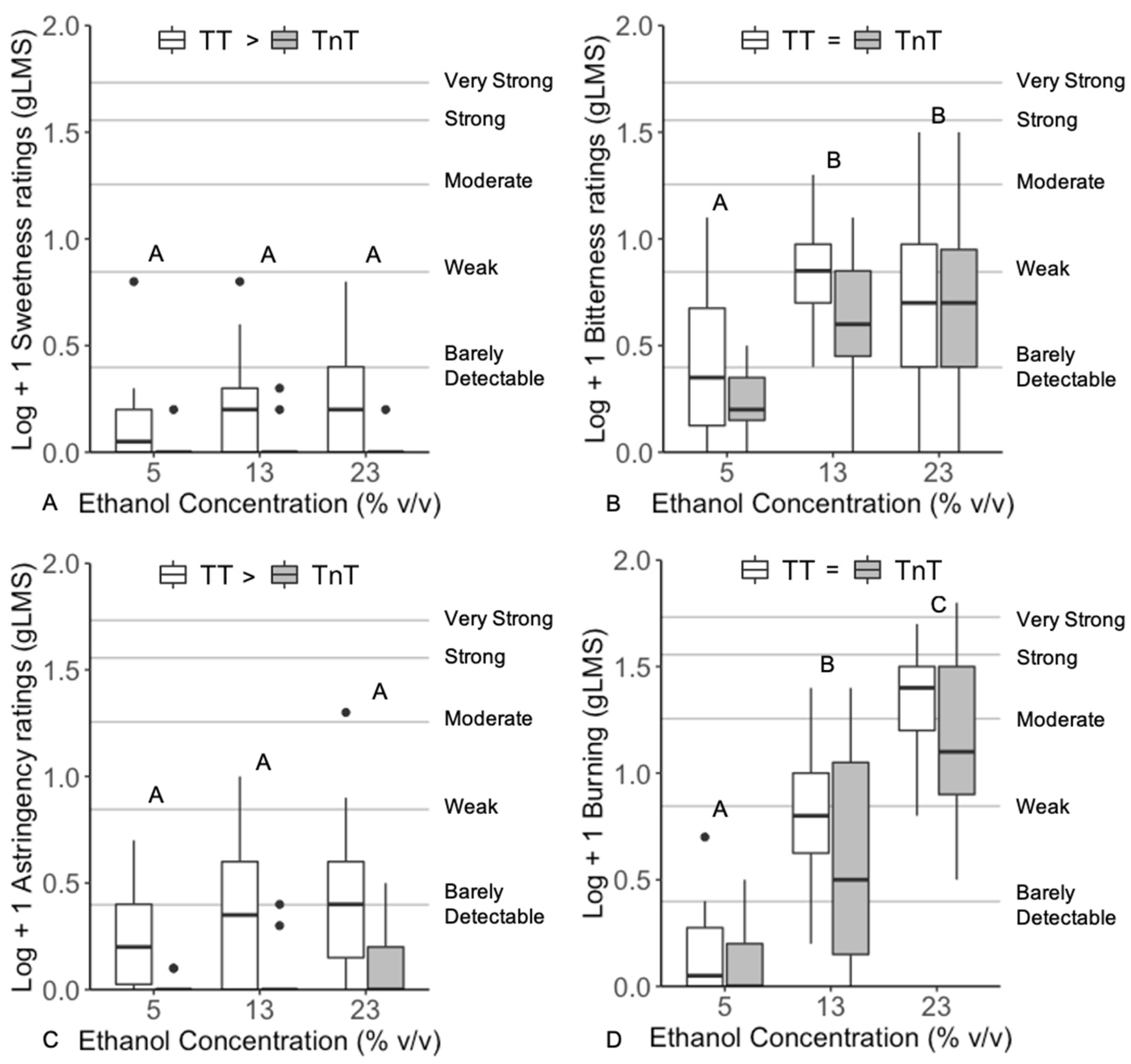
3.2. Unary Solutions
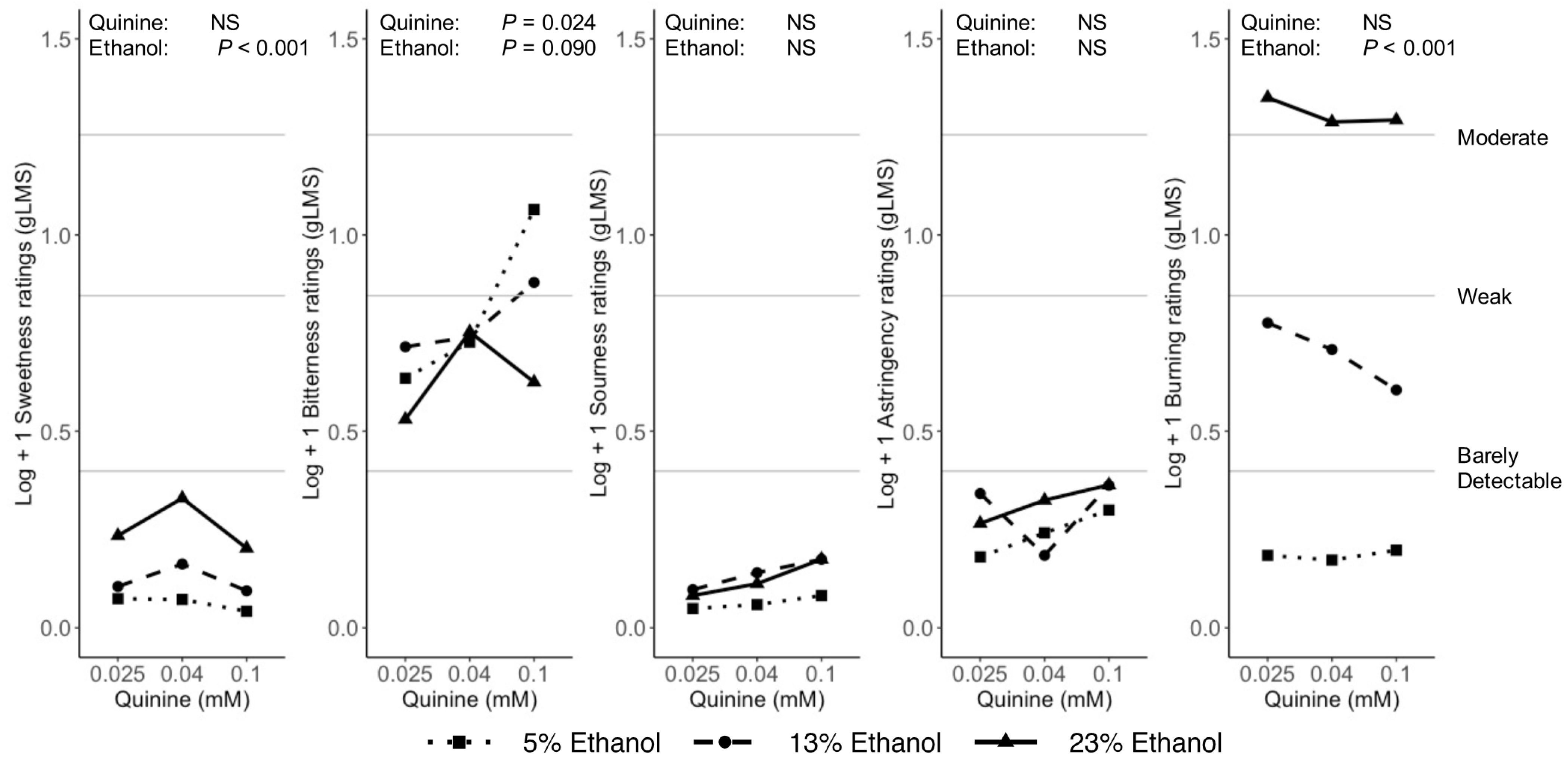
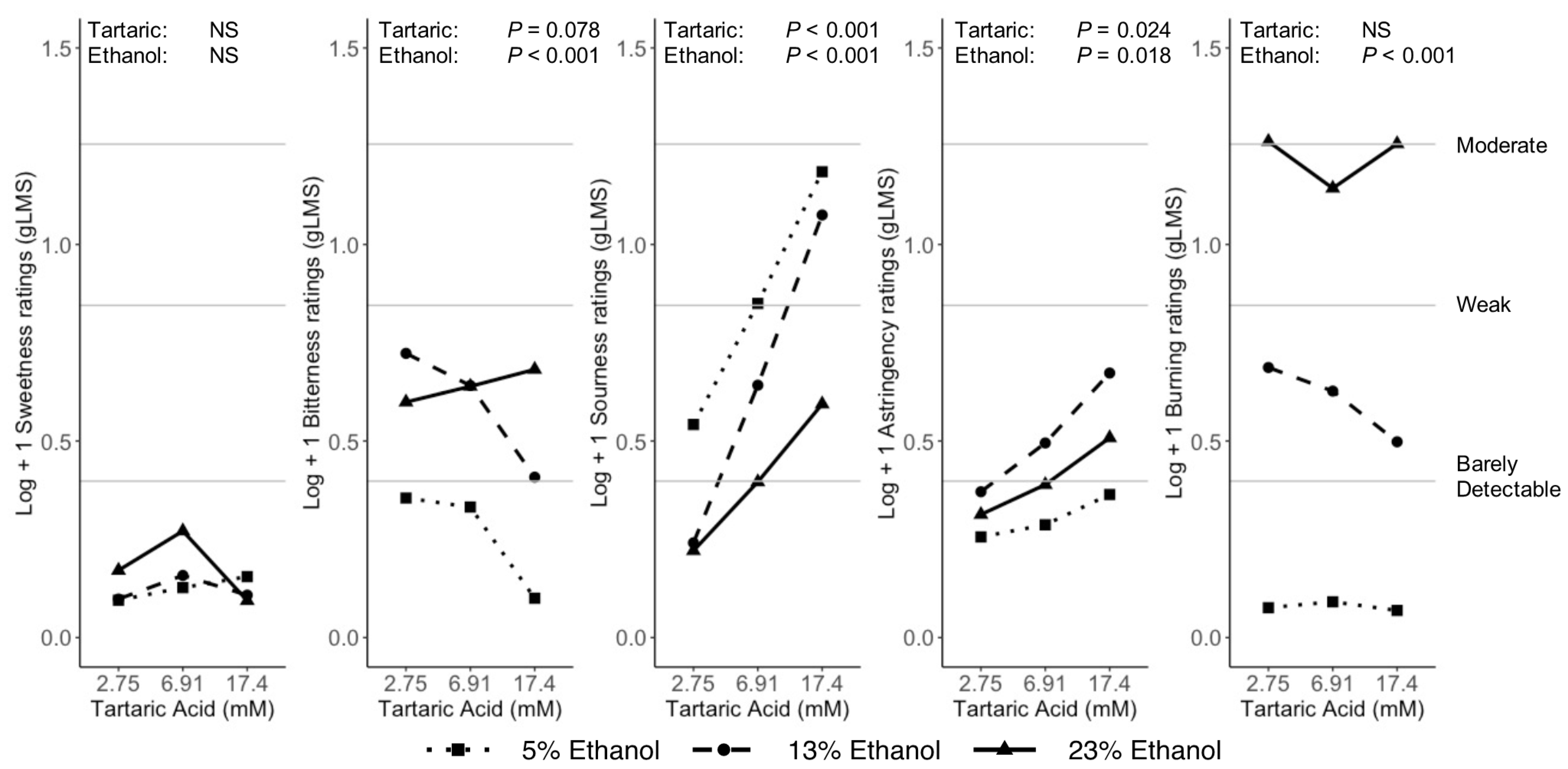
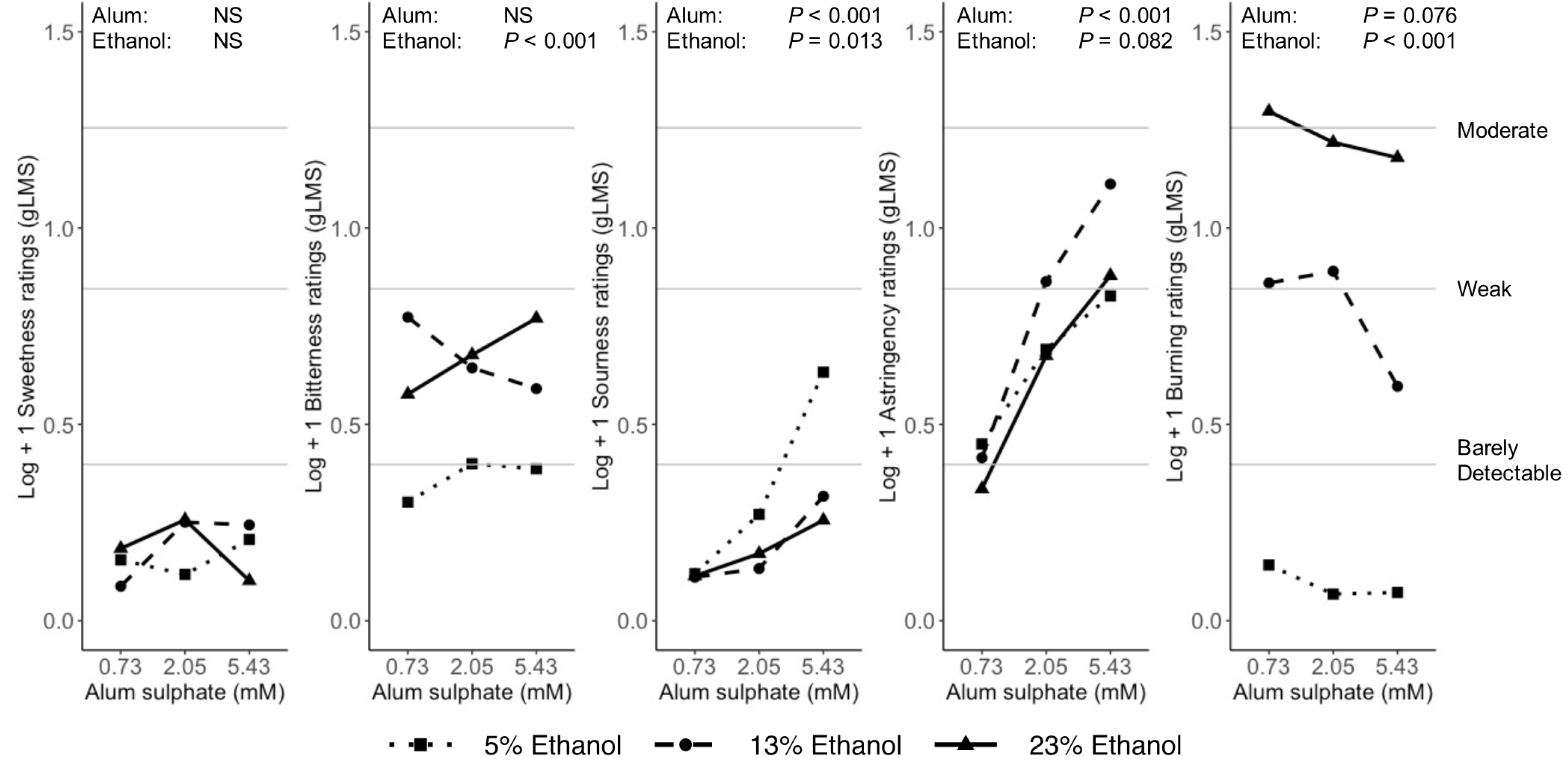
3.3. Binary Mixtures
Index of Interaction
3.4. Other Considerations
4. Discussion
4.1. Unary Solutions
4.2. Binary Mixtures
4.2.1. Sweet
4.2.2. Bitter
4.2.3. Sour
4.2.4. Astringency
4.2.5. Burning/Tingling
4.3. Thermal Taste Status
4.4. Limitations and Other Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; Poznyak, V., Rekve, D., Eds.; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-156563-9. [Google Scholar]
- Barbor, T.F.; Higgins-Biddle, J.C.; Saunders, J.B.; Monteiro, M.G. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care; World Heath Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Park, C.L.; Grant, C. Determinants of positive and negative consequences of alcohol consumption in college students: Alcohol use, gender, and psychological characteristics. Addict. Behav. 2005, 30, 755–765. [Google Scholar] [CrossRef]
- Krenz, M.; Korthuis, R.J. Moderate ethanol ingestion and cardiovascular protection: From epidemiologic associations to cellular mechanisms. J. Mol. Cell Cardiol. 2012, 52, 93–104. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S. Gender differences in risk factors and consequences for alcohol use and problems. Clin. Psychol. Rev. 2004, 24, 981–1010. [Google Scholar] [CrossRef]
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef]
- Fu, D.; Riordan, S.; Kieran, S.; Andrews, R.A.; Ring, H.Z.; Ring, B.Z. Complex relationship between TAS2R receptor variations, bitterness perception, and alcohol consumption observed in a population of wine drinkers. Food Funct. 2019, 10, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Chartier, K.G.; Karriker-Jaffe, K.J.; Cummings, C.R.; Kendler, K.S. Environmental influences on alcohol use: Informing research on the joint effects of genes and the environment in diverse U.S. populations. Am. J. Addict. 2017, 26, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Bruwer, J.; Buller, C. Consumer behaviour insights, consumption dynamics, and segmentation of the Japanese wine market. J. Int. Consum. Mark. 2012, 24, 338–355. [Google Scholar] [CrossRef]
- Small-Kelly, S. Taste responsiveness and beer behavior. MSC Thesis, Brock University, St. Catharines, ON, Canada, 2018. [Google Scholar]
