Abstract
Madeira wine is a fortified Portuguese wine, which has a crucial impact on the Madeira Island economy. The particular properties of Madeira wine result from the unique and specific winemaking and ageing processes that promote the occurrence of chemical reactions among acids, sugars, alcohols, and polyphenols, which are important to the extraordinary quality of the wine. These chemical reactions contribute to the appearance of novel compounds and/or the transformation of others, consequently promoting changes in qualitative and quantitative volatile and non-volatile composition. The current review comprises an overview of Madeira wines related to volatile (e.g., terpenes, norisoprenoids, alcohols, esters, fatty acids) and non-volatile composition (e.g., polyphenols, organic acids, amino acids, biogenic amines, and metals). Moreover, types of aroma compounds, the contribution of volatile organic compounds (VOCs) to the overall Madeira wine aroma, the change of their content during the ageing process, as well as the establishment of the potential ageing markers will also be reviewed. The viability of several analytical methods (e.g., gas chromatography-mass spectrometry (GC-MS), two-dimensional gas chromatography and time-of-flight mass spectrometry (GC×GC-ToFMS)) combined with chemometrics tools (e.g., partial least squares regression (PLS-R), partial least squares discriminant analysis (PLS-DA) was investigated to establish potential ageing markers to guarantee the Madeira wine authenticity. Acetals, furanic compounds, and lactones are the chemical families most commonly related with the ageing process.
1. Introduction
Madeira wine has been produced in Madeira Island for many centuries. It has a crucial impact on the Island economy. On the basis of data provided by the Madeira Wine, Embroidery, and Handicraft Institute, P.I., the commercialization of Madeira wine in 2017 stood at 3.2 million liters, generating first sale receipts of 19.1 million euros. In 1913, Madeira Island completed the official regulatory process, obtaining the status of Demarcated Region of Madeira (DRM), representing one of the oldest Demarcated Regions of Portugal. The singular properties of Madeira wine result from a pool of particular conditions, namely, the terroir, distinctive grape varieties, and its particular winemaking process, which is strictly legislated [1,2]. Nowadays, there are five main Vitis vinifera L. grapes used in winemaking of Madeira wines, namely, Malvasia, Boal, Sercial, Verdelho (white varieties) known as noble varieties, and Tinta Negra (red variety), distributed in approximately 500 hectares (ha) of vines (Figure 1) [3]. About 404 ha is cultivated in Câmara de Lobos (south coast), São Vicente, and Santana (both north coast), representing around 80% of total area of vineyard, namely, 36.88% (185.57 ha), 28.41% (142.97 ha), and 15.09% (75.94 ha), respectively [3,4,5,6,7]. From these V. vinifera L. grapes used in the winemaking of Madeira wines, Tinta Negra is the most representative (around 80% of total production).
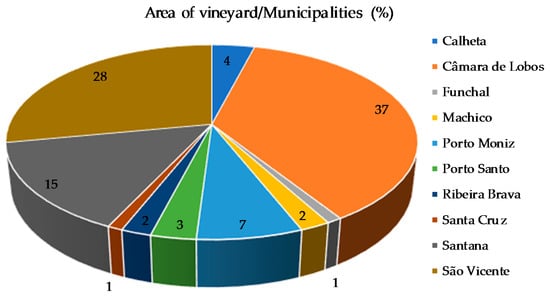
Figure 1.
Percentage of vineyard area by municipalities of Madeira Island [3].
During the fermentation process, the addition of natural grape spirit occurs to obtain an ethanol content of 18%–22% (v/v). On the basis of the fermentation time, wines with different sugar content will be obtained, like as dry (sugar content expressed as 49.1 to 64.8 g glucose per L, obtained from Sercial), medium dry (64.8 to 80.4 g/L, obtained from Verdelho), medium sweet (80.4 to 96.1 g/L, obtained from Boal), and sweet (96.1 to 150 g/L, obtained from Malvasia). Moreover, all types of Madeira wines previously described can be produced using Tinta Negra [7]. After the fermentation process, some wines undergo an ageing process in oak casks, in cellars with a humidity level ranging from 70% to 75% (at >30 °C), while most wines go through a baking process, for example, the wine is put in large coated vats and the temperature is slowly increased at about 5 °C per day, and maintained at 45–50 °C for at least three months. Then, the wine is submitted to a maturation process in oak casks for a minimum of 3 to 20 years or even longer [4,5,6,7]. The ageing process in oak casks is essential to the singular sensorial characteristics of Madeira wine. According to the age, the Madeira wine is classified as a vintage (a specific year of aged in casks, 17, 18, 19, and 20 years old), and blend (an average ageing period of 3, 5, 10, or 15 years old, and are called Finest, Reserve, Old Reserve, and Extra Reserve, respectively) [7].
This review encompasses an overview of Madeira wines related to volatile and non-volatile composition. Moreover, types of aroma compounds (e.g., varietal, pre-fermentative, secondary, and tertiary), the contribution of volatile organic compounds (VOCs) to the overall Madeira wines’ aroma, the change of their content during the ageing process, as well as the establishment of the potential ageing markers will also be reviewed. The non-volatile composition of Madeira wines and the chemical changes that occur during the Madeira wine winemaking process will be also reported.
2. Volatile Profile of Madeira Wines
The volatile composition of wine has a significant role in wine quality as it contributes to several sensations during wine tasting. The odors (owing to compounds that can bind to olfactory receptors) can influence flavor (mixture of aroma and taste) in the mouth retro-nasally, which leads to consumer rejection or acceptance, and subsequently wine valorization [4,8,9]. Until now, more than 1000 VOCs with different polarities, volatilities, and in widespread concentrations (ng/L to mg/L) [10] have been identified in wines. The occurrence of a single compound, at a concentration higher than its odor threshold (OT), is enough to allow a singular aroma, also known as impact odorant. Nonetheless, the aroma comprises the mixture of several hundred of compounds (odorants), which may contribute to the overall wine aroma [4,11,12]. The VOCs’ concentration in wines depends on the grape chemical composition, which is affected by grape variety and ripening stage, climate, and viticultural practices, combined with other parameters from winemaking techniques, storage, and ageing processes [10,13].
Wine aromas can be organized based on their origin in the production process (Figure 2) into the following: primary aroma, from grape variety; pre-fermentative aroma, produced during the processing of grape; secondary aroma, originated during the fermentation process (e.g., alcoholic, malolactic) by yeast and bacteria; tertiary aroma, derived from transformations that occurred during storage and/or the ageing process [10,14].
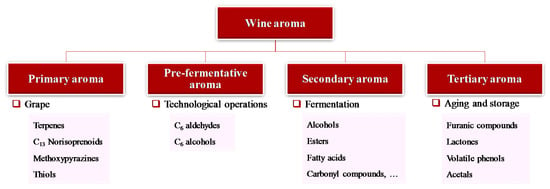
Figure 2.
Classification of wine aroma according to its origin and chemical families predominant in each type of aroma.
2.1. Primary Aroma
Primary aroma, also called varietal aroma, arises from a complex set of compounds that can exist in free forms (VOCs, odorants) and/or as precursors (glycosidically bound compounds, odorless) [13]. The glycosidically bound compounds are formed by free aroma compounds (aglycones) bound to a sugar moiety (glycones) by an O-glycosidic linkage [13,15]. The acid and/or enzymatic hydrolysis can be used to break this linkage, releasing the aglycone, which imparts a pleasant aroma to the wine [15]. They are biosynthesized during grape ripening, which depends on soil, climate, health, and ripening stage. Terpenes (mono- and sesquiterpenic compounds), C13 norisoprenoids, methoxypyrazines, and volatile thiols are the chemical families connected to varietal aroma, and their presence is associated with fruity, floral, and herbaceous aromas [16,17,18].
2.1.1. Terpenes
Terpenes constitutes the chemical family that has been broadly studied in V. vinifera L. grapes [17,19,20,21], and is responsible for fruity and floral aroma. Terpenes are synthesized in berries and stored in the skins. They result from rearrangement, comprising carbocation intermediates of acyclic precursors by terpene synthase/cyclase enzymes [22]. As an example, monoterpenes (C10) are synthesized from geranyl diphosphate (GPP), while sesquiterpenes (C15) and diterpenes (C20) are produced from farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP), respectively [22]. The terpenes levels found in grapes and wine depend on several factors, such as grape variety, maturity of grape berries, geographical region, and winemaking techniques [23]. Around 50 terpenes (monoterpenes and sesquiterpenes) are identified in grapes and 30 in wines [23,24]. The terpenes can occur as hydrocarbons (e.g., α-terpenine), alcohols (e.g., linalool), aldehydes (e.g., linalal), ketones (e.g., geranyl acetone), and/or esters (e.g., geranyl acetate) [14]. The most odorant terpenes in wines are linalool (aroma descriptor: citrus, floral, fruit, green; OT = 25 µg/L [25]), α-terpineol (aroma descriptor: anise floral, fruit, mint; OT = 250 µg/L [26]), geraniol (aroma descriptor: citrus, floral, fruit; OT = 30 µg/L [25]), and citronellol (aroma descriptor: citrus, floral, fresh, green; OT = 100 µg/L [26]). In addition, linalool and citronellol are significant odor compounds of white wines obtained from Muscat varieties and aromatic varieties like ‘Gewürztraminer’ and ‘Riesling’ wines [27], with linalool being identified as an impact odorant in young Vidal wine [28]. On the other hand, according to Campo et al. [29], Madeira wine lacks the most important varietal aromas, such as linalool. Câmara et al. [30,31] observed that α-terpineol, linalool, and citronellol (Table 1) are the predominant terpenes in Malvasia wines—as these varietal compounds are at levels lower than their OTs, they probably do not explain the flowery aroma of this wine. Citronellol can contribute with floral odors to Boal wines, as its concentration is higher than its OT. Perestrelo et al. [4] investigated the VOCs that may be linked to specific descriptors of Madeira wine. In this sense, ten terpenes are identified in Madeira wines, and the citrus and floral odors characteristic of young Madeira wines (three to five years old) can be explained by the presence of α-pinene, limonene, linalool, citronellol, and geraniol, as they are present at relative concentrations higher than their OTs [4]. Usually, the sesquiterpenic compounds are associated with wood, spice, sweet, floral, must, and fresh odors [32]. As far as we know, α-cadinol, γ-eudesmol, farnesol, ι-muurolol, and nerolidol are the sesquiterpenic compounds identified in Madeira wines [33,34].

Table 1.
Predominant volatile organic compounds (VOCs) found in Madeira wines, odor descriptions, origin, and odor thresholds (OTs). HMF, 5-hydroxymethyl-2-furfural.
2.1.2. C13 Norisoprenoids
Norisoprenoids result from chemical, photochemical, and/or enzymatic oxidation of carotenoids (e.g., β-carotene, lutein, neoxanthin, violaxanthin). However, the cleavage is not region-specific and results in the formation of cyclic or linear apocarotenoids with 9, 10, 11, 13, and 15 carbon atoms, which occur in grapes as glycosidically-linked precursors [18,41] (Figure 3).
