Biogenic Amines Determination in “Plant Milks”
Abstract
1. Introduction
- Cereal based: Oat milk, rice milk, corn milk, spelt milk, millet milk;
- Legume based: Soy milk, peanut milk, lupin milk, cowpea milk;
- Nut based: Almond milk, coconut milk, hazelnut milk, pistachio milk, walnut milk;
- Seed based: Sesame milk, flax milk, hemp milk, sunflower milk;
- Pseudo-cereal based: Quinoa milk, teff milk, amaranth milk, buckwheat milk.
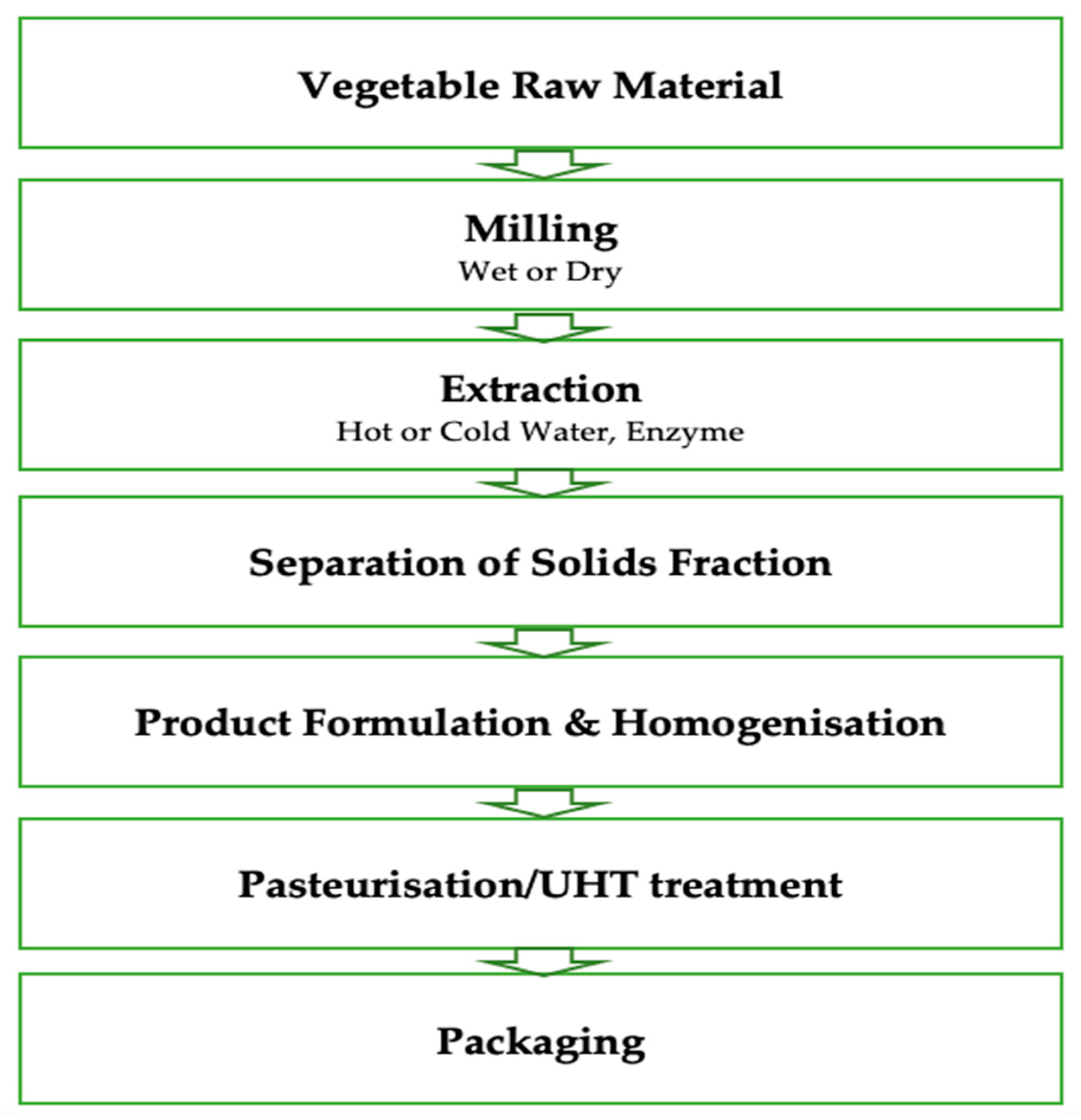
2. Materials and Methods
2.1. Materials
2.2. Sampling
2.3. Biogenic Amines Extraction and Derivatization
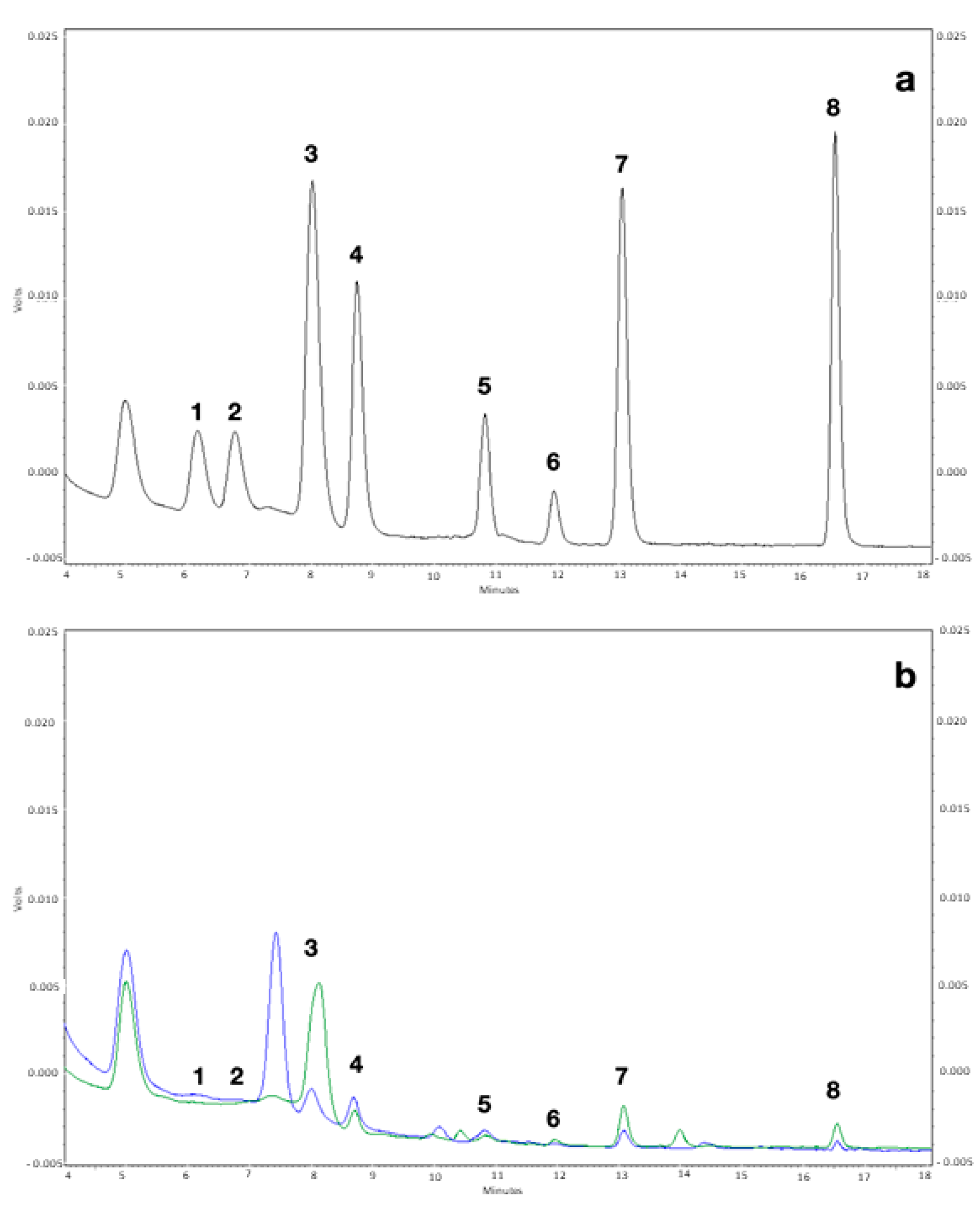
2.4. HPLC Analysis
2.5. Results Expression
3. Results and Discussion