- Thibodeau, M.; Pickering, G.J. The role of taste in alcohol preference, consumption and risk behavior. Crit. Rev. Food Sci. Nutr. 2019, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.; Filipello, F.; Hinreiner, E.; Webb, A. Evaluation of thresholds and minimum difference concentrations for various constituents of wines. II. Sweetness: The effect of ethyl alcohol, organic acids and tannin. Food Tech. 1955, 9, 138–140. [Google Scholar]
- Wilson, C.W.M.; O’Brien, C.; MacAirt, J.G. The effect of metronidazole on the human taste threshold to alcohol. Br. J. Addict. 1973, 68, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Scinska, A.; Koros, E.; Habrat, B.; Kukwa, A.; Kostowski, W.; Beinkowski, P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol. Depend. 2000, 60, 199–206. [Google Scholar] [CrossRef]
- Mattes, R.D.; DiMeglio, D. Ethanol perception and ingestion. Physiol. Behav. 2001, 72, 217–229. [Google Scholar] [CrossRef]
- Allen, A.L.; McGeary, J.E.; Hayes, J.E. Polymorphisms in TRPV1 and TAS2Rs associate with sensations from sampled ethanol. Alcohol. Clin. Exp. Res. 2014, 38, 2250–2560. [Google Scholar] [CrossRef] [PubMed]
- Nolden, A.A.; Hayes, J.E. Perceptual qualities of ethanol depend on concentration, and variation in these percepts associates with drinking frequency. Chem. Percept. 2015, 8, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Nolden, A.A.; McGeary, J.E.; Hayes, J.E. Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiol. Behav. 2016, 156, 117–127. [Google Scholar] [CrossRef]
- Small-Kelly, S.; Pickering, G. Variation in orosensory responsiveness to alcoholic beverages and their constituents—The role of the thermal taste phenotype. Chem. Percept. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Green, B.G. The sensitivity of the tongue to ethanol. Ann. Acad. N. Y. Sci. 1987, 510, 315–317. [Google Scholar] [CrossRef]
- Green, B.G. Spatial and temporal factors in the perception of ethanol irritation on the tongue. Percept. Psychosphys. 1988, 44, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Heatherbell, D.; Vanhanen, L.; Barnes, M. The effect of ethanol concentration on the temporal perception of viscosity and density and white wine. Am. J. Enol. Vitic. 1998, 49, 306–318. [Google Scholar]
- Nurgel, C.; Pickering, G. Contribution of glycerol, ethanol and sugar to the perception of viscosity and density elicited by model white wines. J. Texture Stud. 2005, 36, 303–323. [Google Scholar] [CrossRef]
- Gawel, R.; Van Sluyter, S.; Waters, E.J. The effects of ethanol and glycerol on the body and other sensory characteristics of Riesling wines. Aus. J. Grape Wine Res. 2007, 13, 38–45. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavor perception. Cell 2015, 161, 24–35. [Google Scholar] [CrossRef]
