Why Craft Brewers Should Be Advised to Use Bottle Refermentation to Improve Late-Hopped Beer Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Beer Samples and Aging Procedure
2.3. Short-chain Fatty Acid Analysis
2.4. Static Headspace Analysis of Esters
2.5. Flavor XAD-2 Extraction and Gas Chromatography—Olfactometry Analytical Conditions
2.6. Sensory Analyses
3. Results and Discussion
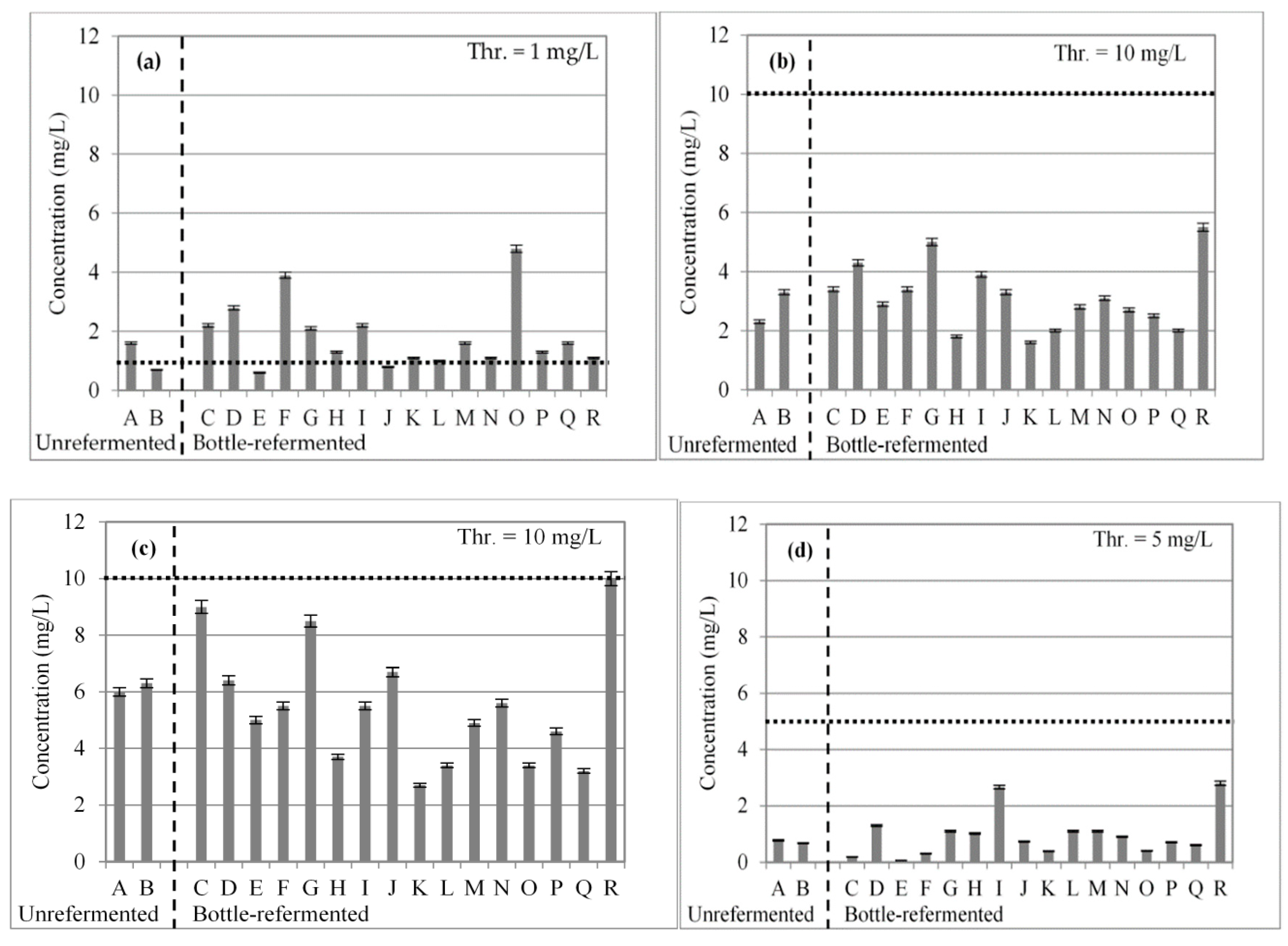
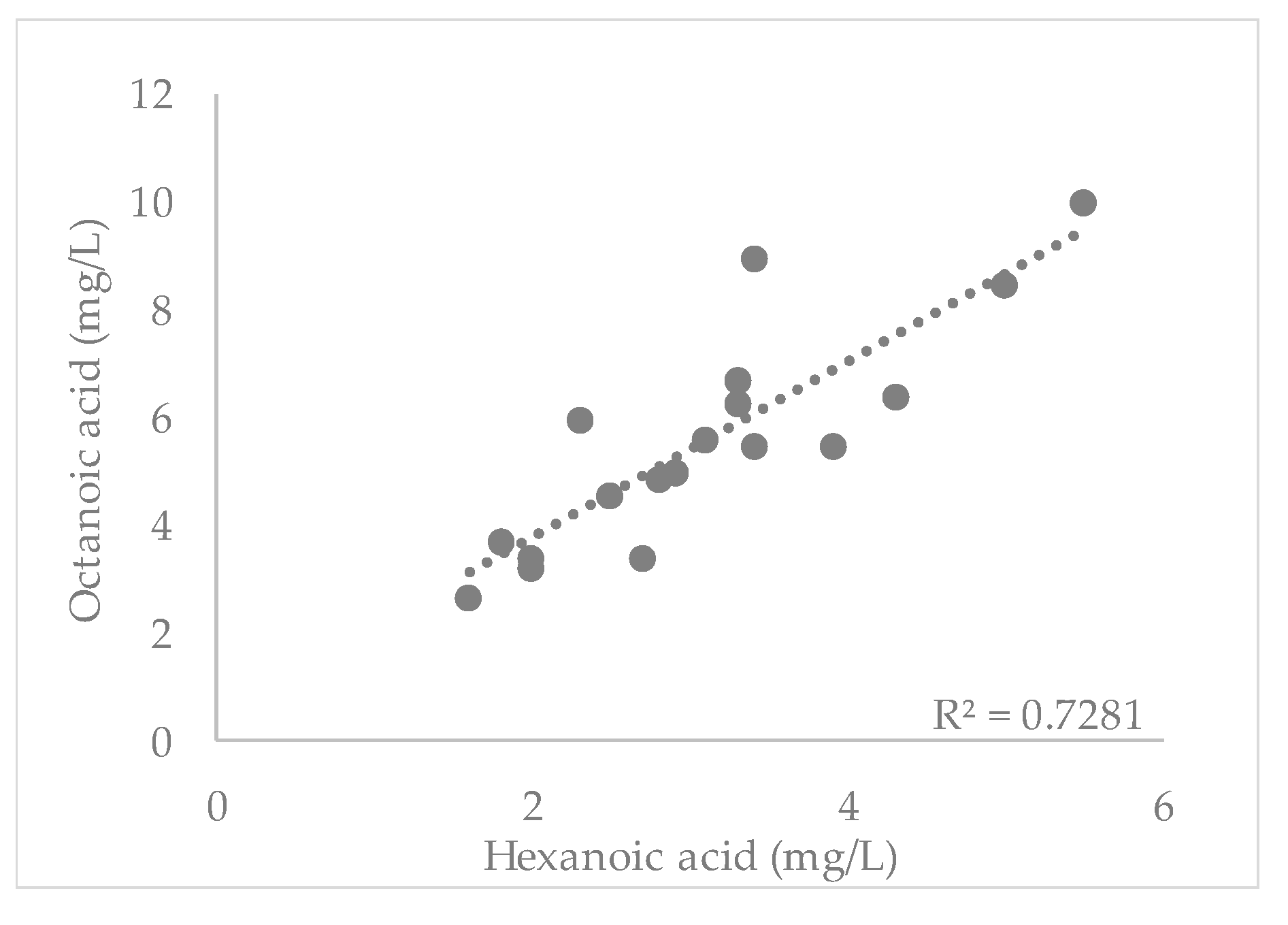
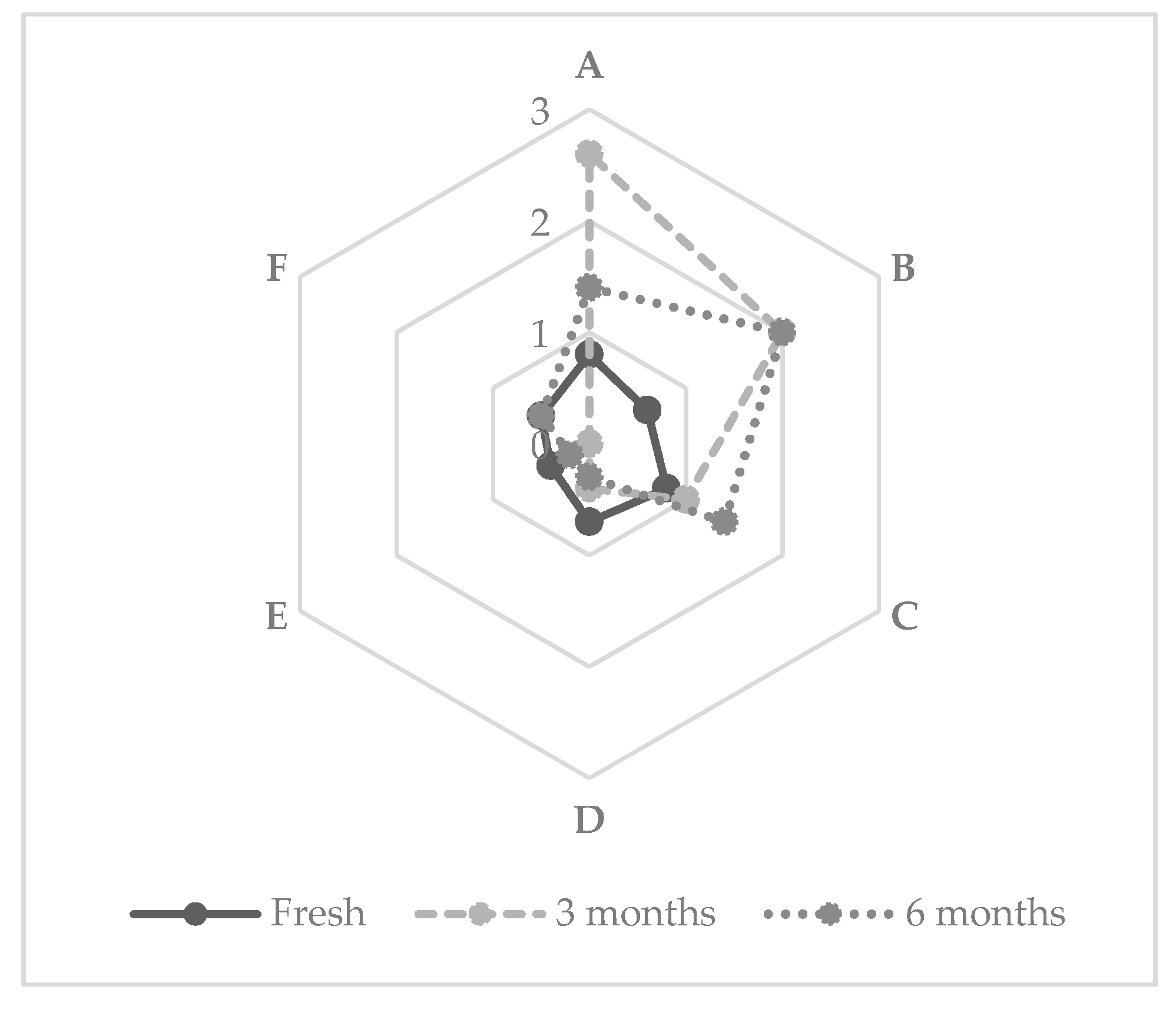
3.1. Short-Chain Fatty Acids
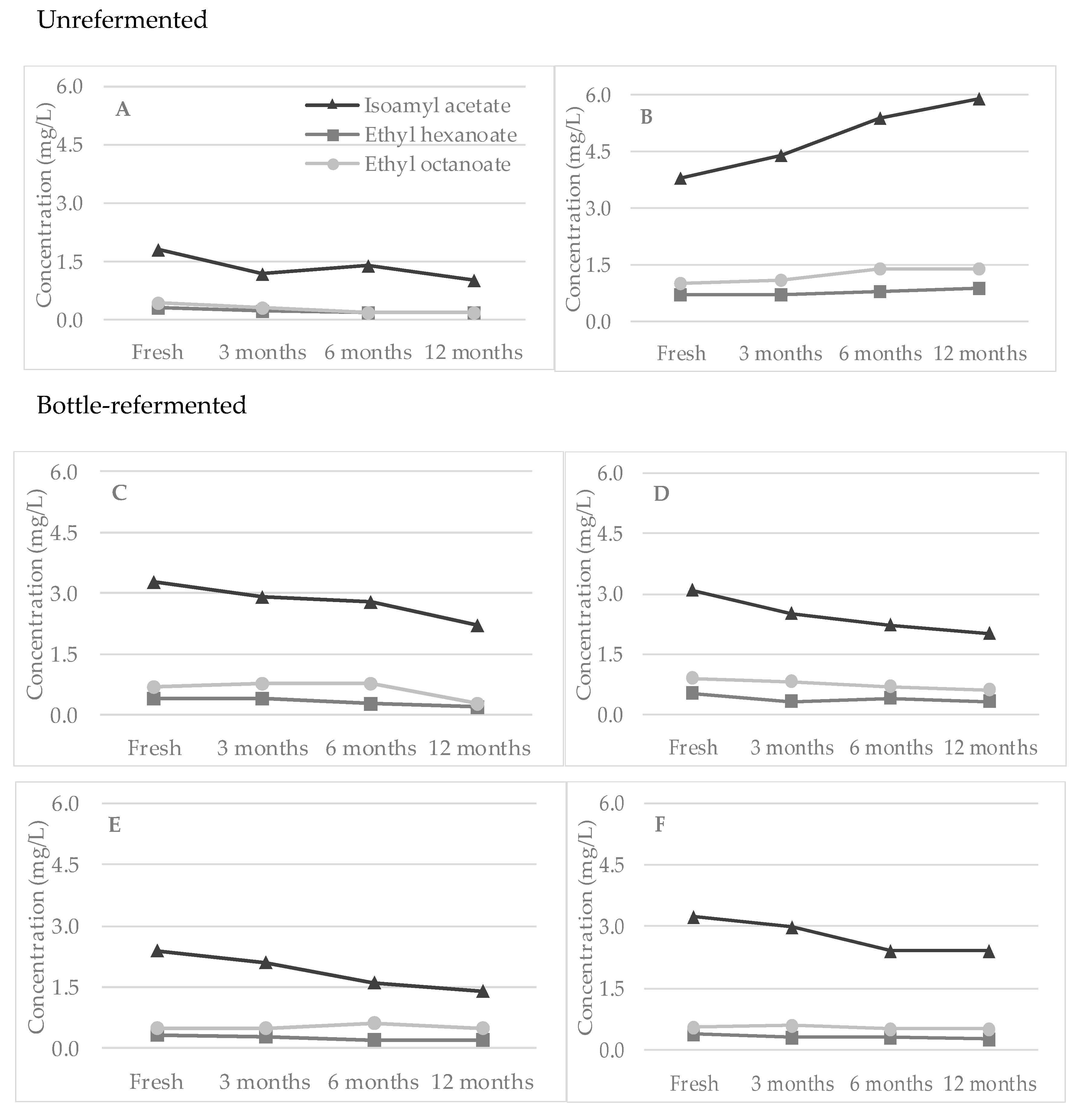
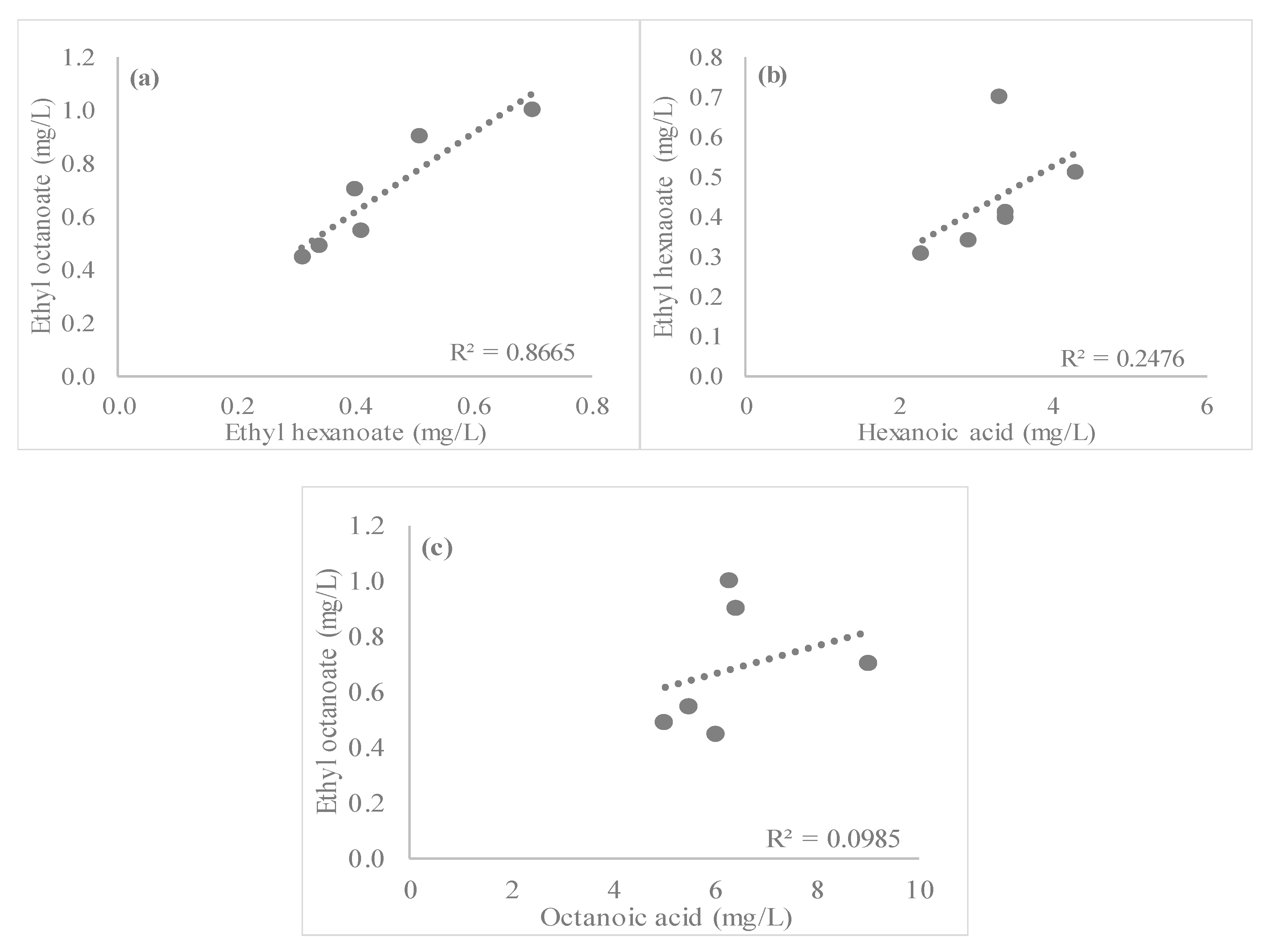
3.2. Esters
3.3. Olfactive Analysis of XAD-2 Extracted Flavors
3.4. Sensory Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2013, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Saerens, M.G.; Verstrepen, K.; Thevelein, J.; Delvaux, F. Ethyl esters production during brewery fermentation: A review. Cerevisia 2008, 33, 82–90. [Google Scholar]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Coghe, S.; Martens, E.; D’Hollander, H.; Dirinck, P.J.; Delvaux, F. Sensory and instrumental flavour analysis of wort brewed with dark specialty malts. J. Inst. Brew. 2004, 110, 94–103. [Google Scholar] [CrossRef]
- Vandecan, S.M.G.; Daems, N.; Schouppe, N.; Saison, D.; Delvaux, F. Formation of flavor, color, and reducing power during the production process of dark specialty malts. J. Am. Soc. Brew. Chem. 2011, 69, 150–157. [Google Scholar] [CrossRef]