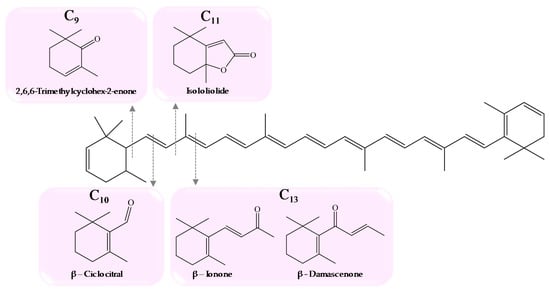
Figure 3.
Formation of C9, C10, C11, and C13 norisoprenoids from breakdown of carotenoids [18].
In grapes, the sun exposure promotes the carotenoids’ synthesis and/or their degradation, resulting mainly in the formation of C13 norisoprenoids. C13 norisoprenoids are important contributors to wine aroma, with floral and fruity aromas, even at low concentration, owing to their very low OTs [38]. β-ionone (aroma descriptor: floral, violet; OT = 0.7 µg/L) and β-damascenone (aroma descriptor: floral, fruit, honey, tobacco; OT = 0.05 µg/L) are considered key aromas in wine [38]. β-ionone arise from both enzymatic and photo-oxidative degradation of β-carotene. β-damascenone is present in wine at a concentration ranging from 1 to 4 μg/L, acting as an aroma enhancer, contributing with fruity aroma of wine esters [42,43]. In addition, β-damascenone and β-ionone can contribute remarkably to the potential aroma of several wine varieties, such as Cabernet Sauvignon, Chardonnay, Chenin Blanc, Semillon, Sauvignon Blanc, Syrah, and Pedro Ximénez [14,44]. Nevertheless, β-damascenone is one of the dominant varietal compounds (concentration ranging from 3 to 9.8 µg/L) in Madeira wines, and according to Câmara et al. [33], this C13 norisoprenoid contribute to wine aroma, as its concentration is above its OT (Table 1). On the other hand, Perestrelo et al. [4] identified seven C13 norisoprenoids, from which β-damascenone, β-ionone, geranyl acetone, and β-ciclocitral may explain the floral aroma related to young Madeira wines obtained from Malvasia, Boal, and Verdelho V. vinifera L. grapes. In addition, vitispirane (aroma descriptor: eucalyptus-like, balsamic; OT = 800 µg/L [34]) and 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN, aroma descriptor: kerosene or petrol-like aromas; OT = 20 µg/L) are also found in Madeira wines in a concentration range from 0.9 to 7.0 µg/L and 0.7 to 12.5 µg/L, respectively [34]. However, their concentrations are also below their OTs, which means that these varietal compounds do not contribute to overall Madeira wine aroma. Nevertheless, TDN has been described as a significant odorant of young Riesling wines [45].
2.1.3. Methoxypyrazines
The methoxypyrazines arise from amino acid metabolism (Figure 4), which originate in the grape and are linked to vegetal, green, and herbaceous aromas of wine [38,46].
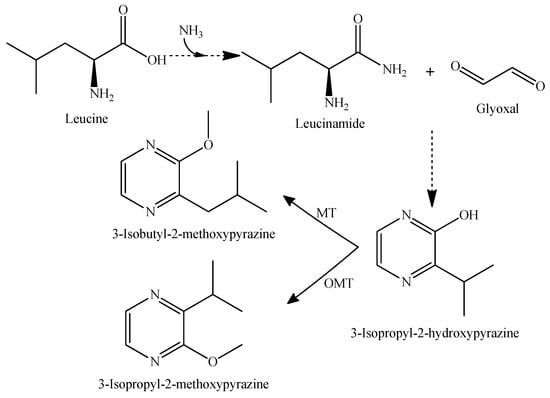
Figure 4.
Proposed pathway for the biosynthesis of 3-isobutyl-2-methoxypyrazine and 3-isopropyl-2-methoxypyrazine [47,48]. MT, methyltransferase; OMT, O-methyltransferase.
3-Isobutyl-2-methoxypyrazine (IBMP, aroma descriptor: bell peppers or green gooseberries), 2-sec-butyl-3-methoxypyrazine (SBMP, aroma descriptor: like pea or bell pepper), and 3-isopropyl-2-methoxypyrazine (IPMP, aroma descriptor: asparagus or green bean) were found in grapes and in wines [14,49]. Methoxypyrazines possess a very low OT (few ng/L), leading to them having an important impact on wine aroma [46]. These varietal compounds predominantly impart in Cabernet Sauvignon and Sauvignon Blanc, but also in Cabernet Franc and Merlot noir [46]. According to Campo et al. [29], the Madeira wine lacks the most relevant varietal aromas, including methoxypyrazines, as these varietal compounds are present at concentrations lower than their OTs.
2.1.4. Volatile Thiols
The volatile thiols, also called mercaptans, can cause defective aromas to wine quality, when present at a high concentration, as they possess the lowest OTs (ng/L or lower) [8]. The most common volatile thiols found in wines include 3-sulfanylhexan-1-ol (aroma descriptor: grapefruit; OT = 60 ng/L [50]), 3-sufanylhexyl acetate (aroma descriptor: passionfruit; OT = 4 ng/L [50]), and 4-methyl-4-sulfanylpentan-2-one (aroma descriptor: boxwood; OT = 0.8 ng/L [50]). Volatile thiols were reported as having a significant positive contribution to Sauvignon Blanc, Merlot, and Cabernet Sauvignon wines [38]. Regarding Madeira wines, only methionol has been identified [51,52], and does not contribute to Madeira wines’ overall aroma, as its concentration is lower than its OT.
2.2. Pre-Fermentative Aroma
Pre-fermentative aroma, expressed as C6 aldehydes and alcohols, is formed by enzymatic action during technological operations (e.g., crushing, stemming, pressing, maceration, clarification) prior to the fermentation, as well as during heating of must heating or grape maceration. These C6 compounds arise from membrane lipids through the lipoxygenase pathway [53,54]. Linoleic and α-linolenic acids resulting from an acyl-hydrolase, in the presence of oxygen, are converted to 13-hydroperoxides by the lipoxygenase activity. Subsequently, a hydroperoxide-lyase promotes the formation of hexanal, from hydroperoxide of linoleic acid, and (Z)-3-hexenal and (E)-2-hexenal from hydroperoxide of α-linolenic acid. Lastly, an alcohol-dehydrogenase reduces the aldehydes to the respective alcohols, for example, 1-hexanol, (Z)-3-hexenol, and (E)-2-hexenol [53].
The C6 alcohols’ concentration depends on grape variety, ripeness stage, treatments prior to fermentation, and temperature/duration of contact with the skins [54,55]. At a low concentration, the (E)-2-hexenol and (E)-3-hexenol (aroma descriptors: fresh, green, grass, leaf; OT = 400 µg/L) contribute positively to the overall aroma of the wine. In the opposite case, at higher levels, these C6 compounds lead to undesirable organoleptic odors, like herbaceous, green fruit, and crushed leaf [54,56]. Oliveira et al. [53] demonstrated that the (E)-3-hexenol/(Z)-3-hexenol ratio evidently discriminates Loureiro wines from those of Alvarinho, Avesso, and Trajadura from Portugal. In addition, the Vinhos Verdes monovarietal wines are discrimated using 1-hexanol/(E)-3-hexenol and 1-hexanol/(Z)-3-hexenol ratios. 1-Hexan-1-ol, (E)-3-hexenol, and (Z)-3-hexenol are found in Madeira wines [57,58,59], but at a concentration lower than their OTs. According to Câmara et al. [60], Boal wines are characterized by (E)-hex-3-en-1-ol and (Z)-hex-2-en-1-ol, whereas (Z)-hex-3-en-1-ol is one of the VOCs associated with Malvasia wines.
2.3. Fermentative Aroma
Fermentative aroma, also known as secondary aroma, arises from the chemical and biochemical changes effected on the wine by the fermentation process. This process can be catalyzed by different yeasts strains, such as Saccharomyces, Kloeckera, Candida, Hansenula, Hanseniospora, Pichia, and Zigossacharomyce, among others, which are part of the grape microbial flora and can start the fermentation. The yeast growth during fermentation depends on pH, fermentation temperature, nitrogen content, sulphur dioxide (SO2), and sugar content [61,62]. The fermentation-derived VOCs represent the major percentage of the total aroma composition of wine [62]. Normally, most of the concentration of fermentative aroma compounds is below the OT, and consequently does not contribute individually, in a significant way, to the typical aroma of wines [62]. Besides ethanol, glycerol, and diols, many other fermentative aroma compounds are formed by yeast metabolism, including alcohols, esters, acids, carbonyl compounds (e.g., ketones, aldehydes), and lactones. Alcohols, esters, and fatty acids are quantitatively the most dominant fermentative aroma compounds.
2.3.1. Alcohols
The alcohols are abundant VOCs arising from amino acids’ decarboxylation/deamination (65%) and from sugars (35%) under the enological conditions [63]. The alcohols’ concentration depends on several factors such as yeast strain, fermentation temperature, pH of must, aeration, and the variety and maturity of grape berries [64]. Nowadays, the alcohols’ concentration range from 140 to 420 mg/L, with 3-methylbutan-1-ol generally accounting for more than 50% of the alcohols’ content [65]. If they arise at a concentration below 300 mg/L, it could contribute positively to the overall wine aroma with the fruity and flowery notes, whereas at concentrations higher than 400 mg/L, a negative effect is noticed as pungent and unpleasant notes [63,66]. The most abundant alcohols identified in Madeira wines are 3-methylbutan-1-ol and 2-phenylethanol, and at the lowest concentration, 2-ethylhexan-1-ol, benzyl alcohol, 1-decanol, and butan-2,3-diol, among others [4,51,52]. Overall, 3-methylbutan-1-ol and 2-phenylethanol have been described to hold the highest odor intensities, contributing positively to wine aroma with flower, honey, and fruit notes [4,29,67,68]. In addition, 2-ethylhexan-1-ol can explain the citrus aroma characteristic of Malvasia, Sercial, and Tinta Negra wines [4].
2.3.2. Esters
The esters are formed by yeasts during fermentation as secondary products of sugar metabolism and represent one of the most significant fermentative aroma compounds influencing flavor [62]. Esters can also be formed through chemical esterification during long-term wine ageing [62]. Generally, ethyl esters of short- and medium-chain-length carboxylic acids (C2–C10) and acetates of short-chain-length alcohols (C4–C6) are reported in wine at the concentration above their OTs (few µg/L) [69,70]. From a sensorial point of view, these fermentative compounds contribute to wines with positive aromas, namely fruity and/or floral. However, if they are present at high concentrations, they can cover the varietal aromas, reducing the wine complexity [62]. The most significant esters for overall wine aroma are ethyl acetate (aroma descriptor: fruity, solvent-like, OT = 7500 µg/L), isoamyl acetate (aroma descriptor: banana, sweet; OT = 30 µg/L), ethyl hexanoate (aroma descriptor: apple; OT = 14 µg/L), and 2-phenylethyl acetate (aroma descriptor: honey, fruity, flowers; OT = 250 µg/L). The citrus odor characteristic of Malvasia, Sercial, and Tinta Negra young wines could be described by the occurrence of some esters, like hexyl acetate and ethyl 3-methylbutanoate [4]. According to Perestrelo et al. [52], the concentration of fatty acid ethyl esters (e.g., ethyl hexanoate, ethyl decanoate) and acetate esters (e.g., isoamyl acetate, ethyl 2-phenylacetate) declined during storage, independently of storage time and temperature. In the opposite case, the concentration of the diprotic acid ethyl esters (e.g., diethyl succinate, ethyl succinate, lactate and 3-hydroxyhexanoate) tended to rise during storage. The same result was reported by Pereira et al. [51] in Madeira wines submitted to traditional accelerated ageing. The esters’ formation depends on several factors, such as must acidity, clarification, aeration, fermentation conditions, and ageing process. Furthermore, the absence of oxygen, low temperatures, and must clarification reduce the esters’ formation.