- Keast, R.S.J.; Breslin, P.A.S. An overview of binary taste-taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef]
- Wilkie, L.M.; Capaldi Phillips, E.D. Heterogeneous binary interactions of taste primaries: Perceptual outcomes, physiology, and future directions. Neurosci. Biobehav. Rev. 2014, 47, 70–86. [Google Scholar] [CrossRef]
- Martin, S.; Pangborn, M. Taste interaction of ethyl alcohol with sweet, salty, sour and bitter compounds. J. Sci. Fd Agric. 1970, 21, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.C.; Goldner, M.C.; Galmarini, M.V. Sourness-sweetness interactions in different media: White wine, ethanol and water. J. Sens. Stud. 2006, 21, 601–611. [Google Scholar] [CrossRef]
- Guirao, M.; Driano, E.J.G.; Evin, D.; Calviño, A. Psychophysical assessments of sourness in citric acid-ethanol mixtures. Pecerpt. Mot. Ski. 2013, 117, 868–880. [Google Scholar] [CrossRef]
- Sowalsky, R.A.; Noble, A. Comparison of the effects of concentration, pH, anion species on astringency and sourness of organic acid. Chem. Senses 1998, 23, 343–349. [Google Scholar] [CrossRef]
- Hoopman, T.; Birch, G.; Serghat, S.; Portmann, M.-O.; Mathlouthi, M. Solute-solvent interactions and the sweet taste of small carbohydrates. Part II: Sweetness intensity and persistence in ethanol-water mixtures. Food Chem. 1993, 46, 147–153. [Google Scholar] [CrossRef]
- Calviño, A.M. Regional Tongue sensitivity for sweetness and pungency of ethanol-aspartame mixtures. Percept. Mot. Ski. 1998, 86, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.G.H.; Arnold, G.M. The phenolics of cider: Bitterness and astringency. J. Sci. Food Agric. 1978, 29, 478–483. [Google Scholar] [CrossRef]
- Hayes, J.E.; Keast, R.S.J. Two decades of supertasting: Where do we stand? Physiol. Behav. 2011, 104, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G.; George, P. “Thermal taste” predicts higher responsiveness to chemical taste and flavour. Chem. Senses 2004, 29, 617–628. [Google Scholar] [CrossRef]
- Bajec, M.R.; Pickering, G.J. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol. Behav. 2008, 95, 581–590. [Google Scholar] [CrossRef]
- Yang, Q.; Hollowood, T.; Hort, J. Phenotypic variation in oronasal perception and the relative effects of PROP and thermal taster status. Food Qual. Prefer. 2014, 38, 83–91. [Google Scholar] [CrossRef]
- Thibodeau, M.; Saliba, A.; Bajec, M.; Pickering, G. Examination and validation of classification schema for determining thermal taste status. Chem. Percept. 2019, 12, 69–89. [Google Scholar] [CrossRef]