- Gros, J.; Peeters, F.; Collin, S. Occurrence of odorant polyfunctional thiols in beers hopped with different cultivars. First evidence of an S-cysteine conjugate in hop (Humulus lupulus L.). J. Agric. Food Chem. 2012, 60, 7805–7816. [Google Scholar] [CrossRef]
- Kankolongo Cibaka, M.-L.; Ferreira, C.S.; Decourrière, L.; Lorenzo-Alonso, C.-J.; Bodart, E.; Collin, S. Dry hopping with the dual-purpose varieties Amarillo, Citra, Hallertau Blanc, Mosaic, and Sorachi Ace: minor contribution of hop terpenol glucosides to beer flavors. J. Am. Soc. Brew. Chem. 2017, 75, 122–129. [Google Scholar]
- Kankolongo Cibaka, M.-L.; Decourrière, L.; Lorenzo-Alonso, C.-J.; Bodart, E.; Robiette, R.; Collin, S. 3-Sulfanyl-4-methylpentan-1-ol in dry-hopped beers: first evidence of glutathione S-Conjugates in hop (Humulus lupulus L.). J. Agric. Food Chem. 2016, 64, 8572–8582. [Google Scholar] [CrossRef]
- Dalgliesh, C.E. Flavour stability. In Proceedings of the European Brewery Convention: Proceedings of the 16th Congress, Amsterdam, The Netherlands, 1977; pp. 623–659. [Google Scholar]
- Noël, S.; Liegeois, C.; Lermusieau, G.; Bodart, E.; Badot, C.; Collin, S. Release of deuterated nonenal during beer aging from labeled precursors synthesized in the boiling kettle. J. Agric. Food Chem. 1999, 47, 4323–4326. [Google Scholar] [CrossRef]
