Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook
Abstract
1. Introduction
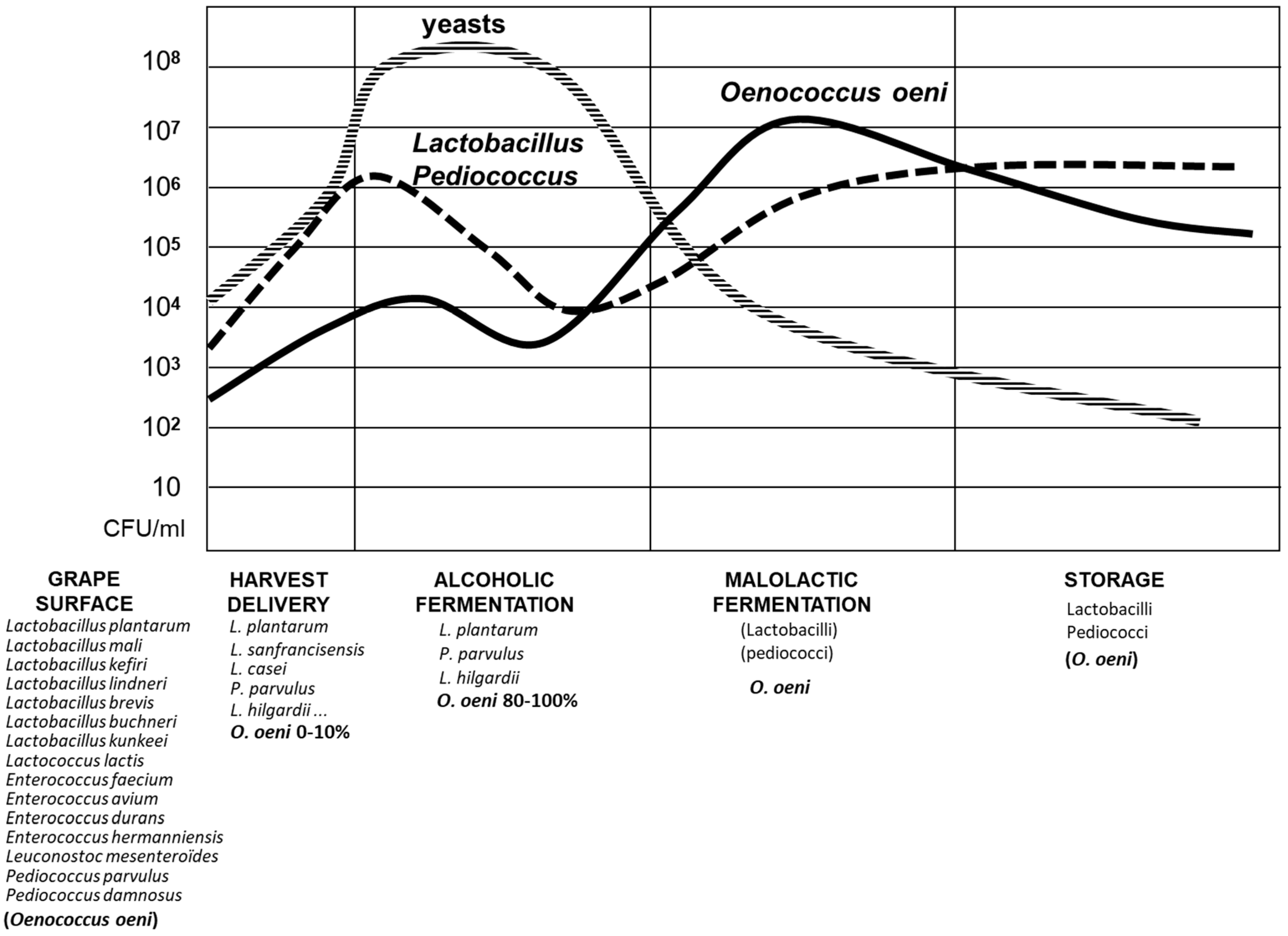
2. Biodiversity of Lactic Acid Bacteria in Wine
3. Selected Wine Lactic Acid Bacteria Starter Cultures and Wine Challenging Factors
3.1. Well-Known Factors that Affect Malolactic Fermentation and Bacteria Vitality
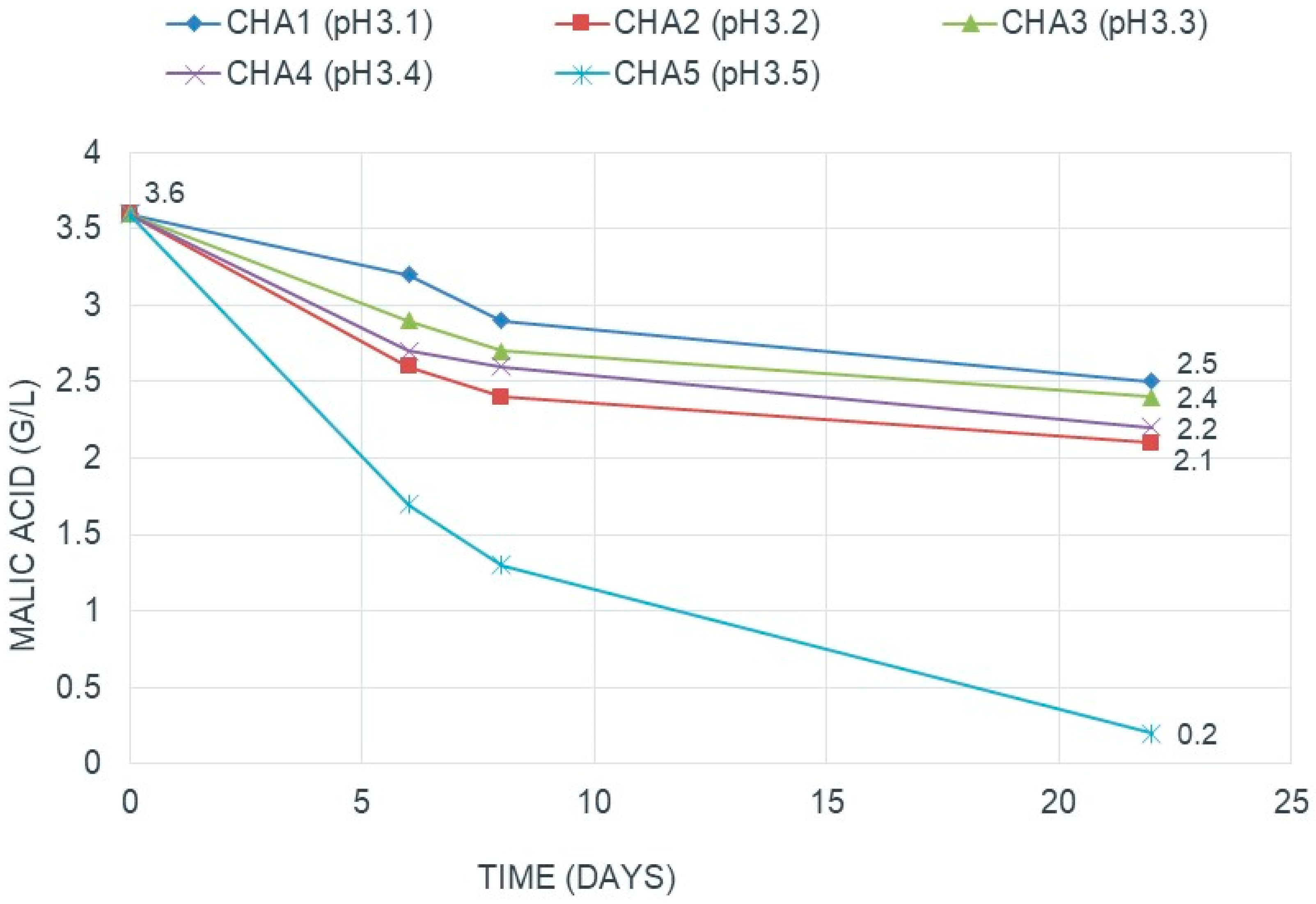
3.1.1. pH
3.1.2. Ethanol
3.1.3. Temperature
3.1.4. Sulphur Doxide
3.2. Lesser-Known Factors that Affect Malolactic Fermentation
3.2.1. Yeast Strain Selection
3.2.2. Organic Acids
3.2.3. Tannins
3.2.4. Nutrient Deficiencies
3.2.5. Residual Lysozyme Activity and Chitosan Formulation
3.2.6. Others
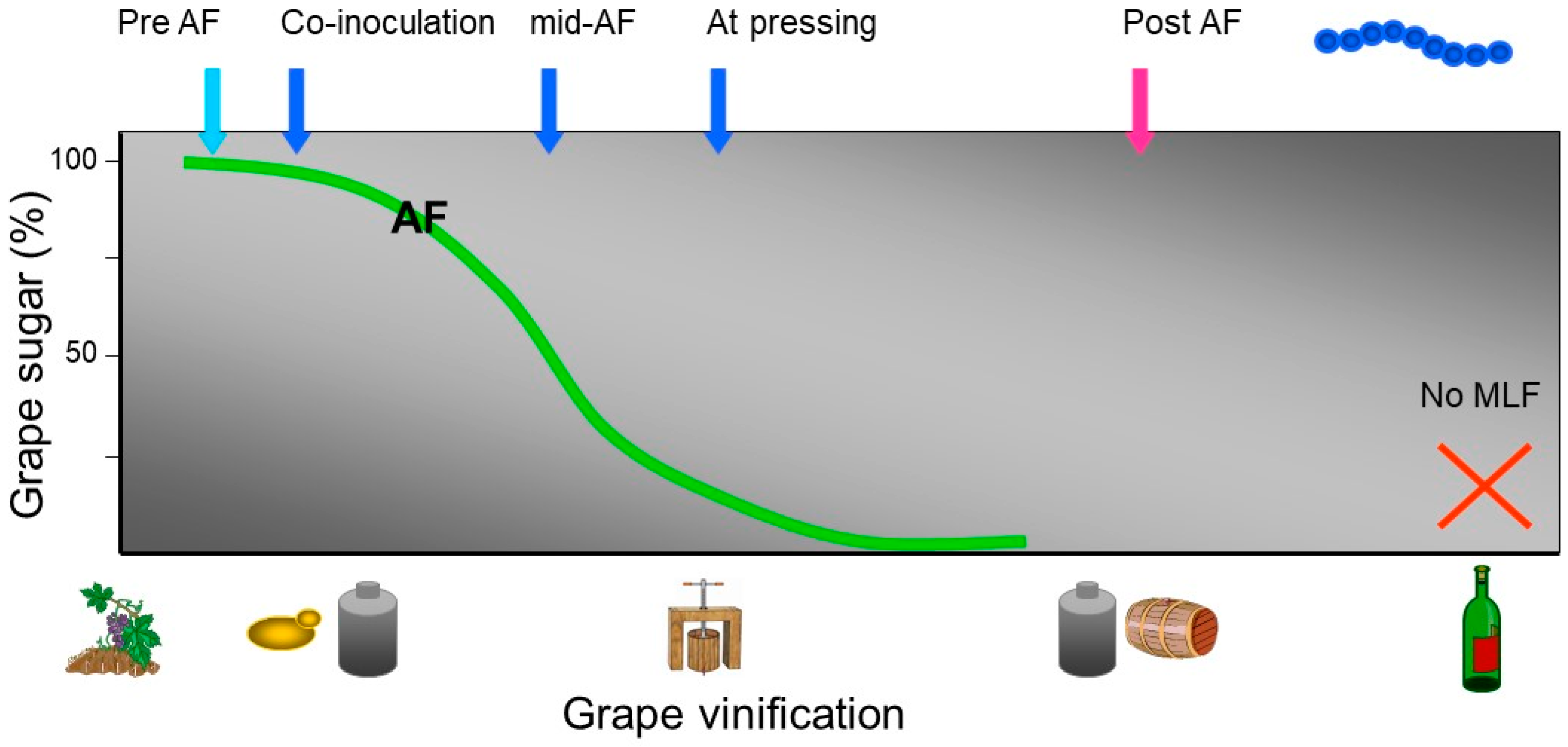
4. Selected Wine Lactic Acid Bacteria Starter Cultures and the Timing of Inoculation
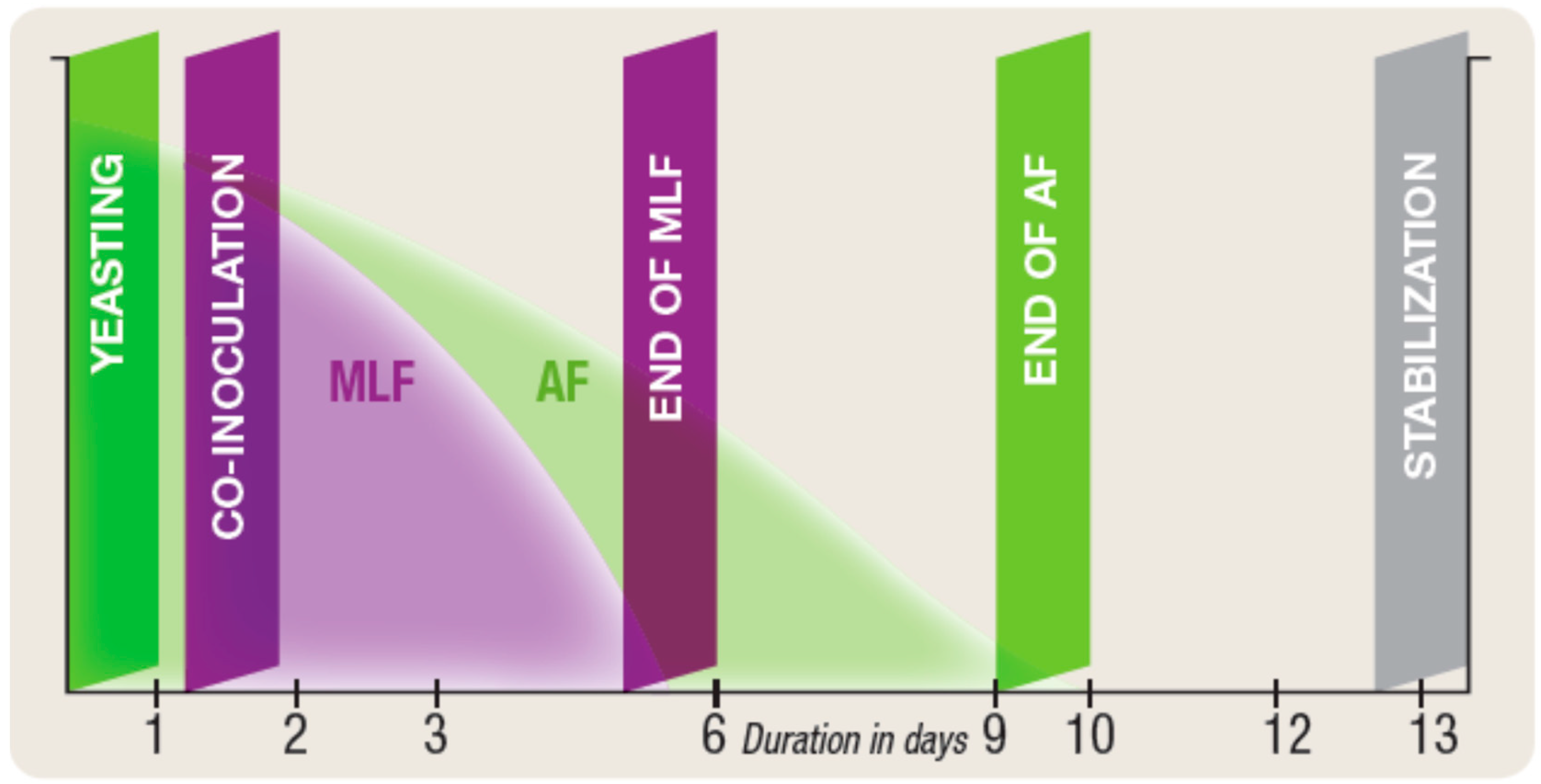
4.1. Co-inoculation with Selected Yeast and Selected Wine Lactic Acid Bacteria
- MLF can be completed in between 3 days and 2 weeks depending on the type of musts and the bacteria used.