- Green, B.G.; Alvarez-Reeves, M.; George, P.; Akirav, C. Chemesthesis and taste: Evidence of independent processing of sensation intensity. Physiol. Behav. 2005, 86, 526–537. [Google Scholar] [CrossRef]
- Bajec, M.R.; Pickering, G.J.; DeCourville, N. Influence of stimulus temperature on orosensory perception and variation with taste phenotype. Chem. Percept. 2012, 5, 243–265. [Google Scholar] [CrossRef]
- Hort, J.; Ford, R.A.; Eldeghaidy, S.; Francis, S.T. Thermal taster status: Evidence of cross-modal integration. Hum. Brain Mapp 2016, 37, 2263–2275. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.J.; Bartolini, J.-A.; Bajec, M.R. Perception of beer flavour associates with thermal taster status. J. Instit. Brew. 2010, 116, 239–244. [Google Scholar] [CrossRef]
- Pickering, G.J.; Moyes, A.; Bajec, M.R.; DeCourville, N. Thermal taster status associates with oral sensations elicited by wine. Aust. J. Grape Wine Res. 2010, 16, 361–367. [Google Scholar] [CrossRef]
- Michon, C.; O’Sullivan, M.; Delahunty, C.; Kerry, J. The investigation of gender-related sensitivity differences in food perception. J. Sens. Stud. 2009, 24, 922–937. [Google Scholar] [CrossRef]
- Mitchell, J.; Castura, J.C.; Thibodeau, M.; Pickering, G. Application of TCATA to examine variation in beer perception due to thermal taste status. Food Qual. Prefer. 2019, 73, 135–142. [Google Scholar] [CrossRef]
- Settle, R.; Meehan, K.; Williams, G.; Doty, R.; Sisley, A. Chemosensory properties of sour tastants. Physiol. Behav. 1986, 32, 619–623. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Booth, B.J.; Losee, M.L.; Pecore, S.D.; Warwick, Z.S. Bitterness of sweetners as a function of concentration. Brain Res. Bull. 1995, 36, 505–513. [Google Scholar] [CrossRef]
- Keast, R.S.J.; Roper, J. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem. Senses 2007, 32, 245–253. [Google Scholar] [CrossRef]
- Low, J.Y.; McBride, R.L.; Lacy, K.E.; Keast, R.S. Psychophysical evaluation of sweetness functions across multiple sweetners. Chem. Senses 2017, 42, 111–120. [Google Scholar] [CrossRef]
- Ickes, C.M.; Cadwallader, K.R. Effects of ethanol on flavour perception in alcoholic beverages. Chem. Percept. 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Lachemeir, D.W.; Kanteres, F.; Rehm, J. Alcoholic beverage strength discrimination by taste may have an upper threshold. Alcohol. Clin. Exp. Res. 2014, 38, 2460–2467. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.-W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef]