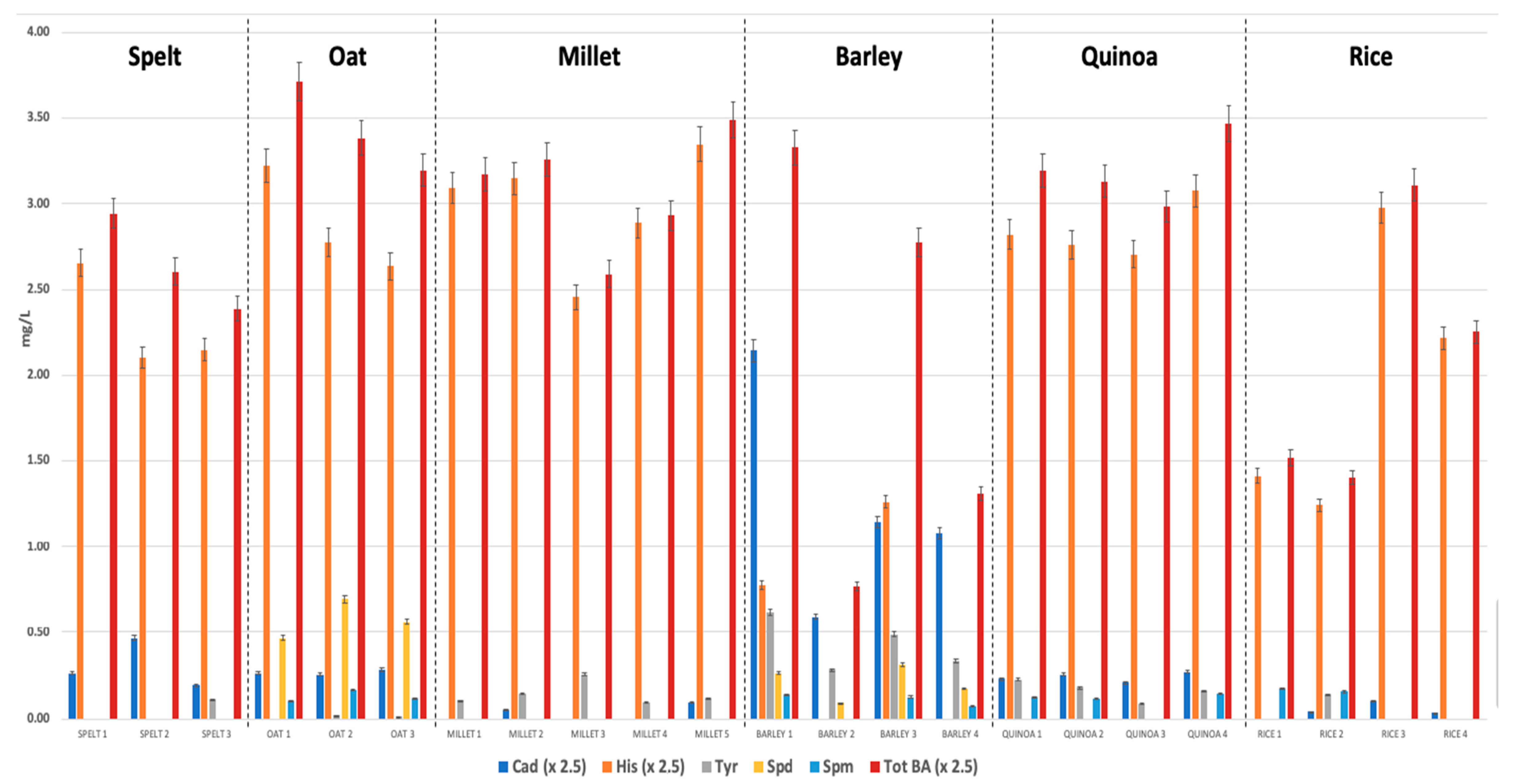
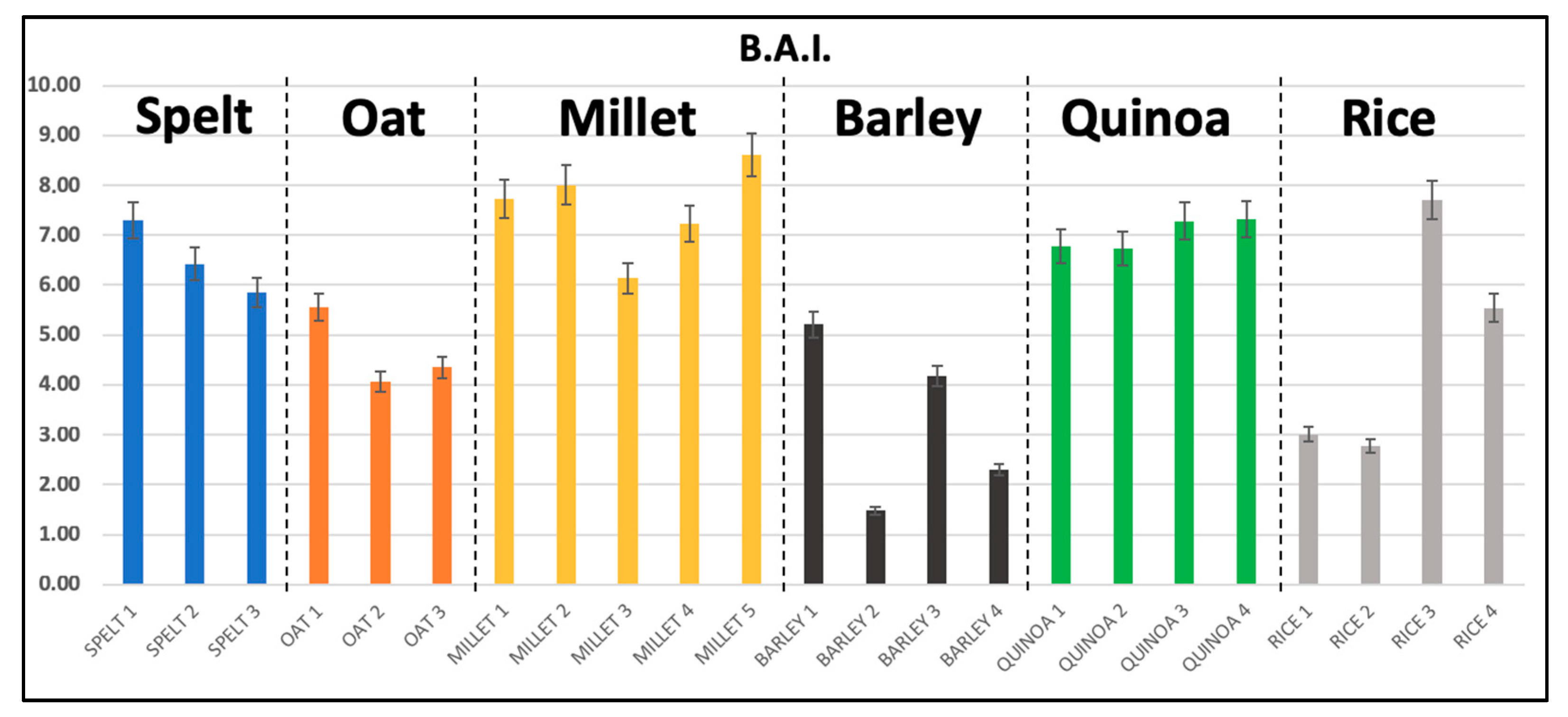
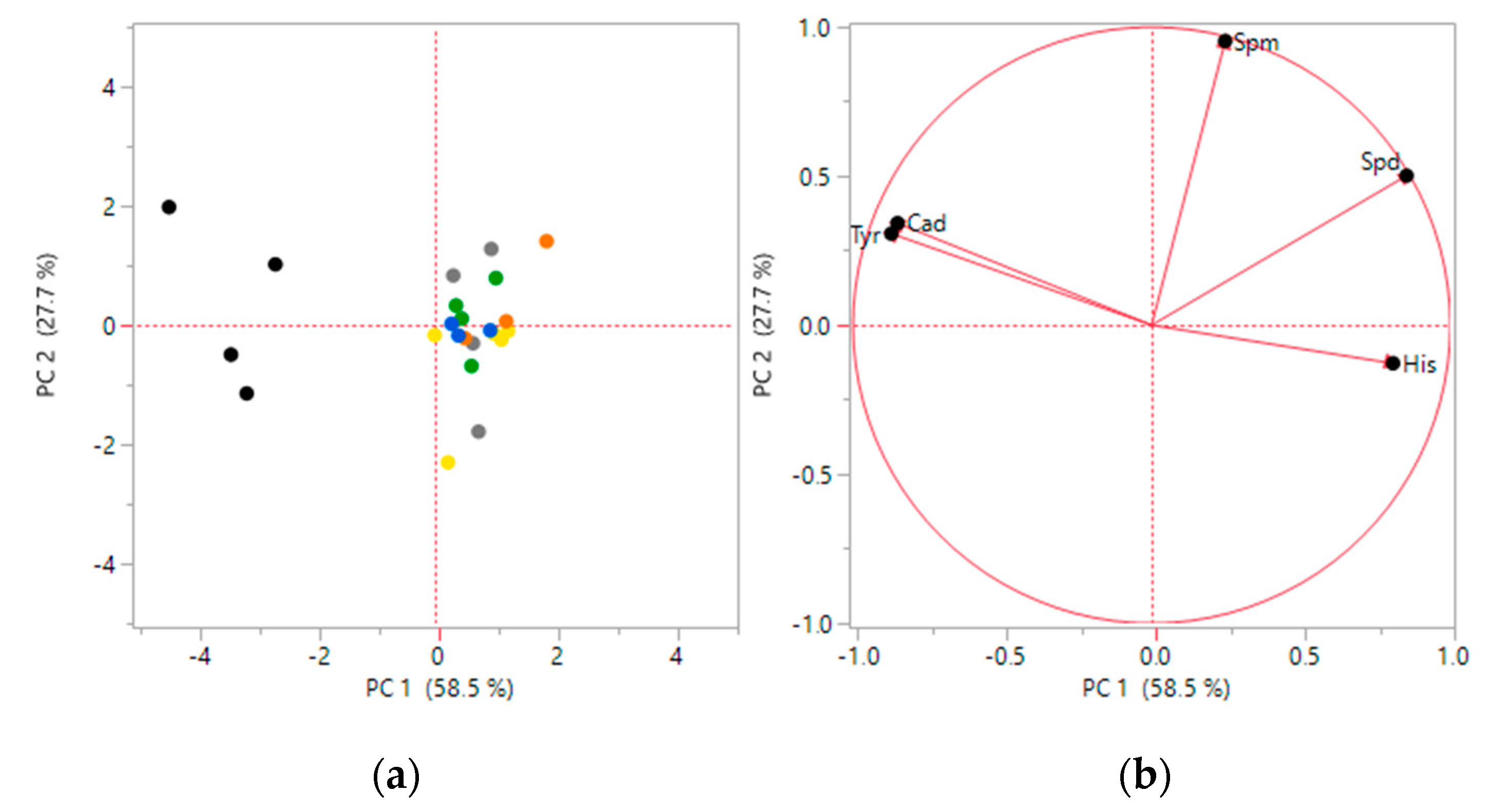
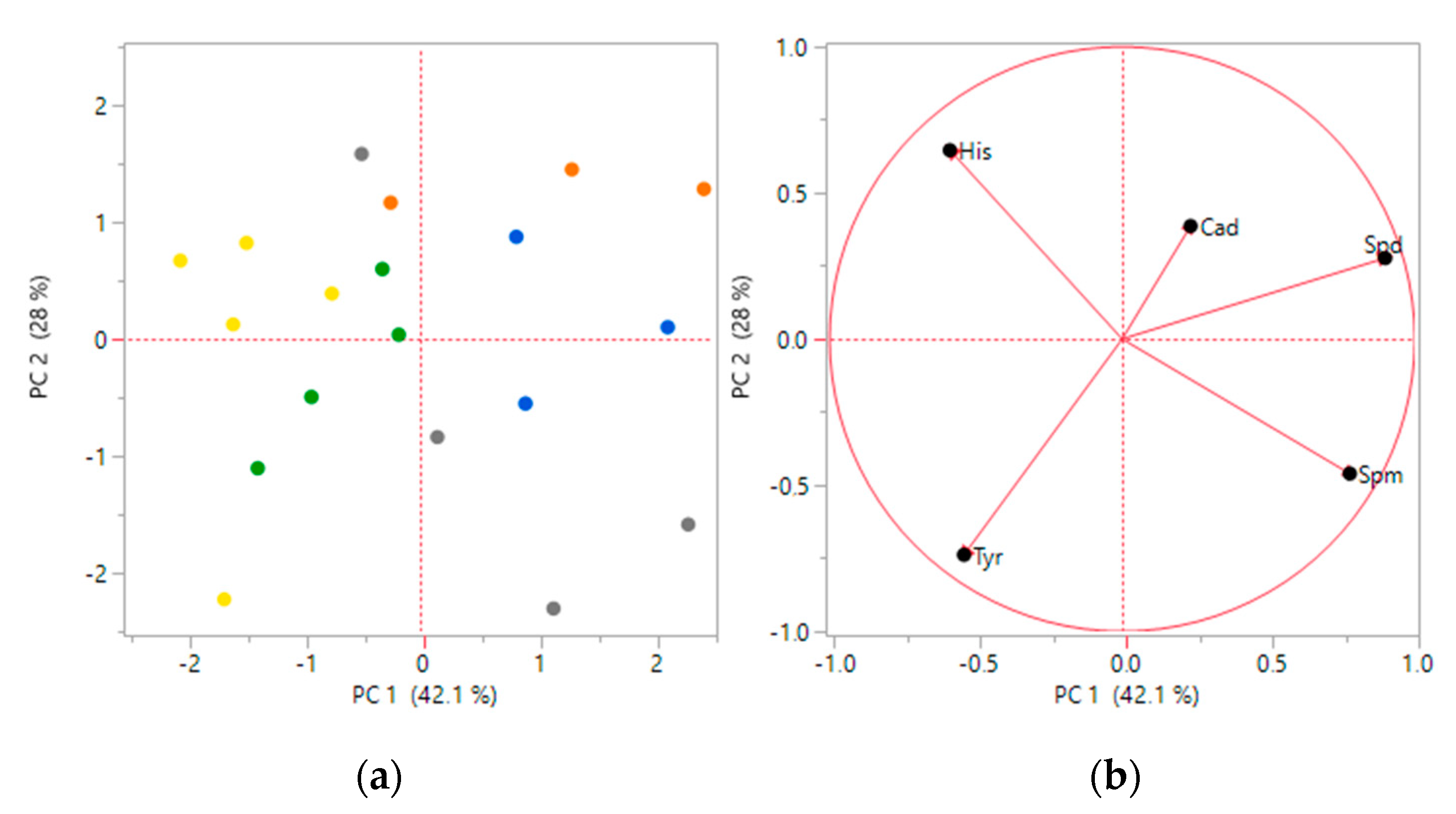
3.1. Biogenic Amines Determination
3.2. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Regulation (EU) No. 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Off. J. Eur. Union 2013, L347, 671–854.
- Lucarini, M. Bioactive Peptides in Milk: From Encrypted Sequences to Nutraceutical Aspects. Beverages 2017, 3, 41. [Google Scholar] [CrossRef]
- FAOSTAT. Milk Consumption—Europe 2007–2017; FAO: Roma, Italy, 2018. [Google Scholar]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Margaret, J.; James, E. Comparison