- Co-inoculation to produce fresh wine styles with low diacetyl content:Co-inoculation always result in more fruit-driven wine styles and very low diacetyl content in wines. Early results also show that in the case of co-inoculation the high content of sugars could repress the metabolism of the diacetyl, as opposed to post-alcoholic fermentation inoculation. Moreover, under the reductive conditions generated by the active yeast, diacetyl produced will be immediately reduced to the less active metabolites, acetoin and butanediol.
- Co-inoculation to limit the development of Brettanomyces and off-flavors:The increase in sugar levels, pH, and sometimes lower SO2 addition can influence the development of spoilage microorganisms, especially Brettanomyces, which can produce phenolic off-odors in wines. It is well known that the period from the end of AF to the start of MLF is particularly conducive to the development of Brettanomyces. Early inoculation with wine bacteria, either right after AF or in co-inoculation (24 h after inoculation with yeast), has proven to be a simple and effective method for preventing the development of Brettanomyces and the production of ethyl phenols off-flavors. Recent studies with IFV in Burgundy (Gerbaux) show co-inoculation with selected O. oeni strains can inhibit the growth of Brettanomyces (below 10 cell/mL) as opposed to the spontaneous control that is still contaminated with 500 cell/mL of Brettanomyces while the MLF is not completed and the wine is not stabilized [65].
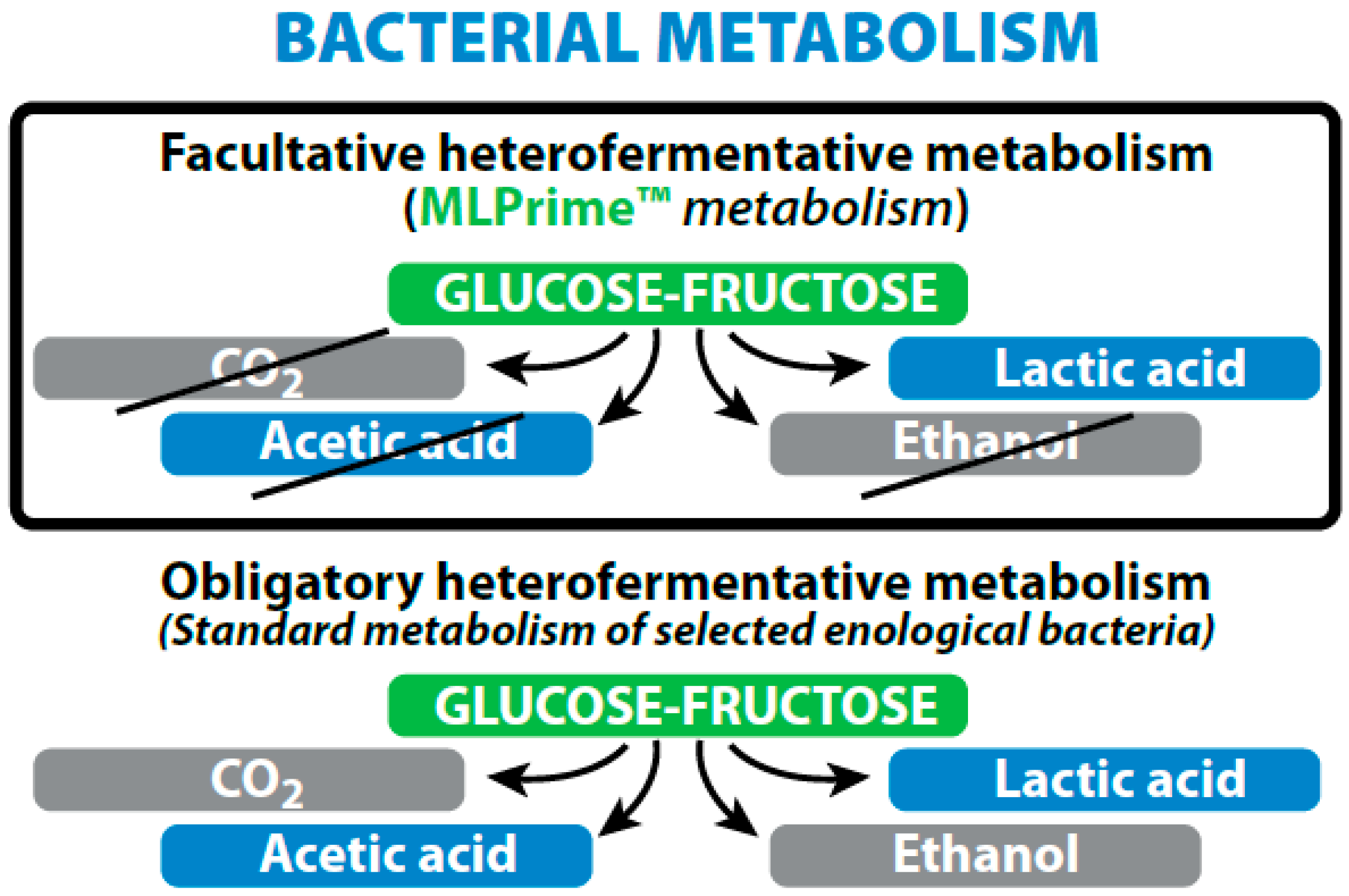
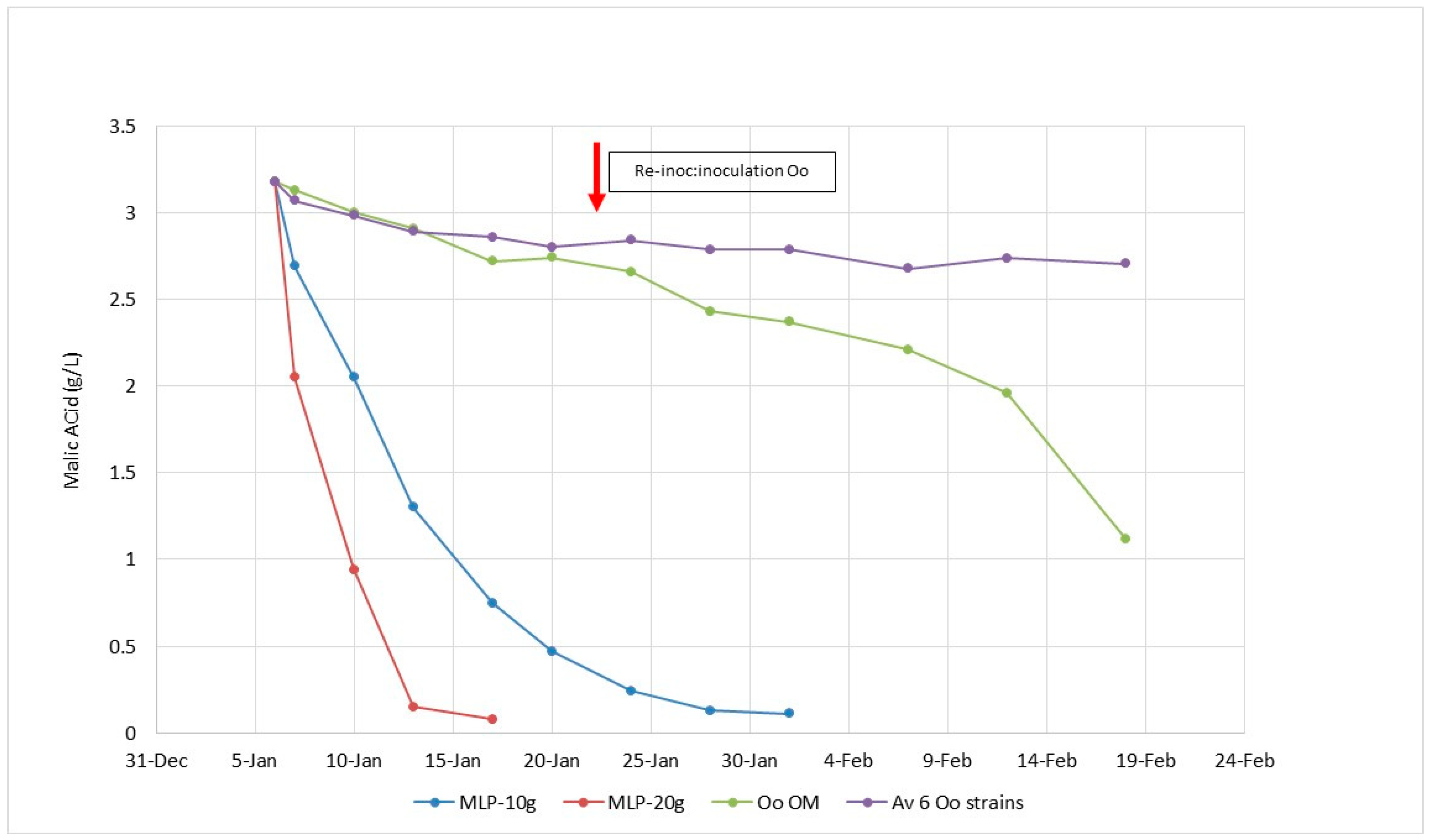
- As a bio-control agent for low acidity/high pH wines, Lactobacillus plantarum with its facultative hetero-fermentative sugar metabolism is ideal as it completes MLF in 3–5 days during the alcoholic fermentation with no risk of increased volatile acidity due to its specific metabolism. It enables early stabilization of wines, as soon as the AF is finished.
- Co-inoculation as a tool for sustainability. In the frame of National Spanish R&D Project (VINySOST) involving six wineries, two companies of auxiliary industry, and several research centers (New strategies vine and winemaking for sustainable management in the production in great surfaces and increase in competitiveness of wineries in the international market—CDTI (strategic program CIEN, call 2014)), one of the studies were focused on the carbon footprint and analysis of life cycle from different axes involving wine producers. Within the study of carbon footprint and energy cost related to malolactic fermentation, co-inoculation with selected wine LAB had been compared to spontaneous MLF. Co-inoculated wine finished MLF with four after termination of the alcoholic fermentation whereas the spontaneous MLF took more than one and a half months to finish MLF. An electrical network analyzer was used after the energy consumption. Co-inoculation reduces the electricity consumption by more than 60%, as there was no need to heat the tanks to achieve a malolactic fermentation.