- Prescott, J.; Swain-Campbell, N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem. Senses 2000, 25, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 9783319242774. [Google Scholar]
- Auguie, B. Miscellaneous Functions for “Grid” Graphics. 2017. Available online: https://cran.r-project.org/web/packages/gridExtra/index.html (accessed on 20 April 2021).
- Hayes, J.E.; Allen, A.L.; Bennett, S.M. Direct comparison of the generalized visual analog scale (gVAS) and general labeled magnitude scale (gLMS). Food Qual. Prefer. 2013, 28, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dorado, R.; Chaya, C.; Hort, J. The impact of PROP and thermal taster status on the emotional response to beer. Food Qual. Prefer. 2018, 68, 420–430. [Google Scholar] [CrossRef]
- Sühnel, J. Evaluation of interaction in olfactory and taste mixtures. Chem. Senses 1993, 18, 131–149. [Google Scholar] [CrossRef]
- Fleming, E.E.; Ziegler, G.R.; Hayes, J.E. Investigating mixture interactions of astringent stimuli using the isobole approach. Chem. Senses 2016, 41, 601–610. [Google Scholar] [CrossRef]
- Wang, G.; Hayes, J.E.; Ziegler, G.R.; Roberts, R.F.; Hopfer, H. Dose-response relationships for vanilla flavor and sucrose in skim milk: Evidence of synergy. Beverages 2018, 4, 73. [Google Scholar] [CrossRef]
- Peleg, H.; Bodine, K.K.; Noble, A.C. The influence of acid on astringency of alum and phenolic compounds. Chem. Senses 1998, 23, 371–378. [Google Scholar] [CrossRef]
- Schifferstein, H.N.J.; Frijters, J.E.R. Contextual and sequential effects on judgments of sweetness intensity. Percept. Psychosphys. 1992, 52, 243–255. [Google Scholar] [CrossRef]
- Ferris, S.; Kempton, R.; Muir, D. Carryover in sensory trials. Food Qual. Prefer. 2003, 14, 299–304. [Google Scholar] [CrossRef]
- Clark, R.A.; Hewson, L.; Bealin-Kelly, F.; Hort, J. The interactions of CO2, ethanol, hop acids and sweetener on flavour perception in a model beer. Chem. Percept. 2011, 4, 42–54. [Google Scholar] [CrossRef]
- Jones, P.R.; Gawel, R.; Francis, I.L.; Waters, E.J. The influence of interactions between major white wine components on the aroma flavour and texture of model white wine. Food Qual. Prefer. 2008, 19, 596–607. [Google Scholar] [CrossRef]
- Cretin, B.N.; Dubourdieu, D.; Marchal, A. Influence of ethanol content on sweetness and bitterness perception in dry wine. LWT 2018, 87, 61–66. [Google Scholar] [CrossRef]
- Poveromo, A.R.; Hopfer, H. Temporal check-all-that-apply (TCATA) reveals matrix interaction effects on flavor perception in a model wine matrix. Foods 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Harwood, W.S.; Parker, M.N.; Drake, M. Influence of ethanol concentration on sensory perception of rums using check-all-that-apply. J. Sens. Stud. 2020, 35, e12546. [Google Scholar] [CrossRef]