- Lermusieau, G.; Noël, S.; Liegeois, C.; Collin, S. Nonoxidative mechanism for development of trans-2-nonenal in beer. J. Am. Soc. Brew. Chem. 1999, 57, 29–33. [Google Scholar] [CrossRef]
- Zufall, C.; Racioppi, G.; Gasparri, M.; Franquiz, J. Flavour stability and ageing characteristics of light stable beers. In Proceedings of the European Brewery Convention Congress, Prague, Czech Republic, 2–5 October 2005; p. 30. [Google Scholar]
- Silva Ferreira, C.; Thibault de Chanvalon, E.; Bodart, E.; Collin, S. Why humulinones are key bitter constituents only after dry hopping: comparison with other Belgian styles. J. Am. Soc. Brew. Chem. 2018, 76, 236–246. [Google Scholar] [CrossRef]
- Clapperton, J.F. Ribes flavor in beer. J. Inst. Brew. 1976, 82, 175–176. [Google Scholar] [CrossRef]
- Thi Thu, H.T.; Nizet, S.; Gros, J.; Collin, S. Occurrence of the ribes odorant 3-sulfanyl-3-methylbutyl formate in aged beers. Flavour Fragr. J. 2013, 28, 147–179. [Google Scholar]
- Scholtes, C.; Nizet, S.; Collin, S. How sotolon can impart a Madeira off-flavor to aged beers. J. Agric. Food Chem. 2015, 63, 2886–2892. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, C.; Nizet, S.; Collin, S. Guaiacol and 4-methylphenol as specific markers of torrefied malts. Fate of volatile phenols in special beers through aging. J. Agric. Food Chem. 2014, 62, 9522–9528. [Google Scholar] [CrossRef] [PubMed]