of the Nutrient Content of Cow’ s Milk and Nondairy Milk Alternatives: What’ s the Difference? Nutr. Today 2018, 53, 153–159. [Google Scholar]
- Ripari, V. Techno-Functional Role of Exopolysaccharides in Cereal-Based, Yogurt-Like Beverages. Beverages 2019, 5, 16. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Kundu, P.; Dhankhar, J.; Sharma, A. Development of Non Dairy Milk Alternative Using Soymilk and Almond Milk. Curr. Res. Nutr. Food Sci. J. 2018, 6, 203–210. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- Cinquina, A.L.; Calì, A.; Longo, F.; De Santis, L.; Severoni, A.; Abballe, F. Determination of biogenic amines in fish tissues by ion-exchange chromatography with conductivity detection. J. Chromatogr. A 2004, 1032, 73–77. [Google Scholar] [CrossRef]
- Vinci, G.; Antonelli, M.L. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 2016, 54, 460–470. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yang, Y.P.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, 94–100. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Suzzi, G. Biogenic Amines: Toxicology and Health Effect. Encycl. Food Health 2015. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Poveda, J.M. Biogenic amines and free amino acids in craft beers from the Spanish market: A statistical approach. Food Control 2019, 96, 227–233. [Google Scholar] [CrossRef]