- Fischer, U.; Noble, A.C. The effect of ethanol, catechin concentration, and pH on sourness and bitterness of wine. Am. J. Enol. Vitic. 1994, 45, 6–10. [Google Scholar]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwitatkowski, M.; Gawel, R.; Williams, P.; Waters, E.; Cheynier, V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual. Prefer. 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Fontoin, H.; Saucier, C.; Teissedre, P.-L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Villamor, R.R.; Evans, M.A.; Ross, C.F. Effects of ethanol, tannin, and fructose concentrations on sensory properties of model red wines. Am. J. Enol. Vitic. 2013, 64, 342–348. [Google Scholar] [CrossRef]
- Gawel, R.; Van Sluyter, S.C.; Smith, P.A.; Waters, E.J. Effect of pH and alcohol on perception of phenolic character in white wine. Am. J. Enol. Vitic. 2013, 64, 425–429. [Google Scholar] [CrossRef]
- Frost, S.C.; Harbertson, J.F.; Heymann, H. A full factorial study on the effect of tannins, acidity, and ethanol on the temporal perception of taste and mouthfeel in red wine. Food Qual. Prefer. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Nurgel, C.; Pickering, G. Modeling of sweet, bitter and irritant sensations and their interactions elicited by model ice wines. J. Sens. Stud. 2006, 21, 505–519. [Google Scholar] [CrossRef]
- Demiglio, P.; Pickering, G.J. The influence of ethanol and pH on the taste and mouthfeel sensations elicited by red wine. J. Food Agric. Environ. 2008, 6, 143–150. [Google Scholar] [CrossRef]
- Thibodeau, M. Alcohol Consumption and Its Association with Thermal Taste Status and Oral Sensations. Ph.D. Thesis, Brock University, St. Catharines, ON, Canada, 2015. [Google Scholar]
- Thibodeau, M.; Bajec, M.; Pickering, G. Orosensory responsiveness and alcohol behaviour. Physiol. Behav. 2017, 177, 91–98. [Google Scholar] [CrossRef]
- Betancur, M.I.; Motoki, K.; Spence, C.; Velasco, C. Factors influencing the choice of beer: A review. Food Res. Int. 2020, 137, 109367. [Google Scholar] [CrossRef]
- Mojet, J.; Christ-Hazelhof, E.; Heifema, J. Taste perception with age: Pleasantness and its relationship with threshold sensitivity and supra-threshold intensity of five taste qualities. Food Qual. Prefer. 2005, 16, 413–423. [Google Scholar] [CrossRef]
- Pickering, G.J.; Kvas, R. Thermal Tasting and Difference Thresholds for Prototypical Tastes in Wine. Chem. Percept. 2016, 9, 37–46. [Google Scholar] [CrossRef]