4.2. Sequential Inoculation with Selected Wine Lactic Acid Bacteria Post-alcoholic Fermentation
5. Advantages of Lactobacillus plantarum Starter Cultures
5.1. Lactobacillus plantarum Starter Cultures for the Induction of Malolactic Fermentation in Must and Wine
5.2. Specific Feature of Lactobacillus plantarum of Oenological Interest
5.3. Mixed Oenococcus oeni and Lactobacillus plantarum Starter Cultures for the Induction of MLF
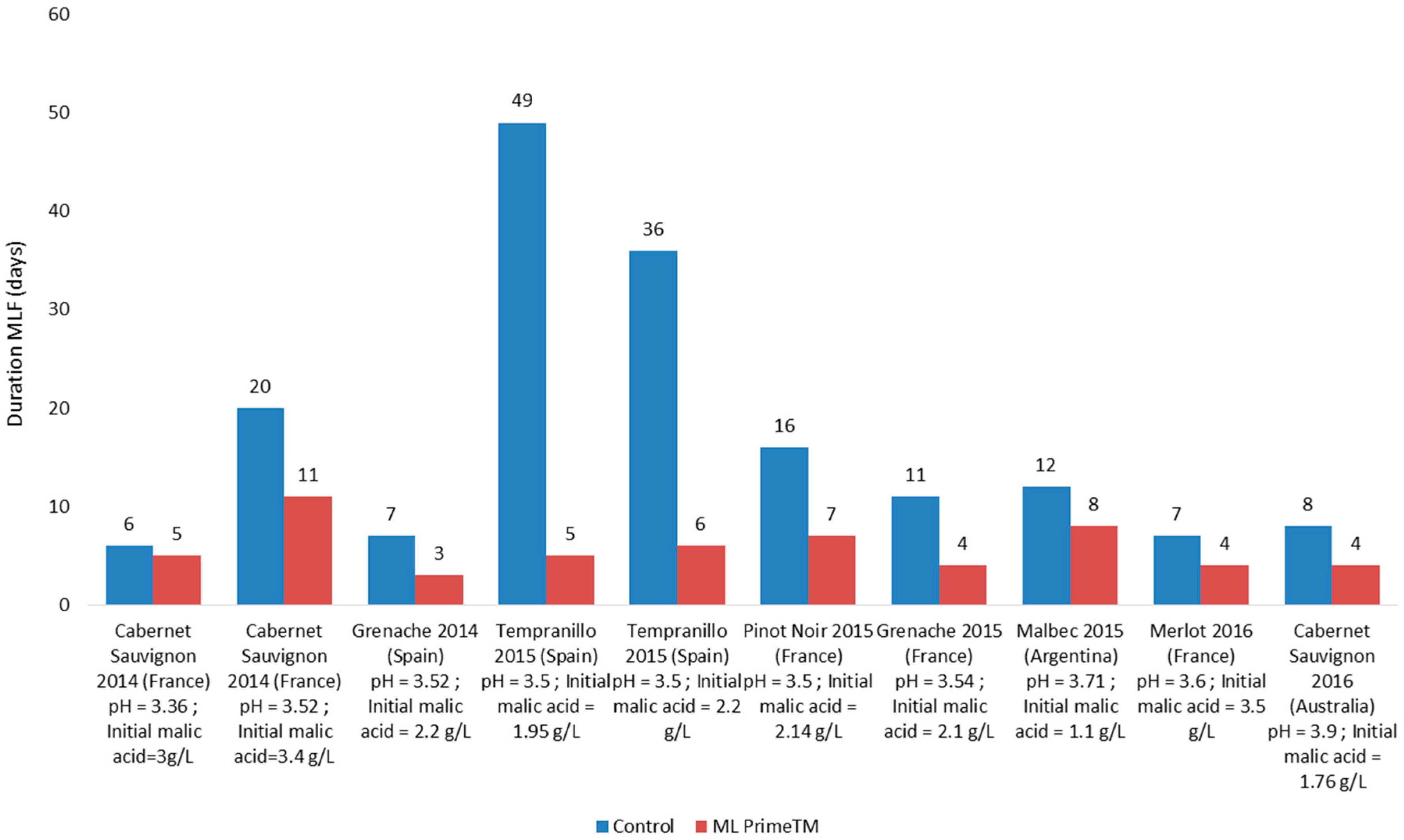
5.4. A New Concept of Lactobacillus plantarum Starter Cultures for High pH Red Wines.
5.4.1. Control of Microbial Contamination
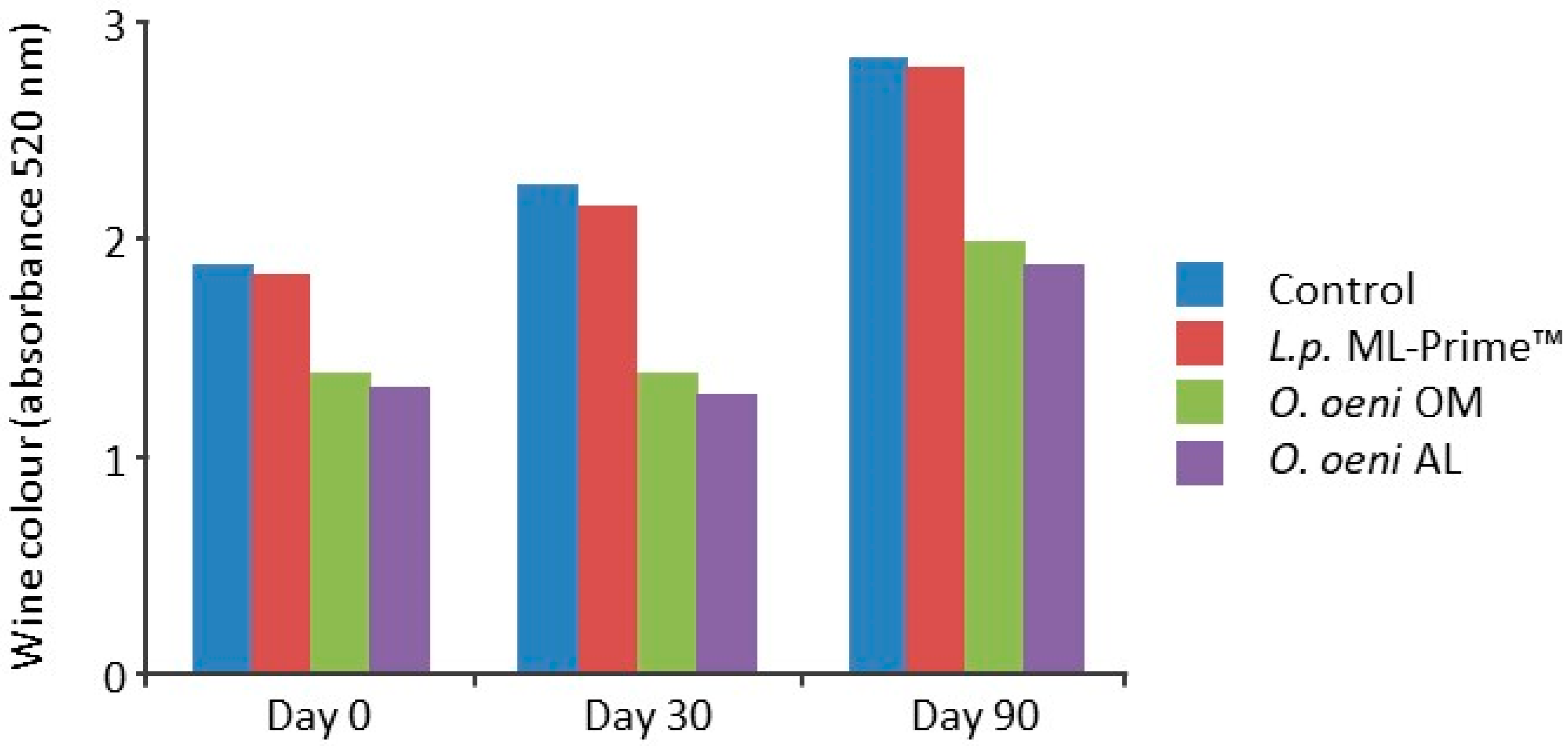
5.4.2. Malolactic Fermentation and Red Wine (Pinot Noir) Color
5.5. A New Concept of Lactobacillus plantarum Starter Cultures for Low pH White Wines
- pH: ≥3.05
- Malic acid content: ≤8 g/L
- Temperature range: from 17 °C to 22 °C
- Total SO2 tolerance in must up to 5 g/hL
- Free SO2 tolerance in wines: less than 10 mg/L
5.6. Interesting Sensory Properties of Lactobacillus plantarum in Wine Application
5.7. Other Applications of Lactobacillus plantarum Apart from the Induction of Malolactic Fermentation
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures-an overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Radler, F. Die mikrobiologischen Grundlagen des Säureabbaus in Wein. Zentralbl Bakteriol Parasitenkd 1966, 120, 237–287. [Google Scholar]
- Peynaud, E.; Domercq, S. Étude de quatre cents souches de coques hétérolactiques isolées de vins. Ann. Inst. Pasteur 1968, 19, 159–170. [Google Scholar]
- Henick-Kling, T. Growth and Metabolism of Leuconostoc oenos and Lactobacillus plantarum in Wine. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 1986. [Google Scholar]
- Davis, C.R.; Wibowo, D.; Fleet, G.H.; Lee, T.H. Properties of wine lactic acid bacteria: Their potential enological significance. Am. J. Enol. Vitic. 1988, 39, 137–142. [Google Scholar]
- Henick-Kling, T. Malolactic Fermentation. In Wine Microbiology and Biotechnology, 1st ed.; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 289–326. [Google Scholar]
- Prahl, C. Method of Inducing the Decarboxylation of Malic Acid in Must or Fruit Juice. European Patent International Application Number PCT/DK89/00009, 24 January 1989. [Google Scholar]
- Prahl, C. La décarboxylation de l’acide L-malique dans le moût par l’ensemencement de lactobacilles homofermentaires. Revue des Œnologues 1989, 54, 13–17. [Google Scholar]
- Bou, M.; Krieger, S. Alcohol-Tolerant Malolactic Strains for the Maturation of Wines with Average or High pH. U.S. Patent 7,625,745, 9 June 2004. [Google Scholar]
- Fumi, M.D.; Krieger-Weber, S.; Déléris-Bou, M.; Silva, A.; du Toit, M. A New Generation of Malolactic Starter Cultures for High pH Wines. WB3 Microorganisms—Malolactic-Fermentation. In Proceedings of the International IVIF Congress, Düsseldorf, Germany, 21–23 March 2010. [Google Scholar]
- Soerensen, K.; Kibenich, A.; Johansen, E. Lactobacillis planaturm Ceels with Improved Resistance to High Concentrations of Ethanol. International Patent Application Number PCT/EP2012/061296, 14 June 2012. [Google Scholar]
- Krieger-Weber, S.; Silvano, A.; Déléris-Bou, M.; Vidal, F.; Dumont, A. Neues Konzept für die Mikroflora. Das Deutsche Weinmagazin 2017, 19, 30–32. [Google Scholar]
- Lerm, E. The Selection and Characterization of Lactic Acid Bacteria to be Used as a Mixed Starter Culture for Malolactic Fermentation. Master’s Thesis, Agricultural Science, Stellenbosch University, Stellenbosch, South Africa, 2010. [Google Scholar]
- Bae, S.; Fleet, G.H.; Heards, G.M. Lactic acid bacteria associated with wine grapes from several Australian vineyards. J. Appl. Microbiol. 2006, 100, 712–727. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1. In The Microbiology of Wine and Vinifications; Ribéreau-Gayon, P., Ed.; Wiley: Chichester, UK, 2006; pp. 149–151. [Google Scholar]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology: Practical Applications and Procedures; Fugelsang, K.C., Edwards, C.G., Eds.; Springer: New York, NY, USA, 1997. [Google Scholar]
- Jackson, R.S. Origin and Growth of Lactic Acid Bacteria. In Wine Science: Principles and Applications; Jackson, R.S., Ed.; Academic Press: Cambridge, MA, USA, 2008; p. 394. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Boulton, R.B., Ed.; Chapman and Hall Publishers: New York, NY, USA, 1996. [Google Scholar]
- Dicks, L.M.T.; Endo, A. Taxonomic status of lactic acid bacteria in wine and key characteristics to differentiate species. S. Afr. J. Enol. Vitic. 2009, 30, 72–90. [Google Scholar] [CrossRef][Green Version]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological properties of Lactobacillus plantarum isolated from grape must fermentations. Food Microbiol. 2016, 57, 187–194. [Google Scholar] [CrossRef]