- Thi Thu, H.T.; Kankolongo Cibaka, M.-L.; Collin, S. Polyfunctional thiols in fresh and aged Belgian special beers: fate of hop S-cysteine conjugates. J. Am. Soc. Brew. Chem. 2015, 73, 61–70. [Google Scholar]
- Vanderhaegen, B.; Neven, H.; Daenen, L.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Furfuryl ethyl ether: important aging flavor and a new marker for the storage conditions of beer. J. Agric. Food Chem. 2004, 52, 1661–1668. [Google Scholar] [CrossRef]
- Derdelinckx, G.; Vanderhasselt, B.; Maudoux, M.; Dufour, J.P. Refermentation in bottles and kegs: A rigorous approach. Brauwelt Int. 1992, 2, 156–164. [Google Scholar]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of chemical and sensory properties during aging of top-fermented beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef]
- Nizet, S.; Gros, J.; Peeters, F.; Chaumont, S.; Robiette, R.; Collin, S. First evidence of the production of odorant polyfunctional thiols by bottle refermentation. J. Am. Soc. Brew. Chem. 2013, 71, 15–22. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Vanbeneden, N.; Daenen, L.; Delvaux, F.; Delvaux, F.R. Decrease of aged beer aroma by the reducing activity of brewing yeast. J. Agric. Food Chem. 2010, 58, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Ernest, C.-H.; Chen, A.; Jamieson, A.M.; Van Gheluwe, G. The release of fatty acids as a consequence of yeast autolysis. J. Am. Soc. Brew. Chem. 1980, 38, 13–17. [Google Scholar]