- Yang, S.; Harlow, L.L.; Puggioni, G.; Redding, C.A. A comparison of different methods of zero-inflated data analysis and an application in health surveys. J. Mod. Appl. Stat. Methods 2017, 16, 518–543. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE Publications Ltd.: London, UK, 2013. [Google Scholar]
- Bartoshuk, L.M.; Conner, E.; Grubin, D.; Karrer, T.; Kochenbach, K.; Palesco, M.; Snow, D.; Pelchat, M.; Danowski, S. PROP supertasters and the perception of ethyl alcohol. Chem. Senses 1993, 18, 526–527. [Google Scholar] [CrossRef]
- Duffy, V.B.; Peterson, J.M.; Bartoshuk, L.M. Associations between taste genetics, oral sensations and alcohol intake. Physiol. Behav. 2004, 82, 435–445. [Google Scholar] [CrossRef]
- Thibodeau, M.; Bajec, M.; Saliba, A.; Pickering, G. Homogeneity of thermal tasters and implications for mechanisms and classification. Physiol. Behav. 2020, 227, 113160. [Google Scholar] [CrossRef] [PubMed]



| Stimulus | Orosensation(s) Elicited | Units | Concentration | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Fructose | Sweet | mM | 140 | 280 | 960 |
| Quinine | Bitter | mM | 0.025 | 0.040 | 0.100 |
| Tartaric acid | Sour (primary), astringent | mM | 2.75 | 6.91 | 17.4 |
| Alum | Astringent (primary), sour | mM | 0.73 | 2.05 | 5.43 |
| Ethanol | Sweet, bitter, astringent, burning 1 | %(v/v) | 5 | 13 | 23 |
| Effect Size (η2p) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimuli | Ethanol | Fructose | Quinine | Tartaric acid | Aluminium sulphate | |||||
| Orosensation | Bitter | Burning/ Tingling | Sweet | Astringent | Sweet | Bitter | Sour | Astringent | Astringent | Sour |
| Factor in ANOVA | ||||||||||
| Thermal taste status (TTS) | 0.04 # | 0.04 # | 0.18 * | 0.22 * | 0.14 * | <0.01 | 0.02 | 0.01 | 0.01 | 0.12 * |
| Stimulus concentration (Conc) | 0.20 * | 0.70 * | 0.03 | 0.06 # | 0.55 * | 0.07 * | 0.26 * | 0.04 | 0.24 * | 0.19 * |
| TTS*Conc | <0.01 | 0.01 | 0.02 | <0.01 | <0.01 | < 0.01 | <0.01 | <0.01 | <0.01 | 0.08 * |
| Ethanol % (v/v) | Quinine (mM) | Mean log(Bitter) in Binary Mixture | Actual log(Ethanol) Concentration (cEtOH) | log(Ethanol) to Achieve the Same log(Bitterness) as in Mixture (CEtOH) | Actual log(1 + Quinine) Concentration (cQuinine) | Concentration of log(1 + Quinine) to Achieve the Same Bitterness as in Mixture (CQuinine) | Index of Interaction (I) | Nature of Interaction |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.025 | 0.635 | 0.699 | 1.141 | 0.011 | 0.014 | 1.41 | Suppression |