- Preti, R.; Bernacchia, R.; Vinci, G. Chemometric evaluation of biogenic amines in commercial fruit juices. Eur. Food Res. Technol. 2016, 242, 2031–2039. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Callejón, R.M.; Troncoso, A.M.; García-Parrilla, M.C. Evaluation of biogenic amines profile in opened wine bottles: Effect of storage conditions. J. Food Compos. Anal. 2017, 63, 139–147. [Google Scholar] [CrossRef]
- La Torre, G.L.; Rando, R.; Saitta, M.; Alfa, M.; Maisano, R.; Dugo, G. Determination of biogenic amine and heavy metal contents in sicilian wine samples. Ital. J. Food Sci. 2010, 22, 28–40. [Google Scholar]
- Linares, D.M.; Del Rio, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Alvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fuste, J.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biologically active amines in fermented and non-fermented commercial soybean products from the Spanish market. Food Chem. 2015, 173, 1119–1124. [Google Scholar] [CrossRef]
- Duflos, G.; Inglebert, G.; Himber, C.; Degremont, S.; Lombard, B.; Brisabois, A. Validation of standard method EN ISO 19343 for the detection and quantification of histamine in fish and fishery products using high-performance liquid chromatography. Int. J. Food Microbiol. 2018, 288, 97–101. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26.
