Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes Using Two Analytical Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- -
- No maceration and free run;
- -
- No maceration and pressure extraction;
- -
- 2-hr maceration and free run;
- -
- 2-hr maceration and pressure extraction.
2.2. Juice Quality Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolics under Maceration and Press Treatments
3.2. Phenolic Analysis Method Comparison
3.3. Standard Analysis of Apple Juice
3.4. Implication of Apple Variety Choices on Cider Style
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jolicoeur, C. The New Cider Maker’s Handbook; Chelsea Green Publishing: White River Junction, VT, USA, 2013; p. 337. [Google Scholar]
- Girschik, L.; Jones, J.E.; Kerslake, F.L.; Robertson, M.; Dambergs, R.G.; Swarts, N.D. Apple variety and maturity profiling of base ciders using UV spectroscopy. Food Chem. 2017, 228, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M. Apple flavor. Hortic. Rev. 1994, 16, 197–234. [Google Scholar]
- Nogueira, A.; Guyot, S.; Marnet, N.; Lequéré, J.M.; Drilleau, J.F.; Wosiacki, G. Effect of alcoholic fermentation in the content of phenolic compounds in cider processing. Braz. Arch. Biol. Technol. 2008, 51, 1025–1032. [Google Scholar] [CrossRef]
- Johansen, K. Cider Production in England and France and Denmark? Brygmesteren 2000, 6, 2–15. [Google Scholar]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Barranco, A.; Abad, B.; Berrueta, L.A.; Gallo, B.; Vicente, F. Polyphenolic profiles of Basque cider apple cultivars and their technological properties. J. Agric. Food Chem. 2004, 52, 2938–2952. [Google Scholar] [CrossRef] [PubMed]
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.F. Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853. [Google Scholar] [CrossRef] [PubMed]
- Eder, R. Wine Presses; University of Life Sciences: Vienna, Austria, 2010. [Google Scholar]
- Cliffe, S.; Fawer, M.S.; Maier, G.; Takata, K.; Ritter, G. Enzyme assays for the phenolic content of natural juices. J. Agric. Food Chem. 1994, 42, 1824–1828. [Google Scholar] [CrossRef]
- Lea, A. Tannin and colour in English cider apples. Flüss. Obst 1984, 8, 1–5. [Google Scholar]