- Valdés La Hens, D.; Bravo-Ferrada, B.M.; Delfederico, L.; Caballero, A.C.; Semorile, L.C. Prevalence of Lactobacillus plantarum and Oenococcus oeni during spontaneous malolactic fermentation in Patagonian wines revealed by polymerase chain reaction-denaturing gradient gel electrophoresis with two targeted genes. Aust. J. Grape Wine Res. 2015, 21, 49–56. [Google Scholar] [CrossRef]
- López-Seijas, J.; García-Fraga, B.; Da Silva, A.F.; Zas-García, X.; Lois, L.C.; Gago-Martínes, A.; Leão-Martins, J.M.; Sieiro, C. Evaluation of malolactic bacteria associated with wines from Albariño variety as potential; starter cultures for quality and safety. Foods 2020, 9, 99. [Google Scholar] [CrossRef]
- Fleet, G.H.; Lafon-Lafourcade, S.; Ribereau-Gayon, P. Evolution of yeasts and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Lonvaud-Funel, A. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Van Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Bartowky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Juega, M.; Costantini, A.; Bonello, F.; Cravero, M.C.; Martinez-Rodriguez, A.J.; Carrascosa, A.V.; Garcia-Moruno, E. Effect of malolactic fermentation by Pediococcus damnosus on the composition and sensory profile of Albariño and Caiño white wines. J. Appl. Microbiol. 2013, 116, 586–595. [Google Scholar] [CrossRef]
- Henick-Kling, T. Yeast and Bacteria Control in Winemaking, Modern Methods of Plant Analysis; Linskens, R.S., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 6, pp. 276–315. [Google Scholar]
- Wibowo, D.; Eschenbruch, R.; Davis, D.R.; Fleet, G.H.; Lee, T.H. Occurrence and growth of lactic acid bacteria in wine: A review. Am. J. Enol. Vitic. 1985, 36, 302–313. [Google Scholar]
- Liu, Y.; Forcisi, S.; Harir, M.; Deleris-Bou, M.; Krieger-Weber, S.; Lucio, M.; Longin, C.; Degueurce, C.; Gougeon, R.D.; Schmitt-Kopplin, P.; et al. New molecular evidence of wine yeast-bacteria interaction unraveled by non-targeted exometabolomic profiling. Metabolomics 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Dittrich, H.H.; Grossmann, M. Der Mirobielle Säureabbau. In Mikrobiologie des Weines, Handbuch der Getränketechnologie, 3rd ed.; Dittrich, H.H., Grossmann, M., Eds.; Ulmer Verlag: Stuttgart, Germany, 2005; pp. 151–169. [Google Scholar]
- Amerine, M.A.; Berg, H.W.; Kunkee, R.E.; Ough, C.S.; Singleton, V.L.; Webb, A.D. The Composition of Grapes and Wines. In The Technology of Wine Making, 4th ed.; Amerine, M.A., Berg, H.W., Eds.; Avi Publishing Company: Westport, CT, USA, 1980. [Google Scholar]
- Nicolas, D. Caractérisation Physiologique et Moléculaire de Préparations Malolactiques de Oenococcus oeni Destinées à L’ensemencement Direct des Vins. Ph.D. Thesis, University of Dijon, Dijon, France, 2005. [Google Scholar]
- Lonvaud-Funel, A. Microbiology of the malolactic fermentation: Molecular aspects. FEMS Microbiol. Lett. 1995, 126, 209–214. [Google Scholar] [CrossRef]
- Cox, D.J.; Henick-Kling, T. Chemiosmotic energy from malolactic fermentation. J. Bacteriol. 1989, 171, 5750–5752. [Google Scholar] [CrossRef]
- Guerzoni, M.E.; Sinigaglia, M.; Gardini, F.; Ferruzzi, M.; Torriani, S. Effects of pH, temperature, ethanol, and malate concentration on Lactobacillus plantarum and Leuconostoc oenos: Modelling of the malolactic activity. Am. J. Enol. Vitic. 1995, 3, 368–374. [Google Scholar]
- Britz, T.J.; Tracey, R.P. The combination effect of pH, SO2, ethanol and temperature on the growth of Leuconostoc oenos. J Appl. Bacteriol. 1990, 68, 23–31. [Google Scholar] [CrossRef]
- Lafon-Lafourcade, S. Chapter III: Lactic Acid Bacteria and the Malolactic Fermentation. In Wine and Brandy in Biotechnology; Rehm, H.J., Redd, G., Eds.; Verlag Chemie: Weinheim, Germany, 1983; pp. 81–163. [Google Scholar]
- Chang, I.S.; Kim, B.H.; Shin, P.K. Use of sulfite and hydrogen peroxide to control bacterial contamination in ethanol fermentation. Appl. Environ. Microbiol. 1997, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Massera, A.; Soria, A.; Catania, A.; Krieger, S.; Combina, M. Simultaneous Inoculation of Malbec (Vitis vinifera) Musts with Yeast and Bacteria: Effects on Fermentation Performance, Sensory and Sanitary Attributes of Wines. Food Technol. Biotechnol. 2009, 47, 192–201. [Google Scholar]
- Abrahamse, C.; Bartowsky, E. Inoculation for MLF reduces overall vinification time. Aust. N. Z. Grapegrow. Winemak. 2012, 577, 41–46. [Google Scholar]
- Azzolini, M.; Tosi, E.; Vagnoli, P.; Krieger, S.; Zapparoli, G. Evaluation of technological effects of yeast-bacterial co-inoculation in red table wine production. Ital. J. Food Sci. 2010, 3, 257–263. [Google Scholar]