2.3.3. Fatty Acids
The fatty acids (C2 to C12) are formed during wine fermentation where the absence of oxygen restrains fatty acid desaturation of yeast. Alternatively, the biosynthesis can be through the direct uptake of unsaturated fatty acids from the grape juice [71]. The acids’ concentration in wine ranges from 500 to 1000 mg/L, with acetic acid usually accounting for more than 90 % of the volatile acids. The acetic acid at a high concentration is responsible for a vinegar-like odor to wine and contributes negatively at concentrations of 0.7–1.1 g/L [72]. Apart from acetic acid, the hexanoic, octanoic, and decanoic acids are some of the most abundant fatty acids in Madeira wine [51,52]. At high concentrations, these acids are linked with rancid, cheesy, and vinegar odors; however, they are generally at concentrations below their OTs in wines [73]. In general, fatty acids decrease during storage, probably because of esterification reactions with ethanol [51] and other alcohols. During storage, the reduction in the concentration of medium- and long-chain fatty acids contributes positively to the Madeira wine aroma [52].
2.3.4. Carbonyl Compounds
The carbonyl compounds include aldehydes and ketones. The aldehydes can be formed by amino acid deamination or transamination, Strecker degradation, microbial activity during fermentation, and fatty acid oxidation [74]. Acetaldehyde (ethanal) is the predominant aldehyde found in wine, generally accounting for more than 90 % of the total aldehydes. This aldehyde at a concentration higher than its OT (100 mg/L) [39] is an off-flavor. In addition, together with other oxidized compounds, it can contribute to the fragrance of sherries and other oxidized wines [75]. Other aldehydes, namely, vanillin (aroma descriptor: vanilla; OT = 995 µg/L), a possible product of ferulic acid degradation; cinnamaldehyde (aroma descriptor: cinnamon; OT = 750 µg/L); and benzaldehyde (aroma descriptor: almond; OT = 350 µg/L), may accumulate in aged oak wines, as they arise mainly through degradation products of wood lignins [29,75].
The ketones are derived from lipid oxidation and the fermentation process, as well as from the citrate and glucose metabolism [74], having a low contribution to wine aroma owing to their high OTs. 2,3-Butanedione (diacetyl), 3-hydroxy-2-butanone (acetoin), and 2,3-pentanedione are the main exceptions. At a high concentration, from 5 to 7 mg/L, diacetal is considered an off-flavor and imparts a buttery, lactic off-odor, whereas in the concentration range from 1 to 4 mg/L, depending on the wine style, it can impart desirable buttery or butterscotch aromas [75,76]. On the other hand, acetoin has a sugary, butter-like character, whereas 2,3-pentanedione and its related diol possess similar aromatic characteristics, changing from buttery to plastic [75]. According to Pereira et al. [57], acetoin and benzaldehyde can be used as indicators of ageing. Moreover, vanillin found in Malvasia of 10 years old is another carbonyl compound of major importance. Acetaldehyde is also found in Madeira wines at concentrations ranging from 18.38 to 116.99 mg/L [60,77].
2.4. Terciary Aroma
Tertiary aroma, also known as ageing/bouquet aroma, is produced after the fermentation process, that is, during wine ageing (wood barrel, bottle ageing) [77,78], which is attended by changes of the organoleptic properties. Wine ageing bouquet is a result of chemical reactions including ester hydrolysis and formation, oxidation, and cyclization [79]. Hydrolysis of glycosidic precursors as well as chemical rearrangements of some VOCs arising from grapes or fermentation are hypothetical crucial phenomena associated with aged wine aroma development [79]. The quality of the ageing bouquet depends on the wine origin (e.g., vineyard soil, microclimate), vintage (reflecting the climatic conditions of the year of production) [78], and diffusion of molecules from the oak to wine, which depends on the oak characteristics (e.g., geographical origin, species of oak, seasoning of the staves, toasting, cask age) [80,81], among others. During the ageing process, the concentration of some varietal and fermentative aroma compounds decreases, allowing the wine to lose its freshness and fruitiness, whereas other odors, namely, caramel, dried fruits, spicy, toasty, and woody odors, appear. The Maillard reactions and diffusion from the oak are the crucial pathways related to these descriptors [4,29]. The furanic compounds, lactones, volatile phenols, and acetals are the main chemical families that have been described as potential ageing markers in Madeira wines (Figure 5) [57,58,77,80,82].
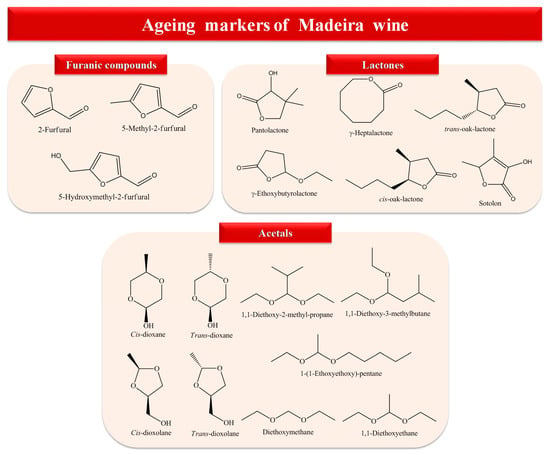
Figure 5.
Potential ageing markers of Madeira wines [57,58,77,80,82].
2.4.1. Furanic Compounds
The furanic compounds arose by three pathways: pyrolysis of carbohydrates, dehydration of sugars by Maillard reaction, and caramelization [83,84,85]. The breakdown of pentoses and/or hexoses catalyzed by heat and Maillard reactions originates the 5-hydroxymethyl-2-furfural (HMF) [86]. Deoxyhexosomes are Amadori reaction intermediates, being part of the transamination and enolization reactions [83].
HMF appears to be stable in wines in the absence of oxygen, for example, in fortified wines submitted to accelerated ageing [51,87]. Nevertheless, because of oxidation reactions (diffusion of oxygen during ageing), HMF may originate 5-methyl-2-furfural and 2,5-furandicarbaldehyde, following a dehydration reaction promoted by the acidic medium [86]. This chemical family has been associated with caramel, spice, brown, and dried fruits odors, with 2-furfural, 5-methyl-2-furfural, and HMF being the predominant furans reported in Madeira wine [68,80,83,88]. Others furans have also been found, namely, 2-ethoxytetrahydrofuran, 2-acetylfuran, ethyl 2-furoate, 5-formylfurfural, 5-ethoxymethyl-2-furfural, and 5-hydroxymethyldihydrofuran-2-one [57,59,77]. In addition, some studies report 2-furfural, 5-methyl-2-furfural, and HMF as potential ageing markers to prevent frauds, as their concentrations increase linearly during the ageing process [77,89,90]. Nevertheless, their contribution to wine aroma is not expected owing to its high OTs.
2.4.2. Lactones
The lactones, special γ-lactones and oak lactones, are the most significant flavor compounds, derived by cyclization of the respective hydroxycarboxylic acids [77,91]. According to Câmara et al. [77], γ-lactones are one of the most relevant ageing aroma compounds as they contribute to wine aroma with pleasant odor, namely, fruit, coconut, caramel, nutty, toast, musky, and sweet odors. Previous studies [77,92] have shown that their concentration tends to increase during storage and the ageing process in oak casks. Oak lactones, like as cis- and trans-oak-lactone, are present in natural oak, and their concentration increases owing to seasoning and toasting [93], and from a sensorial point of view, they are the most significant lactones extractable from oak casks [94]. Perestrelo et al. [80] observed that pantolactone, γ-ethoxybutyrolactone, γ-heptalactone, trans-oak-lactone, and cis-oak-lactone are highest and positively correlated with age for Madeira wines obtained from Malvasia and Boal. In addition, sotolon (3-hydroxy-4,5-dimethyl-2(5H)-furanone) has been considered a key aroma of fortified wines owing to its low OT (few µg/L) and, associated with their characteristic nutty, caramel, and curry odor, depends on its concentration and enantiomeric distribution [95]. According to Câmara et al. [89], the concentration of sotolon increases significantly during ageing from 100 (6 year-old) to 1000 μg/L (25-year-old), and for this reason, can be considered a potential ageing marker and a strong contributor to Madeira wine with spicy aromas. Also, a relation between the amount of sotolon and the sugar content was observed, as the average values obtained for sotolon raised from dry Sercial wines to sweet Malvasia wines.
2.4.3. Volatile Phenols
The volatile phenols, like ethyl and vinylphenols, were also released from oak; nonetheless, their microbiological yeast transformation (e.g., Brettanomyces and Dekkara) from hydroxycinnamic acids of wine is described as the main source [96]. Figure 6 shows the ethylphenols’ formation from hydroxycinnamic precursors by yeasts’ transformation [97].
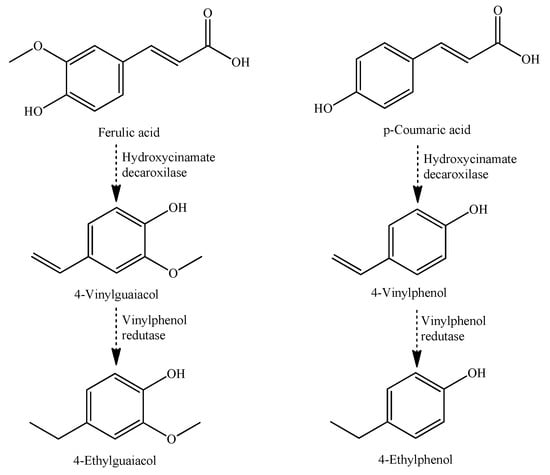
Figure 6.
Formation of ethylphenols from hydrocinnamic precursors by yeast transformation (e.g., Brettanomyces and Dekkera) [97].
Hygienic conditions of the cellar and SO2 treatments of the barrels are some of the parameters taken into consideration to avoid these volatile phenols’ formation [98]. These volatile phenols are connected with olfactory defects in wine, when they exceed their OTs (33 µg/L for 4-ethylguaiacol and 440 µg/L for 4-ethylphenol) [40], Brettanomyces can potentially induce spoilage related to medicinal or barnyard odors [99]. However, at a low concentration, the presence of volatile phenols imparts a singular aged character to some young red wines, by transmitting aroma notes of spices, smoke, and leather [99,100]. A total of 17 volatile phenols are identified in Madeira wines, with o-guaiacol, 4-ethylphenol, and 4-ethylguaiacol being the most predominant [80]. During the ageing process, for Sercial Madeira wines, the relative concentration of volatile phenols increases more remarkably, with a similar trend being reported in the literature [101].