- Singleton, V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: Observations and practical implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar]
- González-Neves, G.; Favre, G.; Gil, G.; Ferrer, M.; Charamelo, D. Effect of cold pre-fermentative maceration on the color and composition of young red wines cv. Tannat. J. Food Sci. Technol. 2015, 52, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Romero-Cascales, I.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The maceration process during winemaking extraction of anthocyanins from grape skins into wine. Eur. Food Res. Technol. 2005, 221, 163–167. [Google Scholar] [CrossRef]
- Poorter, H.; de Jong-Van Berkel, Y. Chemical Determination of Phenolic Compounds. Available online: http://prometheuswiki.org/tiki-pagehistory.php?page=Chemical%20determination%20of%20phenolic%20compounds&preview=9 (accessed on 15 June 2016).
- Kerslake, F.; Longo, R.; Dambergs, R. Discrimination of juice press fractions for sparkling base wines by a UV-Vis spectral phenolic fingerprint and chemometrics. Beverages 2018, 4, 45. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Carew, A.L.; Gill, W.; Close, D.C.; Dambergs, R.G. Microwave maceration with early press off improves phenolics and fermentation kinetics in Pinot Noir. Am. J. Enol. Vitic. 2014, 2014, 13089. [Google Scholar]
- Iland, P. Chemical Analyses of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions Pty Ltd.: Athelstone, Australia, 2000. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin—Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2017, 10, 792–801. [Google Scholar] [CrossRef]
- Pérez-Ilzarbe, J.; Hernández, T.; Estrella, I. Phenolic compounds in apples: Varietal differences. Z. Lebensm. Unters. Forsch. 1991, 192, 551–554. [Google Scholar] [CrossRef]
- Suárez, B.; Picinelli, A.; Mangas, J. Solid-phase extraction and high-performance liquid chromatographic determination of polyphenols in apple musts and ciders. J. Chromatogr. A 1996, 727, 203–209. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; de Mello, J. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kerslake, F.; Jones, J.; Close, D.; Dambergs, R. Bunch exposure effects on the quality of pinot noir and chardonnay fruit and base wines for cool climate sparkiling wine production. In Proceedings of the 18th Symposium of the Group of International Experts of Vitivinicultural Systems for Cooperation (GiESCO 2013), Porto, Portugal, 7–11 July 2013; pp. 471–477. [Google Scholar]