- Leroy, M.J.; Charpentier, M.; Duteurtre, B.; Feuillat, M.; Charpentier, C. Yeast autolysis during Champagne aging. Am. J. Enol. Vitic. 1990, 41, 21–28. [Google Scholar]
- Masschelein, C.A. The biochemistry of maturation. J. Inst. Brew. 1986, 92, 213–219. [Google Scholar] [CrossRef]
- Ormrod, I.H.L.; Lalor, E.F.; Sharpe, F.R. The release of yeast proteolytic enzymes into beer. J. Inst. Brew. 1991, 97, 441–443. [Google Scholar] [CrossRef]
- Neven, H.; Delvaux, F.; Derdelinckx, G. Flavor evolution of top fermented beers. MBAA Tech. Q. 1997, 34, 115–118. [Google Scholar]
- Gassenmeier, K.; Schieberle, P. Potent aromatic compounds in the crumb of wheat bread (French-type)-influence of pre-ferments and studies on the formation of key odorants during dough processing. Z. Lebensm. Unters. Forsch. 1995, 201, 241–248. [Google Scholar] [CrossRef]
- Olsen, E. Brettanomyces: Occurrence, flavour effects and control. In Proceedings of the 23rd Annual New York Wine Industry Workshop, New York, NY, USA, 23–24 March 1994. [Google Scholar]
- Fugelsang, K.C. Wine Microbiology; The Chapman & Hall Enology Library: New York, NY, USA, 1997. [Google Scholar]
- Alvarez., P.; Malcorps, P.; Sa Almeida, A.; Ferreira, A.; Meyer, A.M.; Dufour, J.P. Analysis of free fatty acids, fusel alcohols, and esters in beer: an alternative to CS2 extraction. J. Am. Soc. Brew. Chem. 1994, 52, 127–134. [Google Scholar] [CrossRef]