2.4.4. Acetals
The acetals arose by the acid-catalyzed condensation reaction between aldehydes and alcohols, which is favored at higher pH values, resulting in four heterocyclic acetal alcohol formation: cis- and trans-5-hydroxy-2-methyl-1,3-dioxane (cis- and trans-dioxane), and cis- and trans-4-hydroxymethyl-2-methyl-1,3-dioxalane (cis- and trans-dioxolane); Figure 7 [82,102].
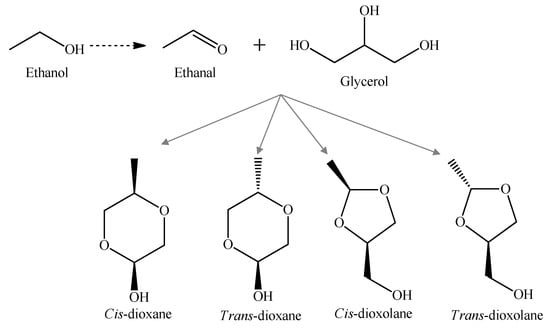
Figure 7.
Formation of heterocylic acetals by the acetalization reaction between acetaldehyde and glycerol [103].
The concentration of these four acetals in Madeira wines increases significantly during the ageing process, and demonstrated a linear trend with age, with correlation coefficients (r) around 0.9 [82]. The ratio between cis and trans forms stays constant independent of the wine age, with these results being in agreement with those obtained for Porto wines [103]. In another study performed by Perestrelo et al. [80], 30 acetals are found in Madeira wines, with diethoxymethane, 1,1-diethoxyethane, 1,1-diethoxy-2-methyl-propane, 1,1-diethoxy-3-methylbutane, and 1-(1-ethoxyethoxy)-pentane being the most predominant. Moreover, the relative concentrations of 1,1-diethoxy-2-methyl-propane, 1-(1-ethoxyethoxy)-pentane, 1,1-diethoxypentane, 2-propyl-1,3-dioxolane, and 1,1-diethoxyhexane show high correlation with age for Madeira wines, and can be used as potential ageing markers to prevent frauds. In general, the Madeira wines obtained from white grape varieties (Malvasia, Boal, Sercial, and Verdelho) showed high acetals content related to red grape varieties (Tinta Negra). Similar results are reported by Cutzach et al. [86] as the high polyphenols’ concentration in red varieties reduced the oxidation reaction and combined easily with acetaldehyde.
3. Non-Volatile Profile of Madeira Wines
The non-volatile profile of wines comprises diverse chemical families, including polyphenols, organic acids, aminoacids, and biogenic amines (BAs), among others. Their concentrations range from few ng/L to g/L results in wine flavor, recognized by several authors [104,105,106]. In this sense, Spanier et al. [105] noted that non-volatile compounds, mainly responsible for taste and tactile sensations, produce the psychological sensory of flavor. It is also shown that a non-volatile wine matrix influences the release of odorants. Moreover, sensory properties are produced by a balance between non-volatile and volatile compounds. The wine qualities have been related to the non-volatile composition of wine, although some studies showed the significance of aroma as well as the volatile composition of wine in the perception of both taste and astringency [104,105].
3.1. Polyphenols
Polyphenols are secondary metabolites in plants, also being present in grapes and wines. They are formed biogenetically from either the shikimate/phenylpropanoid and/or ‘polyketide’ acetate/malonate pathway, resulting in monomeric, polymeric phenols and polyphenols [107]. Their concentrations in wines are influenced by the technological practices, location (e.g., altitude, geological features, soil type, sunlight exposure), climate, ripening stage, and viticultural practices [108,109]. Polyphenols can be classified into two main groups, namely flavonoids (e.g., flavonols, flavones, isoflavones, flavanones, flavanols, anthocyanidins, procyanidins) and non-flavonoids (e.g., phenolic acids, stilbenes, lignans) [110]. These are important components of grapes and wine, as they contribute to sensory properties such as color, astringency, and bitterness, and are also involved in oxidation reactions, protein interactions, and wine-ageing processes [109]. In addition, despite their influence on wine character and quality, they are crucial not only for the wine characterization, but also for the history of the winemaking process [109,110,111]. Generally, the polyphenols’ concentration in white wines is lower than in red wines, with hydroxycinnamic acids (e.g., coumaric, caffeic, ferulic acids) being the most dominant polyphenols class, estimated to be around 190–290 mg/L in white wines and 900–2500 mg/L in red wines [112]. Perhaps, the white wines showed a lower concentration of polyphenols when compared with the red ones, and in turn, their content in caftaric acid is higher. Also, hydroxycinnamic acids and their tartaric esters represent the main class of non-flavonoid phenolics in red wines, whereas in white wines, they are are predominant [108,113]. From the flavonoids, the anthocyanins are responsible for the characteristic red color of grapes and wines, whereas the flavan-3-ols are most related to the astringency, bitterness, and structure of wines [108].
The non-volatile composition of wines has been well investigated regarding the polyphenols’ concentration using different analytical methods [110,113,114,115,116,117,118]. Bravo et al. [118] reported the polyphenols profile of Muscatel sweet wines from Setúbal region in Portugal, identifying 25 polyphenols. Lambert et al. [116] developed a method for the quantification of polyphenols in 12 rose wines. It was possible to identify a total of 152 polyphenols from several chemical families, namely, benzoic acids and derivatives (0.03–10.53 mg/L), hydroxycinnamic acids and derivatives (0.014–53.6 mg/L), stilbenes (0.01–1.41 mg/L), flavonols (0.003–0.44 mg/L), flavan-3-ols (0.001–19.9 mg/L), and anthocyanins (0.0009–4.38 mg/L). Moreover, Tarola et al. [119] analyzed 10 samples of commercially Italian red wines in order to determine the polyphenols’ content. Epicatechin and catechin were the polyphenols identified with highest concentration ranging from 20.45 to 123.30 µg/mL and 55.99–145.72 µg/mL, respectively. Ragusa et al. [115] performed the analysis of polyphenols in Negroamaro and Primitivo red wines from Salento, where gallic acid and catechin were the most dominant with values of 29.4 and 14.2 mg/L, respectively. Pozo-Bayón et al. [113] identified 32 polyphenols in 36 white, blanc de noir, and rose sparkling wines. Hydroxycinnamic acids and their esters were the chemical family with the largest number of compounds detected, including caffeic (trans), caftaric (cis, trans), and p-coumaric acids (cis, trans), among others. From these, trans-caftaric obtained the highest mean concentration of 33.96 mg/L, whereas cis-p-coumaric acid had the lowest (0.52 mg/L). García-Falcón et al. [120] identified 38 polyphenols in red wines (Mencía and Brancellao), with the highest contribution being obtained for hydrobenzoic acids, namely, for gallic acid (7–14 mg/L), hydrocinnamic acids like trans-caftaric acid (46 mg/L), catechins as catechin (37 mg/L), procyanidin B1 (3–36 mg/L), and anthocyanins (0.8–126 mg/L).
With respect to studies involving Madeira wines, numerous reports have been published with the aim of determining the polyphenol content in Madeira table wines as well as in fortified wines [114,121,122,123,124,125,126,127]. As for example, Silva et al. [121] identified 15 polyphenols in red and white wines. The highest concentration in red wines analyzed was obtained for catechin (377 µg/mL), while the lowest concentration was quercetin (2.1 µg/mL). Regarding the white wines, epicatechin (15.9 µg/mL) was the polyphenol with the higher concentration, whereas m-coumaric acid (0.2 µg/mL) presented the lowest. In another study carried out by Silva et al. [122] that investigated the levels of some flavonols, myricetin was the most predominant in all analyzed wines, followed by quercetin and, in the lowest concentration, kaempferol. Myricetin was not detected in any of the white wines under study [122]. Gonçalves et al. [114] quantified hydroxybenzoic acids (e.g., gallic, protocatechuic, gentisic, vanillic and syringic acids) and hydroxycinnamic acids (e.g., o-, p-, m-coumaric, ferulic and cinnamic acids) in red and white wines. The results showed that gallic acid was identified in all analyzed wines, ranging from 6 to 29 µg/mL in red wines, and 1 to 17 µg/mL in white wines. Protocathechuic and vanillic acids were only detected in three wine samples (0.2–1.0 µg/mL), while for syringic acid, the concentration ranged from 2 to 4 µg/mL. With respect to hydroxycinnamic acids, only p-coumaric (1–3 µg/mL) and ferulic acids (0.3–1.0 µg/mL) were detected. In addition, Gonçalves et al. [126] quantified the trans-resveratrol concentration in commercial red and white wines. On the basis of the results, the highest concentration was obtained in red wines (44.4 µg/mL), whereas in white wines, the values were lower than the limit of detection (LOD). A study performed by Paixão et al. [125] assessed the polyphenol content in Madeira wines (red and white) and observed that gallic acid (429 mg/L) was the main polyphenol identified in red wines, whereas in white wines, caffeic acid (14.62 mg/L) was the major compound. Pereira et al. [127] evaluated the polyphenol content in different types of Madeira wines, heated at 45 °C for three months, and 70 °C during one month. Six hydroxybenzoic acids, three hydroxycinnamic acids, one stilbene, three flavonols, and three flavan-3-ols were identified in these wines, with hydroxycinnamates and hydroxybenzoates being the most predominant after a backing process called “estufagem”. The polyphenols’ concentration decreased, except for caffeic, ferulic, p-coumaric, gallic, and syringic acids. Lastly, Rudnitskaya et al. [123] used an electronic tongue (ET) multisensor system and high performance liquid chromatography (HPLC) to study a set of fourteen Madeira wines including wines from different varieties (e.g., Malvasia, Boal, Verdelho, and Sercial). It was verified that protocatechuic acid had higher levels in the analyzed wines, whereas trans-resveratrol the lowest concentration using the multisensor system.