- Somers, T.C.; Ziemelis, G. Spectral evaluation of total phenolic components in Vitis vinifera: Grapes and wines. J. Sci. Food Agric. 1985, 36, 1275–1284. [Google Scholar] [CrossRef]
- Mihalev, K.; Schieber, A.; Mollov, P.; Carle, R. Effect of mash maceration on the polyphenolic content and visual quality attributes of cloudy apple juice. J. Agric. Food Chem. 2004, 52, 7306–7310. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; Skrede, G.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage Conditions. J. Agric. Food Chem. 2002, 50, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.E.; Barrett, D.M. Thermal, high pressure, and electric field processing effects on plant cell membrane integrity and relevance to fruit and vegetable quality. J. Food Sci. 2010, 75, R121–R130. [Google Scholar] [CrossRef] [PubMed]
- Volschenk, H.; Van Vuuren, H.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Kahle, K.; Kraus, M.; Richling, E. Polyphenol profiles of apple juices. Mol. Nutr. Food Res. 2005, 49, 797–806. [Google Scholar] [CrossRef]
- Lea, A.G. Craft Cider Making, 3rd ed.; The Crowood Press LTD: Marlborough, UK, 2015. [Google Scholar]
- Spanos, G.A.; Wrolstad, R.E. Influence of variety, maturity, processing and storage on the phenolic composition of pear juice. J. Agric. Food Chem. 1990, 38, 817–824. [Google Scholar] [CrossRef]
- Alberti, A.; dos Santos, T.P.M.; Zielinski, A.A.F.; dos Santos, C.M.E.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Plavša, T.; Jurinjak, N.; Antunović, D.; Peršurić, Đ.; Ganić, K.K. The influence of skin maceration time on the phenolic composition and antioxidant activity of red wine teran (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 50, 152–158. [Google Scholar]
- Guttart, A.; Hernandez-Orte, P.; Cacho, J. Effects of maceration on the amino acid content of Chardonnay musts and wines. Vitis Geilweilerhof 1997, 36, 43–48. [Google Scholar]
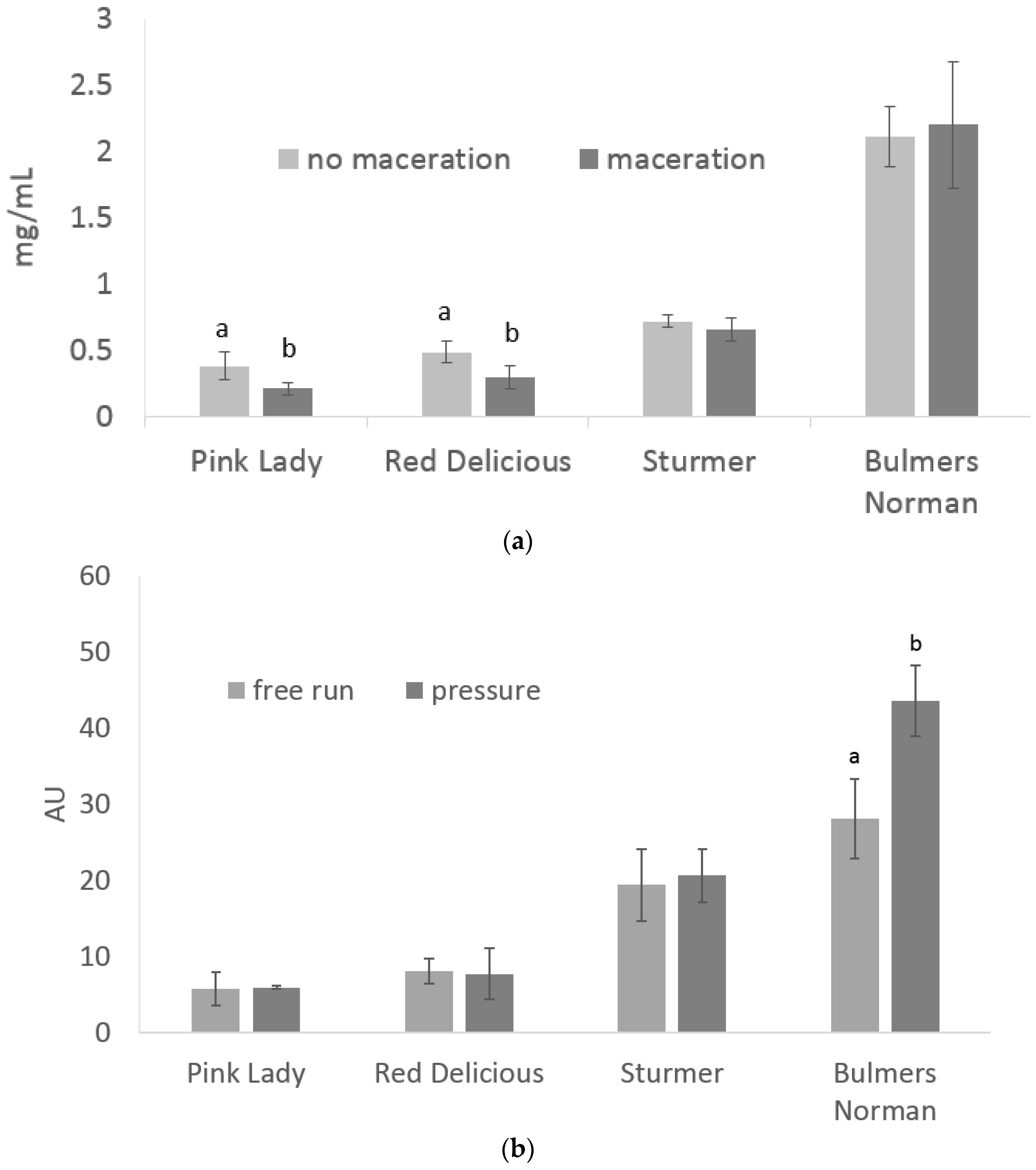
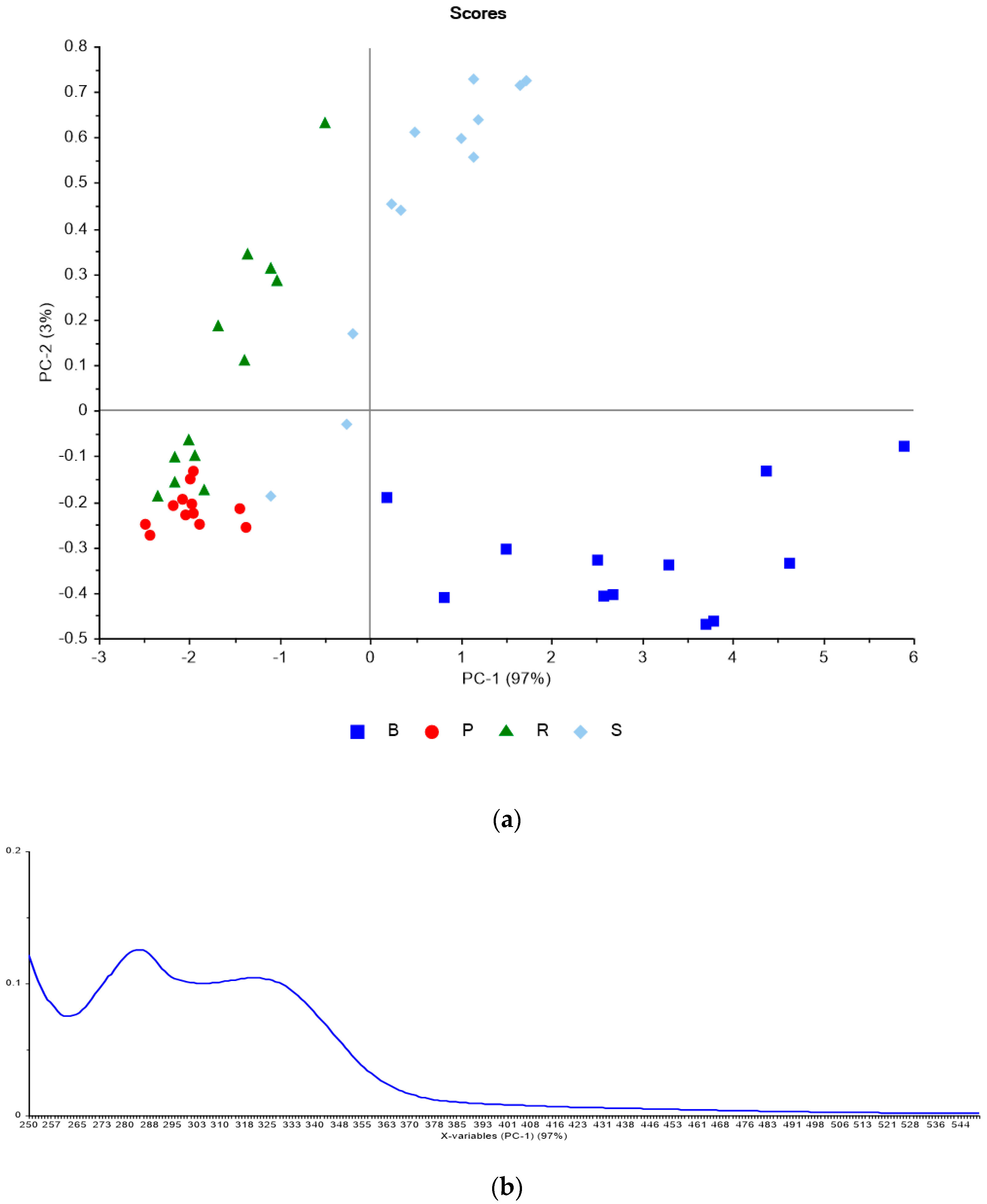