- Lermusieau, G.; Bulens, M.; Collin, S. Use of GC−Olfactometry to identify the hop aromatic compounds in beer. J. Agric. Food Chem. 2001, 49, 3867–3874. [Google Scholar] [CrossRef]
- Licker, J.L.; Acree, T.E.; Henick-Kling, T. What is ‘‘Brett’’ (Brettanomyces) flavour? A preliminary investigation. In Chemistry of Wine Flavour; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; pp. 96–115. [Google Scholar]
- Meilgaard, M. Flavor and threshold of beer volatiles. MBAA Tech. Q. 1974, 11, 87–89. [Google Scholar]
- Engan, S. Organoleptic threshold of some alcohols and esters in beer. J. Inst. Brew. 1971, 78, 33–36. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling sompounds to the aged flavor of lager beer by studying their flavor thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Ullrich, F.; Grosch, W. Identification of the most intensive volatile flavour compounds formed during autoxidation of linoleic acid. Z. Lebensm. Unters. Forsch. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Callemien, D.; Dasnoy, S.; Collin, S. Identification of a stale-beer-like odorant in extracts of naturally aged beer. J. Agric. Food Chem. 2006, 54, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Chevance, F.; Guyot, C.; Dupont, J.; Collin, S. Investigation of the β-damascenone in fresh and aged commercial beers. J. Agric. Food Chem. 2002, 50, 3818–3821. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]