3.2. Organic Acids
The analysis of organic acids in wines is a crucial step of quality control, acting also to screen the evolution of acidity during the winemaking process, with the levels of tartaric acid being a control parameter of wine stabilization. Malic and tartaric acids are abundant in grapes and are used to establish the optimum harvest date, according to the malic/tartaric acid ratio [128]. The main organic acids identified in wines include tartaric, acetic, malic, lactic, and citric. In this sense, acidity in wines originates mainly from two sources, namely from grapes by extraction of organic acids (e.g., malic and tartaric acid) and from the fermentation process, which include succinic, lactic, and acetic acids [129]. Wine acidity influences the ageing potential of wine, as it determines their physical, biochemical, and microbial stability [6]. The determination of the volatile acidity is an important analytical criteria to evaluate in enology. The wine fermentation contributes to the transformation, disappearance, or appearance of different acids [130]. Chidi et al. [131] studied the influence of temperature at pressing on organic acids and concluded that Chardonnay base wines were richer in malic and succinic acids, whereas Pinot noir wines were characterized by higher levels of malic, citric, and pyruvic acids. In addition, pyruvic acid was only detected after the secondary fermentations in wines from both regions. Moreover, Castiñeira et al. [132] developed a capillary electrophoresis method to determine the amount of organic acid in 39 wines from two different Spanish Certified Brands of Origin (CBO). The results showed a predominance of lactic (452–3784 mg/L) and tartaric acids (866–1987 mg/L), followed by succinic (398–897 mg/L), malic (2844 mg/L), and acetic (116–749 mg/L) acids in Ribeira Sacra wines. A similar distribution was observed in the Bierzo wines: lactic acid (179–4037 mg/L), tartaric acid (772–1819 mg/L), succinic acid (389–646 mg/L), malic acid (1513 mg/L), and acetic acid (214–553 mg/L). Moreover, Žulij et al. [133] determined the organic acid composition in Croatian predicate wines, where the dominant organic acids in all analyzed wines were tartaric, malic, citric, and galactaric ranging from 0.09 to 2.98 g/L. Also, Zotou et al. [134] measured the concentration of seven organic acids (tartaric, malic, lactic, acetic, citric, succinic, and galacturonic acids) present in Greek wines and their LODs ranged between 1 and 5 mg/L, while the linear range was between 3 and 2000 mg/L. Moreover, Huang et al. [106] studied the organic acids profile of red wines obtained from different grape varieties. Overall, the main acids identified were tartaric, lactic, and acetic acids, while malic, citric, and succinic acids had a lower contribution in the analyzed samples. Relative to studies carried out in Madeira wines, Pereira et al. [135] carried out the first study for the determination of organic acids in Madeira wines. Tartaric, malic, and lactic acids are the dominant organic acids in all Madeira wines. The overall organic acids varied from 0.06 to 6.27 g/L for oxalic acid, 0.545 to 2.734 g/L for tartaric acid, 0.346 to 0.508 g/L for formic acid, 0.373 to 3.551 g/L for malic acid, 1.331 to 9.839 g/L for lactic acid, 0.666 to 2.207 g/L for acetic acid, 0.236 to 0.509 g/L for citric acid, and 0.063 to 0.152 g/L for succinic acid. Rudnitskaya et al. [123] used HPLC and ET multisensor system to determine the concentration of organic acids in Madeira wines from four varieties, namely, Boal, Malvasia, Verdelho, and Tinta Negra. The results showed that tartaric acid was the organic acid with highest contribution, ranging from 1.506 to 2.733 g/L, whereas formic acid presented the lowest concentration (3–9 mg/L).
3.3. Aminoacids and Biogenic Amines
Aminoacids (AAs) and biogenic amines (BAs) occur together in food matrices and beverages, like wine, taking part in several transformation processes [136]. Representing the main fraction (25–30%) of nitrogen compounds in wines, AAs are an important source of nitrogen during yeast fermentation and are involved in the formation of aroma compounds [137]. Their profile and concentration in wines can be influenced by several factors, such as grape variety, climate, growing conditions (mainly nitrogen fertilization), and winemaking techniques (e.g., ageing process) [137]. In grapes, AAs like leucine, valine, isoleucine, and phenylalanine are precursors of volatile compounds, whereas other AAs including methionine, phenylalanine, tyrosine, and tryptophan can be converted into α-ketoacids and metabolized into alcohols and fatty acids in yeast cells [138]. Depending on the reactions, AAs can originate undesirable compounds in wines, such as ethyl carbamate, biogenic amines, and carbolines (from tryptophan), among others [138].
Several reports have been developed in order to determine the concentration of AAs in wines, such as, for example, Soufleros et al. [139], who established the primary AAs profile in 33 Greek white wines from six grape varieties and different vintages by reversed-phase high performance liquid chromatography (RP-HPLC) using precolumn derivatization with o-phthalaldehyde (OPA) and fluorescence detection (FLD). The total AA content ranged from 68.4 to 2170 mg/L, where arginine, lysine, alanine, and glutamine were the most abundant AAs identified [139]. Moreover, Fiechter et al. [140] studied the evolution of the free AAs profile of on-lees aged white wines, with the arginine (271.7–498.0 mg/L), aspartate (24.8–63.3 mg/L), glutamate (42.8–94.3 mg/L), γ-amino butyric acid (121.4–130.3 mg/L), proline (769.7–895.7 mg/L), lysine (27.5–79.8 mg/L), and leucine (19.1–61.4 mg/L) being the most abundant. Herbert et al. [141] studied the free AAs present in musts and wines from Alentejo sub-regions regarding the grape variety, region, and vintage. White wines had a higher mean concentration (978 mg/L) of total AAs when compared with red wines (368 mg/L). Several reports have studied the AAs content in beverages, such as Madeira wines. The interest in studying these compounds relies on the importance of their presence in wine aroma [2]. Concerning the AAs concentration in Madeira wines, Pereira et al. [136] developed an HPLC-FLD method for their determination. The obtained concentration of AAs identified in Madeira wines ranged from 1.39 mg/L (asparagine) to 459.56 mg/L (arginine) and the LODs ranged from 0.71 mg/L (asparagine) to 8.26 mg/L (lysine). Another investigation performed by Pereira et al. [137] focused on the impact of accelerated ageing on the AAs profile of Madeira wines, and verified that the higher concentration was obtained for alanine (82.3 mg/L), while the lower levels for histidine (2.06 mg/L) in sweet Madeira wine were obtained from Tinta Negra. For dry Madeira wine obtained from Tinta Negra, the higher and lower concentrations were obtained for alanine (27.8 mg/L) and tyrosine (3.90 mg/L), respectively, whereas for Malvasia wine, arginine (73.6 mg/L) was the major AA identified and tyrosine (1.68 mg/L) the minor. More recently, Perestrelo et al. [142] determined the AAs pattern in Verdelho table wines according to vintage. The analyses revealed that the highest content was obtained for phenylalanine (33.2 mg/L), while the lowest was obtained for tryptophan (0.84 mg/L), giving a total AAs of 52.0 mg/L.
Concerning BAs, they arise from amino acids decarboxylation reactions, being formed during the manufacture of food and beverages such as cheese, wine, or beer [143]. They can be divided into several chemical families, namely, aliphatic (e.g., putrescine, cadaverine, ethylamine, methylamine, agmatine, spermidine, spermine), aromatic (e.g., tyramine, β-phenylethylamine), and heterocyclic (e.g., histamine, tryptamine) [144]. They can also contribute to wine smell, taste, and appearance, but in higher levels, they can promote undesirable physiological effects such as nausea and tachycardia, among others [145]. The increase in their concentration in wines can possibly originate from inadequate sanitary conditions at some stages of the winemaking process [137]. The most commonly identified in wines include histamine, cadaverine, agmatine, tyramine, putrescine, and β-phenylethylamine [143]. Among BAs, histamine is the most relevant, not only because of its toxicity, but also because ethanol and other amines (e.g., cadaverine, tyramine, phenylethylamine, tryptamine, agmatine, and putrescine) improve the toxicity by inhibiting enzymes (e.g., methyl transferase, diamine oxidase, monoamine oxidase) that are involved in histamine detoxification in humans [143,145,146]. Some European countries have established legal limits for histamine content in wines, namely, Germany (2 mg/L), Holland (3 mg/L), Belgium (5–6 mg/L), Finland (5 mg/L), France (8 mg/L), Austria (10 mg/L), and Switzerland (10 mg/L) [144,147]. Further, prior studies recommend that the concentration of BAs in wines should be lower than 8 mg/L [137,148,149]. In this sense, some studies have been performed in order to determine the concentration of BAs in wines, namely Ke et al. [138] analyzed commercial wine samples (n = 456) from the China region and determined their BAs content, which included 2-phenylethylamine, putrescine, cadaverine, histamine, tyramine, spermidine, and spermine. From the detected BAs, the higher levels were obtained for putrescine, followed by 2-phenylethylamine, tyramine, spermidine, cadaverine, histamine, and spermine. Moreover, the total concentration of the detected BAs ranged from 1.2 to 52 mg/L. According to a study performed by Esposito et al. [150], the levels of BAs are higher in red wines, especially for putrescine and histamine, rather than in white wines, with concentrations of an average of 7.30 and 2.45 mg/L, respectively. The levels of ABs were determined in red wines made from seven different cultivars by Konakovsky et al. [144], with the putrescine (average 19.4 mg/L, concentration range from 2.9 to 122 mg/L), histamine (7.2 mg/L, 0.5 to 26.9 mg/L), and tyramine (3.5 mg/L, 1.1 to 10.7 mg/L) being the most abundant BAs in wines. Leitão et al. [148] studied 292 commercial Portuguese wines from different regions and observed that BAs presented a content lower than 2.5 mg/L in 13% and 39% of the red and white wines, respectively. Moreover, putrescine was present in 87%, histamine in 99%, and tyramine in 96% of the red wines, whereas cadaverine was present in 73%, histamine in 99%, and putrescine in 81% of white wines.
Regarding the Madeira wines, for the concentration obtained for BAs in a study conducted by Pereira et al. [137], among the six BAs analyzed, only four were detected in the wines under study, namely, histamine, phenylethylamine, isopentylamine, and cadaverine, with values under their limit of quantification (LOQ), on average of 5 mg/L. Additionally, Pereira et al. [136] analyzed nine Madeira wines to determine the histamine, tyramine, tryptamine, phenylethylamine, isopenthylamine, and cadaverine concentrations, and verified that the values obtained were below the LODs. More recently, Perestrelo et al. [142] used Verdelho wines from different vintages in order to determine the concentration of tyramine, tryptamine, histamine, and phenylethylamine. It was observed that the highest content was obtained for phenylethylamine (0.20–3.82 mg/L), followed by histamine (0.20–3.00 mg/L), tyramine (0.08–1.24 mg/L), and tryptamine (0.08–0.14 mg/L).
3.4. Metals
The metallic elemental composition in wines has been widely studied during the last few decades, with potassium (K), calcium (Ca), iron (Fe), zinc (Zn), cadmium (Cd), magnesium (Mg), lead (Pb), mercury (Hg), and tin (Sn) being the most common metals present in wines [151]. Aluminum (Al), nickel (Ni), and manganese (Mn) demonstrated to play a role in the taste of wine [152], whereas copper (Cu), Fe, and Zn, which are essential metals, have been reported to enrich the nutrition quality of wine. In this sense, moderate wine consumption contributes to the intake of these and other essential metals (e.g., Ca, K, Mg) [152,153]. On the other hand, heavy metals (e.g., Cd, arsenic (As)), which can also be part of wines’ composition, may affect human health owing to toxicity reasons [154,155]. Hg, Pb, and Cd are indeed considered toxic, however, when consumed in excess, Cu, Ni, Zn, and Al may also be associated with a certain possibility of toxicity [156]. Thus, characterization and rigorous analytical control of metals concentration in wines is crucial to promote the wines’ quality and to maintain adequate levels of harmful heavy metals [151]. In this sense, the International Organization of Vine and Wine (OIV) has established the maximum acceptable limits for distinct metals present in wines, namely, As (0.20 mg/L), Cd (0.01 mg/L), Cu (1.00 mg/L), Pb (0.15 mg/L), and Zn (5.00 mg/L). Nevertheless, few reports have been conducted related to metals’ content in Madeira wines. Trujillo et al. [157] was the only study aimed to determine the metals’ content in Madeira wine, and the results are shown in Figure 8.