| TSS | YAN | pH | TA | ||
|---|---|---|---|---|---|
| Red Delicious | No Maceration | 11.85 ± 0.28 | 70.78 ± 2.67 | 4.06 ± 0.05 | 2.04 ± 0.10 |
| Maceration | 11.55 ± 0.22 | 69.65 ± 4.90 | 4.05 ± 0.05 | 1.94 ± 0.10 | |
| Free Run | 11.67 ± 0.34 | 70.69 ± 4.38 | 4.07 ± 0.05 | 2.02 ± 0.10 | |
| Pressure | 11.73 ± 0.22 | 69.74 ± 3.48 | 4.04 ± 0.04 | 1.96 ± 0.11 | |
| Bulmer’s Norman | No Maceration | 13.10 ± 0.43 | 38.70 ± 14.70 | 4.18 ± 0.01 | 2.10 ± 0.13 |
| Maceration | 13.17 ± 0.59 | 24.82 ± 10.20 | 4.18 ± 0.06 | 2.08 ± 0.08 | |
| Free Run | 12.92 ± 0.57 | 33.14 ± 20.07 | 4.18 ± 0.04 | 2.06 ± 0.11 | |
| Pressure | 13.35 ± 0.38 | 31.76 ± 6.82 | 4.18 ± 0.05 | 2.12 ± 0.10 | |
| Sturmer | No Maceration | 14.23 ± 0.18 | 37.95 ± 7.83 | 3.34 ± 0.09 | 8.64 ± 0.43 |
| Maceration | 13.72 ± 0.55 | 40.55 ± 5.16 | 3.33 ± 0.02 | 8.30 ± 0.39 | |
| Free Run | 13.83 ± 0.61 | 40.35 ± 6.90 | 3.32 ± 0.05 | 8.32 ± 0.51 | |
| Pressure | 14.12 ± 0.22 | 38.15 ± 6.42 | 3.35 ± 0.07 | 8.61 ± 0.30 | |
| Pink Lady | No Maceration | 11.68 ± 0.19 | 32.95 ± 3.69 | 3.52 ± 0.04 | 5.37 ± 0.10 a |
| Maceration | 11.65 ± 0.35 | 29.05 ± 3.68 | 3.58 ± 0.03 | 5.24 ± 0.06 b | |
| Free Run | 11.52 ± 0.31 | 31.20 ± 4.18 | 3.56 ± 0.05 | 5.25 ± 0.07 a | |
| Pressure | 11.82 ± 0.17 | 30.81± 3.96 | 3.53 ± 0.03 | 5.36 ± 0.10 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Way, M.L.; Jones, J.E.; Swarts, N.D.; Dambergs, R.G. Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes Using Two Analytical Methods. Beverages 2019, 5, 53. https://doi.org/10.3390/beverages5030053
Way ML, Jones JE, Swarts ND, Dambergs RG. Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes Using Two Analytical Methods. Beverages. 2019; 5(3):53. https://doi.org/10.3390/beverages5030053
Chicago/Turabian StyleWay, Madeleine L., Joanna E. Jones, Nigel D. Swarts, and Robert G. Dambergs. 2019. "Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes Using Two Analytical Methods" Beverages 5, no. 3: 53. https://doi.org/10.3390/beverages5030053
APA StyleWay, M. L., Jones, J. E., Swarts, N. D., & Dambergs, R. G. (2019). Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes Using Two Analytical Methods. Beverages, 5(3), 53. https://doi.org/10.3390/beverages5030053