- Jussier, D.; Dubé Morneau, A.; Mira de Orduña, R. Effect of simultaneous inoculation with yeast and bacteria on fermentation kinetics and key wine parameters of cool-climate Chardonnay. Appl. Environ. Microbiol. 2006, 72, 221–227. [Google Scholar] [CrossRef]
- Renouf, V.; La Guerche, S.; Moine, V.; Murat, M.L.; Bowyer, P.K. Malolactic fermentability of wines: The role of octanoic and decanoic acid. Aust. N. Z. Grapegrow. Winemak. 2010, 560, 93–98. [Google Scholar]
- Osborne, J.P.; Charles, G.E. Inhibition of malolactic fermentation by a peptide produced by Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2007, 118, 27–34. [Google Scholar] [CrossRef]
- Nehme, N.; Mathieu, F.; Taillandier, P. Impact of the co-culture of Saccharomyces cerevisiae—Oenococcus oeni on malolactic fermentation and partial characterization of a yeast-derived inhibitory peptidic fraction. Food Microbiol. 2010, 27, 150–157. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Interactions between Lactic Acid Bacteria of Wine and Phenolic Compounds. In Nutritional Aspects II, Synergy between Yeast and Bacteria; Lallemand Technical Meeting: Perugia, Italy, 2001; pp. 27–32. [Google Scholar]
- Vivas, N.; Augustin, M.; Lonvaud-Funel, A. Influence of oak wood and grape tannins on the lactic acid bacterium Oenococcus oeni (Leuconostoc oenos). J. Sci. Food Agric. 2000, 80, 1675–1678. [Google Scholar] [CrossRef]
- Figueiredo, A.R.; Campos, F.; de Freitas, V.; Hogg, T.; Couto, J.A. Effect of phenolic aldehydes and flavonoids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. Food Microbiol. 2008, 25, 105–112. [Google Scholar] [CrossRef]
- Chasseriaud, L.; Krieger-Weber, S.; Déléris-Bou, M.; Sieczkowski, N.; Jourdes, M.; Teissedre, P.L.; Claisse, O.; Lonvaud-Funel, A. Hypotheses on the effects of enological tannins and total red wine phenolic compounds on Oenococcus oeni. Food Microbiol. 2015, 52, 131–137. [Google Scholar] [CrossRef]
- Stivala, M.G.; Villecco, M.B.; Enriz, D.; Fernandez, P.A. Effect of phenolic compounds on viability of 2 wine spoilage lactic acid bacteria. A structure-activity relationship study. Am. J. Enol. Vitic. 2017, 1–12. [Google Scholar] [CrossRef]
- Reguant, C.; Bordons, A.; Arola, L.; Rozes, N. Influence of phenolic compounds on the physiology of Oenococcus oeni from wine. J. Appl. Microbiol. 2000, 88, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Moreno-Arribas, M.V.; Martin-Alvarez, P.J.; Bartolome, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Jimeñez, N.; Esteban-Torres, M.; Mancheño, J.M.; De Las Rivas, B.; Muñoz, R. Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Terrade, N.; Mira de Orduña, R. Determination of the essential nutrient requirements of wine-related bacteria from the genera Oenococcus and Lactobacillus. Int. J. Food Microbiol. 2009, 133, 8–13. [Google Scholar] [CrossRef]
- Liburdi, K.; Benucci, L.; Esti, M. Lysozyme in wine: An overview of current and future applications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1062–1073. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Bağder Elmacı, S.; Gülör, G.; Tokath, M.; Erten, H.; ĺşci, A.; Özċelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 2014. [Google Scholar] [CrossRef] [PubMed]
- Nardi, T.; Vagnoli, P.; Minacci, A.; Gautier, S.; Sieczkowski, N. Evaluating the impact of fungal-origin chitosan preparation on Brettanomyces bruxellensis in context of wine aging. Wine Stud. 2014, 3, 13–15. [Google Scholar] [CrossRef]
- Schildberger, B. Influence of Specific Botryticides on Malolactic Fermentation. In Mitteilungen Klosterneuburg, Rebe und Wein, Obstbau und Früchteverwertung; Wissensbericht; Lehr- und Forschungszentrum für Wein und Ostbau: Klosterneuburg, Austria, 2011; pp. 168–172. [Google Scholar]
- Beelman, R.B.; Kunkee, R.E. Inducing co-inoculation malolactic-alcoholic fermentation in red table wines. In Proceedings of the Australian Society of Viticulture and Oenology Seminar on Malolactic Fermentation; Australian Wine Research Institute: Urrbrae, South Australia, 1985; pp. 97–112. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, E.; Ribéreau-Gayon, P.; Sudraud, P. Sciences et Techniques du Vin; Dunod: Paris, France, 1975; Volume 2. [Google Scholar]
- Krieger, S.; Zapparoli, G.; Veneri, G.; Tosi, E.; Vagnoli, P. Simultaneous and sequential alcoholic and malolactic fermentations: A comparison for Amarone-style wines. Aust. N. Z. Grapegrow. Winemak. 2007, 517, 71–77. [Google Scholar]
- Sieczkowski, N. Maîtrise et intérêt de la co-inoculation levures-bactéries. Revue Française D’Oenologie 2004, 207, 24–28. [Google Scholar]
- Gerbaux, V.; Briffox, C.; Dumont, A.; Krieger, S. Research Note: Influence of Inoculation with Malolactic Bacteria on Volatile Phenols in Wines. Am. J. Enol. Vitic. 2009, 60–62, 233–235. [Google Scholar]
- Kunkee, R.E. Malolactic fermentation. Adv. Appl. Microbiol 1967, 9, 235–279. [Google Scholar]