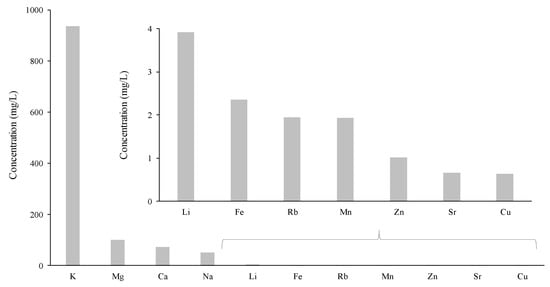
Figure 8.
Concentration of metals in Madeira wine (adapted from the work of [157]).
The Madeira wine is aged in casks, which contribute greatly to the contamination of Al, Cd, Cr, Cu, Fe, and Zn [157,158]. Trujillo et al. [157] found significant differences between Madeira wines and Azores wines, where the former presented higher concentrations of Fe, Cu, and Zn than the latter. When in excess, such elements have a negative effect on the organoleptic properties [151], influencing the wine quality. Therefore, technological processes are essential to decrease the concentration of toxic metals in wines. In addition, according to Trujillo et al. [157], the higher values of Na content in Madeira wines compared with the previous literature is the result of the influence of the sea on the vines. Nevertheless, all metals reported lower levels than the acceptable limits established by the OIV.
4. Conclusions
The current review provides an overview of the chemical composition of Madeira wines, namely on volatile and non-volatile profiles. Regarding the volatile profile, up to 100 VOCs from different origins—varietal (e.g., terpenes, C13 norisoprenoids), pre-fermentative (C6 aldehydes and alcohols), fermentative (e.g., alcohols, esters, fatty acids, carbonyl compounds), and ageing (e.g., furanic compounds, lactones, acetals, volatile phenols)—are identified. However, several authors reported that, during the ageing process, the Madeira wine loses its freshness and fruitiness odors (e.g., terpenes, esters), and other aroma descriptors arise such as caramel, dried fruit, spice, toast, and wood, suggesting the formation of VOCs by Maillard reaction (e.g., furanic compounds), and diffusion from the oak to wines (e.g., lactones, volatile phenols). Some VOCs that belong to furans, lactones, volatile phenols, and acetals, such as 2-furfural, HMF, ethyl 2-furoate, 5-methylfurfural, cis-oak-lactone and trans-oak-lactone, diethoxymethane, 1,1-diethoxyethane, 1,1-diethoxy-2-methyl-propane, 1-(1-ethoxyethoxy)-pentane, trans-dioxane, cis-dioxane, and 2-propyl-1,3-dioxolane, have been described as potential aging markers in Madeira wine, independently of the variety and type of wine. The establishment of potential age markers is crucial to detect frauds and to ensure the wine authenticity, as the economic value of Madeira wine is greatly connected with its age. On the other hand, the results related to non-volatile composition showed that Madeira wines can have a positive effect on human health, based on the estimation values for antioxidant capacity and polyphenols’ concentration, such as trans-resveratrol, myricetin, catechin, and epicatechin, among others. In addition, the levels of biogenic amines and metals were below the legal limits set by some European Union countries (<8 mg/L) and OIV, respectively.
Author Contributions
C.S. wrote the section related to non-volatile composition, namely, polyphenols, aminoacids, biogenic amines, and organic acids; C.G. wrote the metals sub-section; M.C. wrote the introduction section; J.S.C., original draft preparation, review and editing; R.P. wrote the volatile composition section, review and editing of the review. All authors have read and agree to the published version of the manuscript.
Funding
This work was supported by FCT-Fundação para a Ciência e a Tecnologia (projects PEst-OE/QUI/UI0674/2019, CQM, Portuguese Government funds); through Madeira 14-20 Program, project PROEQUIPRAM—Reforço do Investimento em Equipamentos e Infraestruturas Científicas na RAM (M1420-01-0145-FEDER-000008); and by Agência Regional para o Desenvolvimento da Investigação, Tecnologia e Inovação (ARDITI) through the support granted under the M1420-01-0145-FEDER-000005—Centro de Química da Madeira—CQM+ (Madeira 14-20).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Secretaria Regional do Ambiente e Recursos Naturais. Jornal Oficial. Portaria n. 40/2015 Região Autónoma da Madeira. Available online: https://www.ivv.gov.pt/np4/529/?newsId=8410&fileName=Portaria_40_2015_terras_madeirenses.pdf (accessed on 15 December 2019).
- Secretaria Regional do Ambiente e Recursos Naturais. Jornal Oficial. Portaria n. 39/2015 Região Autónoma da Madeira. Available online: https://www.ivv.gov.pt/np4/529/?newsId=8409&fileName=Portaria_39_2015_madeira.pdf (accessed on 15 December 2020).
- BKO, F. A Região Demarcada da Madeira—Características e património vitícola. Available online: https://docplayer.com.br/48548205-A-regiao-demaracada-da-madeira-caracteristicas-e-patrimonio-viticola.html (accessed on 15 December 2019).
- Perestrelo, R.; Silva, C.; Câmara, J.S. Madeira wine volatile profile. A platform to establish madeira wine aroma descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Pereira, J.; Câmara, J.S. Wines: Madeira, Port and Sherry fortified wines—The Sui Generis and notable peculiarities. Major differences and chemical patterns. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 534–555. ISBN 9780123849533. [Google Scholar]
- Perestrelo, R.; Albuquerque, F.; Rocha, S.M.; Câmara, J.S. Distinctive characteristics of madeira wine regarding its traditional winemaking and modern analytical methodologies. Adv. Food Nutr. Res. 2011, 63, 207–249. [Google Scholar]
- Perestrelo, R.; Câmara, J.S.; Rocha, S.M. Impact of Winemaking Process in Madeira Wine Composition: From Aging Markers to Ethyl Carbamate (a Contaminant); Nova Science Publishers: New York, NY, USA, 2011; ISBN 9781614706359. [Google Scholar]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Analysis of potent odour-active volatile thiols in foods and beverages with a focus on wine. Molecules 2019, 24, 2472. [Google Scholar] [CrossRef]
- Mihnea, M.; González-SanJosé, M.L.; Ortega-Heras, M.; Pérez-Magariño, S. A comparative study of the volatile content of Mencía wines obtained using different pre-fermentative maceration techniques. LWT-Food Sci. Technol. 2015, 64, 32–41. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Aroma compounds in wine. In Grape and Wine Biotechnology; InTech: London, UK, 2016. [Google Scholar]
- Mestres, M.; Busto, O.; Guasch, J.; Guasch, G. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; De La Hoz, K.S.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of vine foliar treatments on the varietal aroma of Monastrell wines. Food Chem. 2014, 163, 258–266. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, G.J.; Wang, X.J.; Kong, C.L.; Liu, J.; Tao, Y.S. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chem. 2019, 284, 155–161. [Google Scholar] [CrossRef]
- Darriet, P.; Thibon, C.; Dubourdieu, D. Aroma and aroma precursors in grape berry. In The Biochemistry of the Grape Berry; Bentham Science Publishers Ltd.: United Arab Emirates, 2012; pp. 111–136. ISBN 9781608055401. [Google Scholar]
- Coelho, E.; Rocha, S.M.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Screening of variety- and pre-fermentation-related volatile compounds during ripening of white grapes to define their evolution profile. Anal. Chim. Acta 2007, 597, 257–264. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M. Carotenoid breakdown products the-norisoprenoids-in wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Establishment of the varietal profile of Vitis vinifera L. grape varieties from different geographical regions based on HS-SPME/GC-qMS combined with chemometric tools. Microchem. J. 2014, 116, 107–117. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Câmara, J.S. Unraveling Vitis vinifera L. grape maturity markers based on integration of terpenic pattern and chemometric methods. Microchem. J. 2018, 142, 367–376. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellín, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Wedler, H.B.; Pemberton, R.P.; Tantillo, D.J. Carbocations and the complex flavor and bouquet of wine: Mechanistic aspects of terpene biosynthesis in wine grapes. Molecules 2015, 20, 10781–10792. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene content of wine from the aromatic grape variety “Irsai Oliver” (Vitis vinifera L.) depends on maceration time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Santiago, A.C.; Munoz, R.; Garcia, R.G. Molecular Wine Microbiology; Elsevier Inc.: London, UK, 2011; ISBN 9780123750211. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, the Chemistry of Wine: Stabilization and Treatments, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 2, ISBN 9780470010396. [Google Scholar]
- Chisholm, M.G.; Guiher, L.A.; Zaczkiewicz, S.M. Aroma characteristics of aged Vidal blanc wine. Am. J. Enol. Vitic. 1995, 46, 56–62. [Google Scholar]
- Campo, E.; Ferreira, V.; Escudero, A.; Marqués, J.C.; Cacho, J. Quantitative gas chromatography-olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal. Chim. Acta 2006, 563, 180–187. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Development of headspace solid-phase microextraction-gas chromatography–mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Classification of Boal, Malvazia, Sercial and Verdelho wines based on terpenoid patterns. Food Chem. 2007, 101, 475–484. [Google Scholar] [CrossRef][Green Version]
- Coelho, E.; Rocha, S.M.; Delgadillo, I.; Coimbra, M.A. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Câmara, J.S.; Herbert, P.; Marques, J.C.; Alves, M.A. Varietal flavour compounds of four grape varieties producing Madeira wines. Anal. Chim. Acta 2004, 513, 203–207. [Google Scholar] [CrossRef]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Acree, T.; Arn, H. Flavornet Home Page. Available online: http://www.flavornet.org/ (accessed on 14 February 2019).
- Leffingwell, D.; Leffingwell, J.C. Odor Detection Thresholds of GRAS Flavor Chemicals. Available online: http://www.leffingwell.com (accessed on 25 November 2019).