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Chiacchierini, E.; Restuccia, D.; Vinci, G. Evaluation of two different extraction methods for chromatographic determination of bioactive amines in tomato products. Talanta 2006, 69, 548–555. [Google Scholar] [CrossRef]
- Al Bulushi, I.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation-A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Preti, R.; Letizia, M.; Bernacchia, R.; Vinci, G. Fast determination of biogenic amines in beverages by a core—Shell particle column. Food Chem. 2015, 187, 555–562. [Google Scholar] [CrossRef]
- Basheer, C.; Wong, W.; Makahleth, A.; Tameem, A.A.; Salhin, A.; Saad, B.; Lee, H.K. Hydrazone-based ligands for micro-solid phase extraction-high performance liquid chromatographic determination of biogenic amines in orange juice. J. Chromatogr. A 2011, 28, 4332–4339. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Polo, L.; Pardo, I. Biogenic amines in wines from three Spanish regions. J. Agric. Food Chem. 2005, 53, 1119–1124. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Zhang, G.; Zhao, Y.; Xue, C. A survey of biogenic amines in chinese red wines. Food Chem. 2007, 105, 1530–1535. [Google Scholar]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Guo, X.-L.; Li, E.H.; Wang, L.F.; Liu, Q.; Li, X.Y.; Yu, J.; Zhong, W.; Song, Y.D.; Pan, S.Y. Analysis of Biogenic Amines in Pickled Vegetable by a New Pre-column Derivatization of RP-HPLC. Mod. Food Sci. Technol. 2017, 877, 507–512. [Google Scholar]
- Kalač, P.; Švecová, S.; Pelikánová, T. Levels of biogenic amines in typical vegetable products. Food Chem. 2002, 77, 349–351. [Google Scholar] [CrossRef]
- Mietz, J.L.; Karmas, E. Chemical quality index of canned tuna as determined by high-pressure liquid chromatography. J. Food Sci. 1977, 42, 155–158. [Google Scholar] [CrossRef]
- Custódio, F.B.; Theodoro, K.H.; Gloria, M.B.A. Bioactive amines in fresh beef liver and influence of refrigerated storage and pan-roasting. Food Control 2016, 60, 151–157. [Google Scholar] [CrossRef]
- Guebel, D.V.; Torres, N.V. Principal Component Analysis (PCA). Encycl. Syst. Biol. 2013. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—Existing and emerging approaches. J. Food Sci. 2010, 75, 139–150. [Google Scholar] [CrossRef]
| Milk Sample | % Cereal | Milk Sample | % Cereal |
|---|---|---|---|
| SPELT 1 | 16.0 | BARLEY 1 | 17.0 |
| SPELT 2 | 15.0 | BARLEY 2 | 15.0 |
| SPELT 3 | 15.0 | BARLEY 3 | 17.0 |
| OATS 1 | 14.0 | BARLEY 4 | 15.0 |
| OATS 2 | 11.0 | QUINOA 1 | 8.0 |
| OATS 3 | 10.0 | QUINOA 2 | 8.0 |
| MILLET 1 | 16.0 | QUINOA 3 | 4.0 |
| MILLET 2 | 16.0 | QUINOA 4 | 8.0 |
| MILLET 3 | 15.0 | RICE 1 | 12.5 |
| MILLET 4 | 15.0 | RICE 2 | 12.0 |
| MILLET 5 | 17.0 | RICE 3 | 17.0 |
| RICE 4 | 14.0 |
| CAD | HIS | TYR | SPD | SPM | TOT BA | BAI | |