| Beer | Alcohol (% vol) | Real Extract (°P) | pH | Bitterness (BU) | Color (EBC) | Sensorial Characteristics |
|---|---|---|---|---|---|---|
| A | 6.5 | 4.6 | 4.2 | 15 | 12.5 | Butter, apple, hop, green |
| B | 12.3 | 6.6 | 4.4 | 20 | 25 | Alcohol, banana, cheese, phenols |
| C * | 7.9 | 5.1 | 4.5 | 21 | 16.5 | Butter, sulfur, hop |
| D * | 8.8 | 5.8 | 4.4 | 14 | 66 | Malt, sulfur, green |
| E * | 8.1 | 4.2 | 4.4 | 29 | 14.5 | Lemon, banana, apple, spicy, phenols |
| F * | 7.5 | 3.7 | 4.5 | 24 | 15.5 | Orange, pineapple, spicy, phenols |
| Log3FD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | C | E | F | |||||||
| Compound | RI | Odor | Fresh | Aged | Fresh | Aged | Fresh | Aged | Fresh | Aged |
| Ethyl butyrate | 778 | Red fruits | 1 | 1 | 4 | 6 | nd | 3 | 3 | 4 |
| 3-Methyl-2-buten-1-thiol | 809 | Garlic, hoppy | 7 | 8 | 7 | 8 | 10 | 10 | 10 | 10 |
| Isovaleric acid | 811 | Sweat, rancid | 2 | 2 | 2 | 2 | 4 | 5 | 5 | 5 |
| 2-Methylbutanoic acid | 828 | Sweat | 2 | 2 | 2 | 2 | 4 | 5 | 3 | 6 |
| 2-Methyl-3-furanthiol | 850 | Broth | 5 | 7 | 5 | 2 | 10 | 10 | 10 | 10 |
| Isoamyl acetate | 854 | Fruity, banana | 1 | 1 | 2 | 2 | nd | nd | nd | nd |
| Ethyl hexanoate | 979 | Fruity, candy | 2 | 2 | 4 | 4 | 3 | 4 | 4 | 4 |
| Furaneol | 1037 | Cotton candy | 4 | 5 | 6 | 7 | 5 | 5 | 4 | 7 |
| Linalool | 1089 | Flowery, coriander | 7 | 6 | 6 | 7 | nd | nd | 5 | 2 |