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-active compounds of American, French, Hungarian and Russian oak woods, studied by GC–MS and GC–O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 1–20. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of Chinese rice wine by gas chromatography–olfactometry, chemical quantitative analysis, and aroma reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, J.; Qian, M.; Li, H. Characterization of the key aroma compounds in Chinese Syrah wine by gas chromatography-olfactometry-mass spectrometry and aroma reconstitution studies. Molecules 2017, 22, 1045. [Google Scholar] [CrossRef]
- Mathieu, S.; Terrier, N.; Procureur, J.; Bigey, F.; Günata, Z. A carotenoid cleavage dioxygenase from Vitis vinifera L.: Functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J. Exp. Bot. 2005, 56, 2721–2731. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Cacho, J.; Ferreira, V. The chemical characterization of the aroma of dessert and sparkling white wines (Pedro Ximénez, Fino, Sauternes, and Cava) by gas chromatography-olfactometry and chemical quantitative analysis. J. Agric. Food Chem. 2008, 56, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and Non-Riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Boss, P.K. Two O-methyltransferases involved in the biosynthesis of methoxypyrazines: Grape-derived aroma compounds important to wine flavour. Plant Mol. Biol. 2010, 74, 77–89. [Google Scholar] [CrossRef]
- Sala, C.; Mestres, M.; Martí, M.P.; Busto, O.; Guasch, J. Headspace solid-phase microextraction analysis of 3-alkyl-2-methoxypyrazines in wines. J. Chromatogr. A 2002, 953, 1–6. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Tominaga, T. Polyfunctional thiol compounds. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; pp. 275–293. ISBN 9780387741161. [Google Scholar]
- Pereira, V.; Cacho, J.; Marques, J.C. Volatile profile of Madeira wines submitted to traditional accelerated ageing. Food Chem. 2014, 162, 122–134. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Impact of storage time and temperature on volatomic signature of Tinta Negra wines by LLME/GC-ITMS. Food Res. Int. 2018, 109, 99–111. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Mozzon, M.; Savini, S.; Boselli, E.; Thorngate, J.H. The herbaceous character of wines. Ital. J. Food Sci. 2016, 28, 190–207. [Google Scholar]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; Du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar]
- Ferrandino, A.; Carlomagno, A.; Baldassarre, S.; Schubert, A. Varietal and pre-fermentative volatiles during ripening of Vitis vinifera cv Nebbiolo berries from three growing areas. Food Chem. 2012, 135, 2340–2349. [Google Scholar] [CrossRef]
- Pereira, A.C.; Reis, M.S.; Saraiva, P.M.; Marques, J.C. Aroma ageing trends in GC/MS profiles of liqueur wines. Anal. Chim. Acta 2010, 659, 93–101. [Google Scholar] [CrossRef]
- Pereira, A.C.; Reis, M.S.; Saraiva, P.M.; Marques, J.C. Madeira wine ageing prediction based on different analytical techniques: UV–vis, GC-MS, HPLC-DAD. Chemom. Intell. Lab. Syst. 2011, 105, 43–55. [Google Scholar] [CrossRef]
- Pereira, A.C.; Reis, M.S.; Saraiva, P.M.; Marques, J.C. Analysis and assessment of Madeira wine ageing over an extended time period through GC-MS and chemometric analysis. Anal. Chim. Acta 2010, 660, 8–21. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Multivariate analysis for the classification and differentiation of Madeira wines according to main grape varieties. Talanta 2006, 68, 1512–1521. [Google Scholar] [CrossRef][Green Version]
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Xu, B.W.; Chen, B.Y.; Zhao, W.Q.; Xue, C.H. Ultrasound as an effective technique to reduce higher alcohols of wines and its influencing mechanism investigation by employing a model wine. Ultrason. Sonochemistry 2020, 61, 104813–104823. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Higher alcohols concentration and its relation with the biological aging evolution. Eur. Food Res. Technol. 2006, 222, 629–635. [Google Scholar] [CrossRef]
- Pisarnitskii, A.F. Formation of wine aroma: Tones and imperfections caused by minor components (review). Appl. Biochem. Microbiol. 2001, 37, 552–560. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Avizcuri, J.M.; Ballester, J.; Fernández-Zurbano, P.; Ferreira, V.; Peyron, D.; Valentin, D. Sensory-active compounds influencing wine experts’ and consumers’ perception of red wine intrinsic quality. LWT-Food Sci. Technol. 2015, 60, 400–411. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Perestrelo, R.; Rodriguez, E.; Câmara, J.S.J.S. Impact of storage time and temperature on furanic derivatives formation in wines using microextraction by packed sorbent tandem with ultrahigh pressure liquid chromatography. LWT-Food Sci. Technol. 2017, 76, 40–47. [Google Scholar] [CrossRef]
- Aznar, M.; Arroyo, T. Analysis of wine volatile profile by purge-and-trap-gas chromatography-mass spectrometry. Application to the analysis of red and white wines from different Spanish regions. J. Chromatogr. A 2007, 1165, 151–157. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Liu, P.-T.; Duan, C.-Q.; Yan, G.-L. Comparing the effects of different unsaturated fatty acids on fermentation performance of Saccharomyces cerevisiae and aroma compounds during red wine fermentation. Molecules 2019, 24, 538. [Google Scholar] [CrossRef]
- König, H.; Unden, G.; Fröhlich, J. Biology of Microorganisms on Grapes, in Must and in Wine; Springer: New York, USA, 2009; ISBN 9783540854623. [Google Scholar]
- Louw, L.; Tredoux, A.G.J.; van Rensburg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H.H. Fermentation-derived aroma compounds in varietal young wines from South Africa. South Afr. J. Enol. Vitic. 2010, 31, 213–225. [Google Scholar] [CrossRef][Green Version]
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M.T. Food aroma compounds. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Elsevier Inc.: London, UK, 2017; pp. 297–334. ISBN 9780128052570. [Google Scholar]
- Jackson, R.S. Chemical constituents of grapes and wine. In Wine Science; Elsevier: London, UK, 2000; pp. 232–280. [Google Scholar]
- Clark, S.; Winter, C.K. Diacetyl in foods: A review of safety and sensory characteristics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 634–643. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in volatile composition of Madeira wines during their oxidative ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef]
- Le Menn, N.; van Leeuwen, C.; Picard, M.; Riquier, L.; de Revel, G.; Marchand, S. Effect of vine water and nitrogen status, as well as temperature, on some aroma compounds of aged red Bordeaux wines. J. Agric. Food Chem. 2019, 67, 7098–7109. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, sesquiterpenes and terpenoids content of Valpolicella wines during aging: Investigating aroma potential in relationship to evolution of tobacco and balsamic aroma in aged wine. Front. Chem. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.; Câmara, J.S.; Rocha, S.M. In-depth search focused on furans, lactones, volatile phenols, and acetals as potentialage markers of Madeira wines by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction. J. Agric. Food Chem. 2011, 59, 3186–3204. [Google Scholar] [CrossRef]
- Koussissi, E.; Dourtoglou, V.G.; Ageloussis, G.; Paraskevopoulos, Y.; Dourtoglou, T.; Paterson, A.; Chatzilazarou, A. Influence of toasting of oak chips on red wine maturation from sensory and gas chromatographic headspace analysis. Food Chem. 2009, 114, 1503–1509. [Google Scholar] [CrossRef]
- Câmara, J.S.; Marques, J.C.; Alves, A.; Ferreira, A.C.S. Heterocyclic acetals in Madeira wines. Anal. Bioanal. Chem. 2003, 375, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.O.; Guedes De Pinho, P.; Machado, B.P.; Hogg, T.; Marques, J.C.; Câmara, J.S.; Albuquerque, F.; Silva Ferreira, A.C.; Oliveira, E.; Silva, H.; et al. Impact of forced-aging process on Madeira wine flavor. J. Agric. Food Chem. 2008, 56, 11989–11996. [Google Scholar] [CrossRef]
- Alañón, M.E.; Rubio, H.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Monosaccharide anhydrides, new markers of toasted oak wood used for ageing wines and distillates. Food Chem. 2010, 119, 505–512. [Google Scholar] [CrossRef]
- Ortu, E.; Caboni, P. Levels of 5-hydroxymethylfurfural, furfural, 2-furoic acid in sapa syrup, Marsala wine and bakery products. Int. J. Food Prop. 2017, 20, S2543–S2551. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study of the formation mechanisms of some volatile compounds during the aging of sweet fortified wines. J. Agric. Food Chem. 1999, 47, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Albuquerque, F.M.; Ferreira, A.C.; Cacho, J.; Marques, J.C. Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Res. Int. 2011, 44, 71–76. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Câmara, J.S. Quantification of furanic derivatives in fortified wines by a highly sensitive and ultrafast analytical strategy based on digitally controlled microextraction by packed sorbent combined with ultrahigh pressure liquid chromatography. J. Chromatogr. A 2015, 1381, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Marques, J.C.; Alves, M.A.; Silva Ferreira, A.C. 3-Hydroxy-4,5-dimethyl-2(5H)-furanone levels in fortified Madeira wines: Relationship to sugar content. J. Agric. Food Chem. 2004, 52, 6765–6769. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Baumes, R.; Bayonove, C.; Razungles, A. Volatile compounds involved in the aroma of sweet fortified wines (Vins Doux Naturels) from Grenache Noir. J. Agric. Food Chem. 1998, 46, 3230–3237. [Google Scholar] [CrossRef]
- Pérez-Olivero, S.J.; Pérez-Pont, M.L.; Conde, J.E.; Pérez-Trujillo, J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2014, 2014, 863019. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Goñi, D.T.; Azpilicueta, C.A. Accumulation of volatile compounds during ageing of two red wines with different composition. J. Food Eng. 2004, 65, 349–356. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-Huerta, C.; Herrera, P.; González-Sanjosé, M.L. Changes in wine volatile compounds of varietal wines during ageing in wood barrels. Anal. Chim. Acta 2004, 513, 341–350. [Google Scholar] [CrossRef]
- Guchu, E.; Díaz-Maroto, M.C.; Díaz-Maroto, I.J.; Vila-Lameiro, P.; Pérez-Coello, M.S. Influence of the species and geographical location on volatile composition of spanish oak wood (Quercus petraea Liebl. and Quercus robur L.). J. Agric. Food Chem. 2006, 54, 3062–3066. [Google Scholar] [CrossRef]
- Gaspar, J.M.; Freitas, A.I.; Zhao, Q.; Leça, J.M.; Pereira, V.; Marques, J.C. Is sotolon relevant to the aroma of madeira wine blends? Biomolecules 2019, 9, 720. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Ortega-Heras, M.; Pérez-Magariño, S.; González-Huerta, C. Volatile compounds of red wines macerated with Spanish, American, and French oak chips. J. Agric. Food Chem. 2009, 57, 6383–6391. [Google Scholar] [CrossRef] [PubMed]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Martorell, N.; Martí, M.P.; Mestres, M.; Busto, O.; Guasch, J. Determination of 4-ethylguaiacol and 4-ethylphenol in red wines using headspace-solid-phase microextraction-gas chromatography. J. Chromatogr. A 2002, 975, 349–354. [Google Scholar] [CrossRef]
- Kheir, J.; Salameh, D.; Strehaiano, P.; Brandam, C.; Lteif, R. Impact of volatile phenols and their precursors on wine quality and control measures of Brettanomyces/Dekkera yeasts. Eur. Food Res. Technol. 2013, 237, 655–671. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Vilela, A.; Cosme, F.; Nunes, F.M. 4-Ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: Microbial formation, prevention, remediation and overview of analytical approaches. Crit. Rev. Food Sci. Nutr. 2019, 59, 1367–1391. [Google Scholar] [CrossRef]