- Silva, A.; Lambri, M.; Fumi, M.D. Ochratoxin: A Decontamination by Lactic Acid Bacteria in Wine: Adsorption or Biodegradation? In Proceedings of the Oeno 2007 VIII Symposium International d’Oenologie—Bordeaux, Paris, France, 25–27 June 2007; pp. 24–27. [Google Scholar]
- Iorizzo, M.; Testa, B.; Lombardi, A.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Knoll, C.; Divol, B.; Du Toit, M. Genetic screening of lactic acid bacteria of oenological origin for bacteriocin-encoding genes. Food Microbiol. 2008, 25, 983–991. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Selection and Characterisation of Oenococcus oeni and Lactobacillus plantarum South African Wine Isolates for Use as Malolactic Fermentation Starter Cultures. S. Afr. J. Enol. Vitic. 2011, 32, 280–295. [Google Scholar] [CrossRef]
- Gerbaux, V.; Briffox, C. Influence de l’ensemencement en bactéries lactiques sur l’évolution de la couleur des vins de Pinot noir pendant l’élevage. Revue des Œnologues 2003, 103, 19–23. [Google Scholar]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.J.; Jones, G.P. Formation of vitisin A during red wine fermentation and maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Burns, T.R.; Osborne, J.P. Loss of Pinot noir wine color and polymeric pigment after malolactic fermentation and potential causes. Am. J. Enol. Vitic. 2015, 66, 130–137. [Google Scholar] [CrossRef]
- Bartowsky, E.; Krieger-Weber, S. Management of malolactic fermentation to enhance red wine colour. Grapegrow. Winemak. 2020, 673, 66–70. [Google Scholar]
- Mtshali, P.S.; Divol, B.T.; Van Rensburg, P.; Du Toit, M. Genetic screening of wine-related enzymes in Lactobacillus species isolated from South African wines. J. Appl. Microbiol. 2010, 108, 1389–1397. [Google Scholar] [CrossRef]
- Spano, G.; Rinaldi, A.; Ugliano, M.; Moio, L.; Beneduce, L.; Massa, S. A β-glucosidase gene isolated from wine Lactobacillus plantarum is regulated by abiotic stresses. J. Appl. Microbiol. 2005, 98, 855–861. [Google Scholar] [CrossRef]
- Lucio Costa, O. Acidificón Biológica de Vinos de pH Elevado Mediante la Utilización de Bacterias Lácticas. Ph.D. Thesis, University of Valencia, Valencia, Spain, 2014. [Google Scholar]
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Heras, H.M.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT Food Sci. Technol. 2016, 73, 334–341. [Google Scholar] [CrossRef]
- Saerens, S.; Edwards, N.; Soerensen, K.; Badaki, M.; Swieger, J.H. Production of Low Alcohol Fruit Beverage. International Patent Application Number PCT/EP2015/051163, 21 January 2015. [Google Scholar]
- Edwards, N.; Saerens, S.; Swieger, J.H. The Use of Lactobacillus plantarum As an Anti-Microbial Agent in the Process of Winemaking. International Patent Application Number PCT/EP2015/051161, 21 January 2015. [Google Scholar]
| Species | Lactobacillus plantarum | Oenococcus oeni |
|---|---|---|
| Fermentation of sugars (hexoses) | Homo-fermentative = 2 × lactate | Hetero-fermentative = Lactate + acetate + CO2 |
| Wine parameter for best performance | pH > 3.5 Alcohol < 15.5% (v/v) Total SO2 < 50 ppm Temperature 20–26 °C | pH > 3.1 Alcohol < 15.5% (v/v) Total SO2 < 50 ppm Temperature > 17 °C |
| Genetic preposition for enzyme activities | MOST strains: Malolactic enzyme/ Glycosidase/Protease/Esterase/Lipase/Citrate lyase | Only very FEW strains: Malolactic enzyme Esterase/Protease/ Citratelyase/Methionine synthase c |
| Genetic preposition for bacteriocins production | Good potential | Only a FEW strains |
| Types of Wines | Reds–Traditional Vinification (Short or Medium Maceration–Thermovinification (Liquid Phase) Initial Sugar/Potential Alcohol up to 260 g/L/15,5% (v/v)) |
|---|---|
| Timing of Bacteria Inoculation | Only co-inoculation Addition of ML Prime™, 24 h after adding yeast |
| SO2 Addition on Grapes/Must | ≤5 g/hL |
| pH Acid Malic Content | ≥ 3.4 maximum 3 g/L |
| Temperature During AF | 20° to 26 °C |
| Pre-MLF | Post-MLF | |
|---|---|---|
| Control | 8.37 | 9.2 |
| L. plantarum ML-Prime™ | 8.37 | 7.67 |
| O. oeni OM | 8.37 | 2.33 |
| O. oeni AL | 8.37 | 1.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages 2020, 6, 23. https://doi.org/10.3390/beverages6020023
Krieger-Weber S, Heras JM, Suarez C. Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages. 2020; 6(2):23. https://doi.org/10.3390/beverages6020023
Chicago/Turabian StyleKrieger-Weber, Sibylle, José María Heras, and Carlos Suarez. 2020. "Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook" Beverages 6, no. 2: 23. https://doi.org/10.3390/beverages6020023
APA StyleKrieger-Weber, S., Heras, J. M., & Suarez, C. (2020). Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages, 6(2), 23. https://doi.org/10.3390/beverages6020023