| trans-2-Nonenal | 1127 | Cardboard | nd | 6 | nd | 5 | 3 | 4 | 4 | 5 |
| Citronellol | 1216 | Fruity, flowery | nd | nd | 1 | 4 | 3 | 2 | nd | 8 |
| 4-Vinylguaiacol | 1294 | Clove | 3 | 4 | 4 | 4 | 5 | 6 | 4 | 6 |
| γ-Nonalactone | 1327 | Coconut | nd | 3 | 0 | 4 | 1 | 2 | 3 | 5 |
| Vanillin | 1365 | Vanilla | 0 | nd | 6 | 7 | nd | 4 | 1 | 5 |
| β-Damascenone | 1374 | Fruity, apricot | 2 | 3 | 4 | 5 | 4 | 4 | 3 | 6 |
| 4-Vinylsyringol | 1543 | Clove | 3 | 3 | 0 | 3 | 2 | 3 | 5 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva Ferreira, C.; Bodart, E.; Collin, S. Why Craft Brewers Should Be Advised to Use Bottle Refermentation to Improve Late-Hopped Beer Stability. Beverages 2019, 5, 39. https://doi.org/10.3390/beverages5020039
Silva Ferreira C, Bodart E, Collin S. Why Craft Brewers Should Be Advised to Use Bottle Refermentation to Improve Late-Hopped Beer Stability. Beverages. 2019; 5(2):39. https://doi.org/10.3390/beverages5020039
Chicago/Turabian StyleSilva Ferreira, Carlos, Etienne Bodart, and Sonia Collin. 2019. "Why Craft Brewers Should Be Advised to Use Bottle Refermentation to Improve Late-Hopped Beer Stability" Beverages 5, no. 2: 39. https://doi.org/10.3390/beverages5020039
APA StyleSilva Ferreira, C., Bodart, E., & Collin, S. (2019). Why Craft Brewers Should Be Advised to Use Bottle Refermentation to Improve Late-Hopped Beer Stability. Beverages, 5(2), 39. https://doi.org/10.3390/beverages5020039