| 5 | 0.040 | 0.727 | 0.699 | 1.310 | 0.017 | 0.022 | 1.31 | Suppression |
| 5 | 0.100 | 1.065 | 0.699 | 1.934 | 0.041 | 0.053 | 1.14 | Suppression |
| 13 | 0.025 | 0.715 | 1.114 | 1.289 | 0.011 | 0.021 | 1.38 | Suppression |
| 13 | 0.040 | 0.740 | 1.114 | 1.334 | 0.017 | 0.023 | 1.57 | Suppression |
| 13 | 0.100 | 0.879 | 1.114 | 1.590 | 0.041 | 0.036 | 1.84 | Suppression |
| 23 | 0.025 | 0.530 | 1.362 | 0.947 | 0.011 | 0.004 | 4.24 | Suppression |
| 23 | 0.040 | 0.753 | 1.362 | 1.357 | 0.017 | 0.024 | 1.70 | Suppression |
| 23 | 0.100 | 0.625 | 1.362 | 1.122 | 0.041 | 0.013 | 4.50 | Suppression |
| Ethanol % (v/v) | Alum Sulphate (mM) | Mean log(Astringency) in Binary Mixture | Actual log(Ethanol) (cEtOH) | log(Ethanol) to Obtain the Mean log(Astringency) in Binary Mixture (CEtOH) | Actual log(Aluminium Sulphate) (calum) | log(Aluminium Sulphate) to Obtain the Mean log(Astringency) in Binary Mixture (CAlum) | Index of Interaction (I) | Nature of Interaction |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.73 | 0.450 | 0.699 | 1.920 | −0.137 | −0.042 | 3.65 | Suppression |
| 5 | 2.05 | 0.691 | 0.699 | 2.907 | 0.312 | 0.265 | 1.42 | Suppression |
| 5 | 5.43 | 0.827 | 0.699 | 3.463 | 0.735 | 0.437 | 1.88 | Suppression |
| 13 | 0.73 | 0.415 | 1.114 | 1.774 | −0.137 | −0.087 | 2.20 | Suppression |
| 13 | 2.05 | 0.864 | 1.114 | 3.614 | 0.312 | 0.484 | 0.95 | Additive |
| 13 | 5.43 | 1.112 | 1.114 | 4.631 | 0.735 | 0.799 | 1.16 | Suppression |
| 23 | 0.73 | 0.336 | 1.362 | 1.450 | −0.137 | −0.187 | 1.67 | Suppression |
| 23 | 2.05 | 0.675 | 1.362 | 2.842 | 0.312 | 0.245 | 1.75 | Suppression |
| 23 | 5.43 | 0.879 | 1.362 | 3.675 | 0.735 | 0.503 | 1.83 | Suppression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibodeau, M.; Pickering, G. Perception of Aqueous Ethanol Binary Mixtures Containing Alcohol-Relevant Taste and Chemesthetic Stimuli. Beverages 2021, 7, 23. https://doi.org/10.3390/beverages7020023
Thibodeau M, Pickering G. Perception of Aqueous Ethanol Binary Mixtures Containing Alcohol-Relevant Taste and Chemesthetic Stimuli. Beverages. 2021; 7(2):23. https://doi.org/10.3390/beverages7020023
Chicago/Turabian StyleThibodeau, Margaret, and Gary Pickering. 2021. "Perception of Aqueous Ethanol Binary Mixtures Containing Alcohol-Relevant Taste and Chemesthetic Stimuli" Beverages 7, no. 2: 23. https://doi.org/10.3390/beverages7020023
APA StyleThibodeau, M., & Pickering, G. (2021). Perception of Aqueous Ethanol Binary Mixtures Containing Alcohol-Relevant Taste and Chemesthetic Stimuli. Beverages, 7(2), 23. https://doi.org/10.3390/beverages7020023