- Perez-Prieto, L.J.; De la Hera-Orts, M.L.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Oak-matured wines: Influence of the characteristics of the barrel on wine colour and sensory characteristics. J. Sci. Food Agric. 2003, 83, 1445–1450. [Google Scholar] [CrossRef]
- Peterson, A.L.; Gambuti, A.; Waterhouse, A.L. Rapid analysis of heterocyclic acetals in wine by stable isotope dilution gas chromatography-mass spectrometry. Tetrahedron 2015, 71, 3032–3038. [Google Scholar] [CrossRef]
- Da Silva Ferreira, A.C.; Barbe, J.-C.; Bertrand, A. Heterocyclic acetals from glycerol and acetaldehyde in Port wines: Evolution with aging. J. Agric. Food Chem. 2002, 50, 2560–2564. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Campo, E.; Avizcuri, J.M.; Valentin, D.; Fernández-Zurbano, P.; Ferreira, V. Contribution of non-volatile and aroma fractions to in-mouth sensory properties of red wines: Wine reconstitution strategies and sensory sorting task. Anal. Chim. Acta 2012, 732, 64–72. [Google Scholar] [CrossRef]
- Breslin, P.A.S. Human gustation and flavor. In Food Flavors and Chemistry: Advances of the New Millennium; Royal Society of Chemistry: London, UK, 2001; pp. 33–64. [Google Scholar]
- Huang, X.Y.; Jiang, Z.T.; Tan, J.; Li, R. Geographical origin traceability of red wines based on chemometric classification via organic acid profiles. J. Food Qual. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, V.-C.; Paun, N.; Ionete, R.-E. The evolution of polyphenols from grapes to wines. In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; InTech: London, UK, 2017; pp. 119–141. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Gonçalves, J.; Mendes, B.; Silva, C.L.; Câmara, J.S. Development of a novel microextraction by packed sorbent-based approach followed by ultrahigh pressure liquid chromatography as a powerful technique for quantification phenolic constituents of biological interest in wines. J. Chromatogr. A 2012, 1229, 13–23. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. J. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol extracts from red wine and grapevine: Potential effects on cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.; Hernández, M.; Martín-Alvarez, P.; Polo, M. Study of low molecular weight phenolic compounds during the aging of sparkling wines manufactured with red and white grape varieties. J. Agric. Food Chem. 2003, 51, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Silva, C.L.; Castilho, P.C.; Câmara, J.S. An attractive, sensitive and high-throughput strategy based on microextraction by packed sorbent followed by UHPLC-PDA analysis for quantification of hydroxybenzoic and hydroxycinnamic acids in wines. Microchem. J. 2013, 106, 129–138. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC analysis of phenols in Negroamaro and Primitivo red wines from Salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A high-throughput UHPLC-QqQ-MS method for polyphenol profiling in rosé wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef]
- Flamini, R. Recent applications of mass spectrometry in the study of grape and wine polyphenols. Int. Sch. Res. Not. 2012, 2013, 1–45. [Google Scholar] [CrossRef]
- Bravo, M.N.; Silva, S.; Coelho, A.V.; Vilas Boas, L.; Bronze, M.R. Analysis of phenolic compounds in Muscatel wines produced in Portugal. Anal. Chim. Acta 2006, 563, 84–92. [Google Scholar] [CrossRef]
- Tarola, A.M.; Milano, F.; Giannetti, V. Simultaneous determination of phenolic compounds in red wines by HPLC-UV. J. Anal. Lett. 2007, 40, 2433–2445. [Google Scholar] [CrossRef]
- García-Falcón, M.S.; Pérez-Lamela, C.; Martínez-Carballo, E.; Simal-Gándara, J. Determination of phenolic compounds in wines: Influence of bottle storage of young red wines on their evolution. Food Chem. 2007, 105, 248–259. [Google Scholar] [CrossRef]
- Silva, C.L.; Pereira, J.; Wouter, V.G.; Giró, C.; Câmara, J.S. A fast method using a new hydrophilic-lipophilic balanced sorbent in combination with ultra-high performance liquid chromatography for quantification of significant bioactive metabolites in wines. Talanta 2011, 86, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Gonçalves, J.L.; Câmara, J.S. A sensitive microextraction by packed sorbent-based methodology combined with ultra-high pressure liquid chromatography as a powerful technique for analysis of biologically active flavonols in wines. Anal. Chim. Acta 2012, 739, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rudnitskaya, A.; Rocha, S.M.; Legin, A.; Pereira, V.; Marques, J.C. Evaluation of the feasibility of the electronic tongue as a rapid analytical tool for wine age prediction and quantification of the organic acids and phenolic compounds. The case-study of Madeira wine. Anal. Chim. Acta 2010, 662, 82–89. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Paixão, N.; Pereira, V.; Marques, J.C.; Câmara, J.S. Quantification of polyphenols with potential antioxidant properties in wines using reverse phase HPLC. J. Sep. Sci. 2008, 31, 2189–2198. [Google Scholar] [CrossRef]
- Gonçalves, J.; Câmara, J.S. New method for determination of (E)-resveratrol in wine based on microextraction using packed sorbent and ultra-performance liquid chromatography. J. Sep. Sci. 2011, 34, 2376–2384. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.; Cacho, J.; Marques, J. Polyphenols, antioxidant potential and color of fortified wines during accelerated ageing: The Madeira wine case study. Molecules 2013, 18, 2997–3017. [Google Scholar] [CrossRef]
- Lena Do Nascimento Silva, F.; Schmidt, E.M.; Messias, C.L.; Eberlin, M.N.; Helena Frankland Sawaya, A.C. Quantitation of organic acids in wine and grapes by direct infusion electrospray ionization mass spectrometry. Anal. Methods 2015, 7, 53–62. [Google Scholar] [CrossRef]
- Regmi, U.; Palma, M.; Barroso, C.G. Direct determination of organic acids in wine and wine-derived products by Fourier transform infrared (FT-IR) spectroscopy and chemometric techniques. Anal. Chim. Acta 2012, 732, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC-Trends Anal. Chem. 2019, 120, 115630–115644. [Google Scholar] [CrossRef]
- Chidi, B.S.; Mafata, M.; Notshokovu, N.Z.; van Jaarsveld, F. Impact of grape temperature at pressing on organic acids and oenological characteristics of méthode cap classique wines. South Afr. J. Enol. Vitic. 2018, 39, 106–115. [Google Scholar] [CrossRef]
- Castin, A.; Penã, R.M.; Herrero, C.; Garcıá-Martı, S.; El Sabio, A.X. Analysis of organic acids in wine by capillary electrophoresis with direct UV detection. J. Food Compos. Anal. 2002, 15, 319–331. [Google Scholar] [CrossRef]
- Mihaljevic Žulj, M.; Puhelek, I.; Jagatic Korenika, A.M.; Maslov Bandic, L.; Pavlesic, T.; Jeromel, A. Organic Acid Composition in Croatian Predicate Wines. Agric. Conspec. Sci. 2015, 80, 113–117. [Google Scholar]
- Zotou, A.; Loukou, Z.; Karava, O. Method development for the determination of seven organic acids in wines by reversed-phase high performance liquid chromatography. Chromatographia 2004, 60, 39–44. [Google Scholar] [CrossRef]
- Pereira, V.; Câmara, J.S.; Cacho, J.; Marques, J.C. HPLC-DAD methodology for the quantification of organic acids, furans and polyphenols by direct injection of wine samples. J. Sep. Sci. 2010, 33, 1204–1215. [Google Scholar] [CrossRef]
- Pereira, V.; Pontes, M.; Câmara, J.S.; Marques, J.C. Simultaneous analysis of free amino acids and biogenic amines in honey and wine samples using in loop orthophthalaldeyde derivatization procedure. J. Chromatogr. A 2008, 1189, 435–443. [Google Scholar] [CrossRef]
- Pereira, V.; Pereira, A.C.; Pérez Trujillo, J.P.; Cacho, J.; Marques, J.C. Amino acids and biogenic amines evolution during the Estufagem of fortified wines. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Bouloumpasi, E.; Tsarchopoulos, C.; Biliaderis, C.G. Primary amino acid profiles of Greek white wines and their use in classification according to variety, origin and vintage. Food Chem. 2003, 80, 261–273. [Google Scholar] [CrossRef]
- Fiechter, G.; Mayer, H.K. UPLC analysis of free amino acids in wines: Profiling of on-lees aged wines. J. Chromatogr. B 2011, 879, 1361–1366. [Google Scholar] [CrossRef]
- Herbert, P.; Cabrita, M.J.; Ratola, N.; Laureano, O.; Alves, A. Free amino acids and biogenic amines in wines and musts from the Alentejo region. Evolution of amines during alcoholic fermentation and relationship with variety, sub-region and vintage. J. Food Eng. 2005, 66, 315–322. [Google Scholar] [CrossRef]
- Perestrelo, R.; Bordiga, M.; Locatelli, M.; Silva, C.; Câmara, J.S. Polyphenols, biogenic amines and amino acids patterns in Verdelho wines according to vintage. Microchem. J.
- Nalazek-Rudnicka, K.; Wasik, A. Development and validation of an LC–MS/MS method for the determination of biogenic amines in wines and beers. Monatsh. Chem. 2017, 148, 1685–1696. [Google Scholar] [CrossRef]
- Konakovsky, V.; Focke, M.; Hoffmann-Sommergruber, K.; Schmid, R.; Scheiner, O.; Moser, P.; Jarisch, R.; Hemmer, W. Levels of histamine and other biogenic amines in high-quality red wines. Food Addit. Contam. Part A 2011, 28, 408–416. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.; Alvarez, M. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Smi, A.Y.; Du Toit, M. Biogenic amines and the winemaking process. In Managing Wine Quality; Woodhead Publishing: London, UK, 2010; pp. 494–522. [Google Scholar]
- Leitão, M.C.; Marques, A.P.; San Romão, M.V. A survey of biogenic amines in commercial Portuguese wines. Food Control 2005, 16, 199–204. [Google Scholar] [CrossRef]
- Miranda, A.; Pereira, V.; Leça, J.M.; Marques, J.C. Analytical methodologies for the determination of biogenic amines in wines: An overview of the recent trends. J. Anal. Bioanal. Sep. Tech. 2017, 2, 52–57. [Google Scholar]
- Esposito, F.; Montuori, P.; Schettino, M.; Velotto, S.; Stasi, T.; Romano, R.; Cirillo, T. Level of biogenic amines in red and white wines, dietary exposure, and histamine-mediated symptoms upon wine ingestion. Molecules 2019, 24, 3629. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Rutkowska, M.; Cieślik, B.; Tyburcy, A.; Namieśnik, J. Determination of selected metals in fruit wines by spectroscopic techniques. J. Anal. Methods Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tariba, B. Metals in wine—Impact on wine quality and health outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Dalipi, R.; Borgese, L.; Zacco, A.; Tsuji, K.; Sangiorgi, E.; Piro, R.; Bontempi, E.; Depero, L.E. Determination of trace elements in Italian wines by means of total reflection X-ray fluorescence spectroscopy. Int. J. Environ. Anal. Chem. 2015, 95, 1208–1218. [Google Scholar] [CrossRef]
- Seeger, T.S.; Rosa, F.C.; Bizzi, C.A.; Dressler, V.L.; Flores, E.M.M.; Duarte, F.A. Feasibility of dispersive liquid-liquid microextraction for extraction and preconcentration of Cu and Fe in red and white wine and determination by flame atomic absorption spectrometry. Spectrochim. Acta-Part B At. Spectrosc. 2015, 105, 136–140. [Google Scholar] [CrossRef]
- Bocková, J.; Tian, Y.; Yin, H.; Delepine-Gilon, N.; Chen, Y.; Veis, P.; Yu, J. Determination of metal elements in wine using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2017, 71, 1750–1759. [Google Scholar] [CrossRef]
- Blesic, M.; Drmac, M.; Batinic, K.; Spaho, N.; Smajic Murtic, M.; Zele, M. Levels of selected metals in wines from different Herzegovinian viticultural localities. Croat. J. Food Sci. Technol. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Pérez Trujillo, J.P.; Conde, J.E.; Pérez Pont, M.L.; Câmara, J.; Marques, J.C. Content in metallic ions of wines from the Madeira and Azores archipelagos. Food Chem. 2011, 124, 533–537. [Google Scholar] [CrossRef]
- Pohl, P. What do metals tell us about wine? TrAC-Trends Anal. Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).