|---|---|---|---|---|---|---|---|
| Spelt | 0.77 ± 0.35 | 5.76 ± 0.77 | 0.11 * | n.d. | n.d. | 6.61 ± 0.70 | 6.53 ± 0.73 |
| range | 0.49 − 1.17 | 5.26 − 6.64 | − | − | − | 5.97 − 7.36 | 5.86 − 7.3 |
| Oat | 0.67 ± 0.03 | 7.20 ± 0.77 | n.d. | 0.57 ± 0.11 | 0.13 ± 0.03 | 8.58 ± 0.66 | 4.66 ± 0.79 |
| range | 0.64 − 0.71 | 6.59 − 8.06 | - | 0.46 − 0.69 | 0.1 − 0.17 | 7.99 − 9.29 | 4.07 − 5.56 |
| Millet | 0.17 ± 0.05 | 7.47 ± 0.85 | 0.17 ± 0.05 | n.d. | n.d. | 7.72 ± 0.86 | 7.58 ± 0.90 |
| range | n.d. − 0.23 | 6.14 − 8.37 | n.d. − 0.25 | − | − | 6.47 − 8.72 | 6.22 − 8.60 |
| Barley | 3.10 ± 1.63 | 2.55 ± 0.86 | 0.43 ± 0.15 | 0.21 ± 0.10 | 0.12 ± 0.02 | 5.11 ± 3.01 | 3.20 ± 1.81 |
| range | 1.47 − 5.36 | n.d. − 3.15 | 0.28 − 0.62 | n.d. − 0.31 | n.d. − 0.14 | 1.92 − 8.32 | 1.27 − 5.21 |
| Quinoa | 0.60 ± 0.07 | 7.10 ± 0.41 | 0.16 ± 0.05 | n.d. | 0.12 ± 0.02 | 7.98 ± 0.50 | 6.88 ± 0.30 |
| range | 0.52 − 0.68 | 6.77 − 7.69 | 0.10 − 0.22 | − | n.d. − 0.14 | 7.46 − 8.67 | 6.68 − 7.32 |
| Rice | 0.15 ± 0.09 | 4.90 ± 2.00 | 0.14 * | n.d. | 0.15 ± 0.02 | 5.18 ± 1.97 | 4.68 ± 2.19 |
| range | n.d. − 0.25 | 3.10 − 7.44 | − | − | n.d. − 0.17 | 3.51 − 7.77 | 2.78 − 7.33 |
| Milk Sample | CAD | HIS | TYR | SPD | SPM | TOT BA | BAI |
|---|---|---|---|---|---|---|---|
| Barley-Millet | <0.0001 | <0.0001 | 0.0001 | - | 0.1398 | 0.0192 | <0.0001 |
| Barley-Rice | <0.0001 | 0.0005 | <0.0001 | - | 0.4689 | 0.3356 | 0.0089 |
| Barley-Quinoa | 0.0001 | <0.0001 | 0.0004 | - | 0.6063 | 0.0170 | 0.0002 |
| Barley-Oat | 0.0003 | <0.0001 | <0.0001 | 0.0060 | 0.4164 | 0.0108 | <0.0001 |
| Barley-Spelt | 0.0004 | 0.0002 | <0.0001 | - | - | 0.1513 | 0.0001 |
| Rice-Millet | 0.9982 | 0.0152 | 0.3533 | - | 0.0674 | 0.0613 | 0.0038 |
| Rice-Oat | 0.3115 | 0.0489 | 0.3970 | - | 0.9310 | 0.0312 | 0.0468 |
| Rice-Quinoa | 0.3355 | 0.0424 | 0.2401 | - | 0.8000 | 0.0517 | 0.0783 |
| Rice-Spelt | 0.2337 | 0.4413 | 0.9916 | - | - | 0.3356 | 0.0486 |
| Oat-Spelt | 0.8598 | 0.2295 | 0.4021 | - | - | 0.2211 | 0.0009 |
| Oat-Quinoa | 0.9017 | 0.9299 | 0.0642 | - | 0.7298 | 0.6858 | 0.0012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbi, L.; Ciano, S.; Rapa, M.; Ruggieri, R. Biogenic Amines Determination in “Plant Milks”. Beverages 2019, 5, 40. https://doi.org/10.3390/beverages5020040
Gobbi L, Ciano S, Rapa M, Ruggieri R. Biogenic Amines Determination in “Plant Milks”. Beverages. 2019; 5(2):40. https://doi.org/10.3390/beverages5020040
Chicago/Turabian StyleGobbi, Laura, Salvatore Ciano, Mattia Rapa, and Roberto Ruggieri. 2019. "Biogenic Amines Determination in “Plant Milks”" Beverages 5, no. 2: 40. https://doi.org/10.3390/beverages5020040
APA StyleGobbi, L., Ciano, S., Rapa, M., & Ruggieri, R. (2019). Biogenic Amines Determination in “Plant Milks”. Beverages, 5(2), 40. https://doi.org/10.3390/beverages5020040






