Effect of Asparaginase Enzyme in the Reduction of Asparagine in Green Coffee
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. Pretreatment with Steam
2.3. Enzymatic Treatment
2.4. Asparagine Extraction
2.5. Derivatization of Amino Acids
2.6. Chromatographic Analysis
3. Results and Discussion
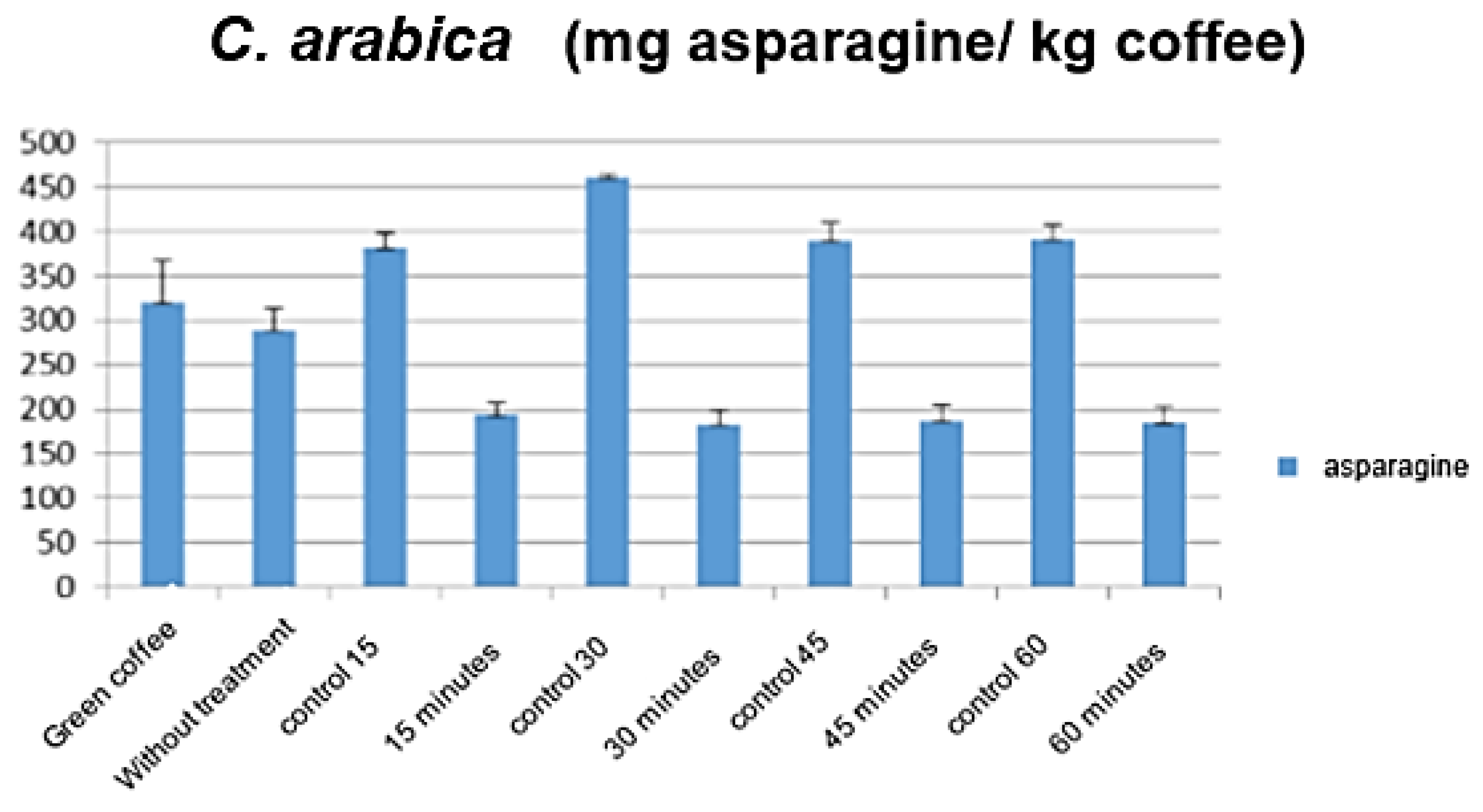
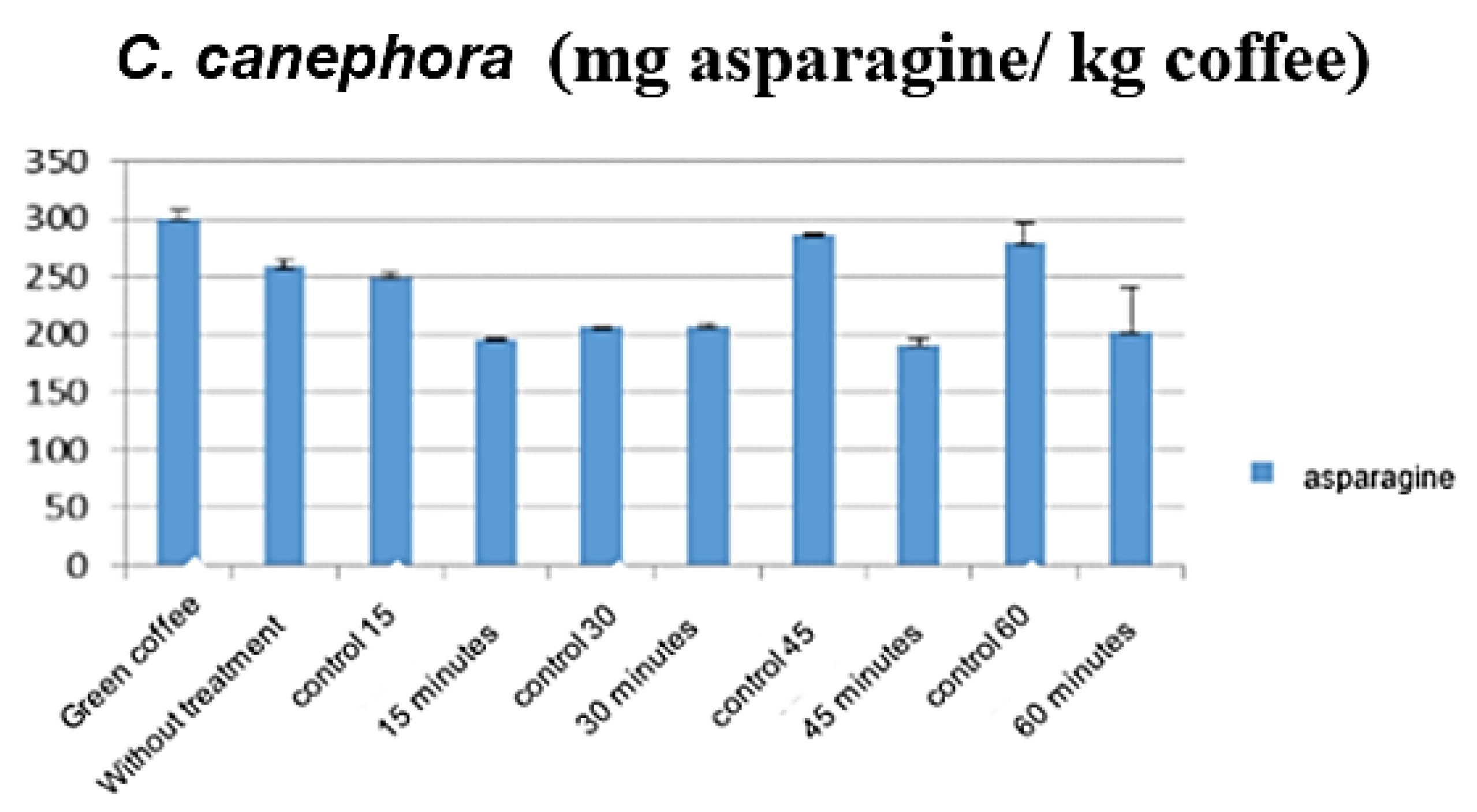
3.1. Influence of Stream Pretreatment
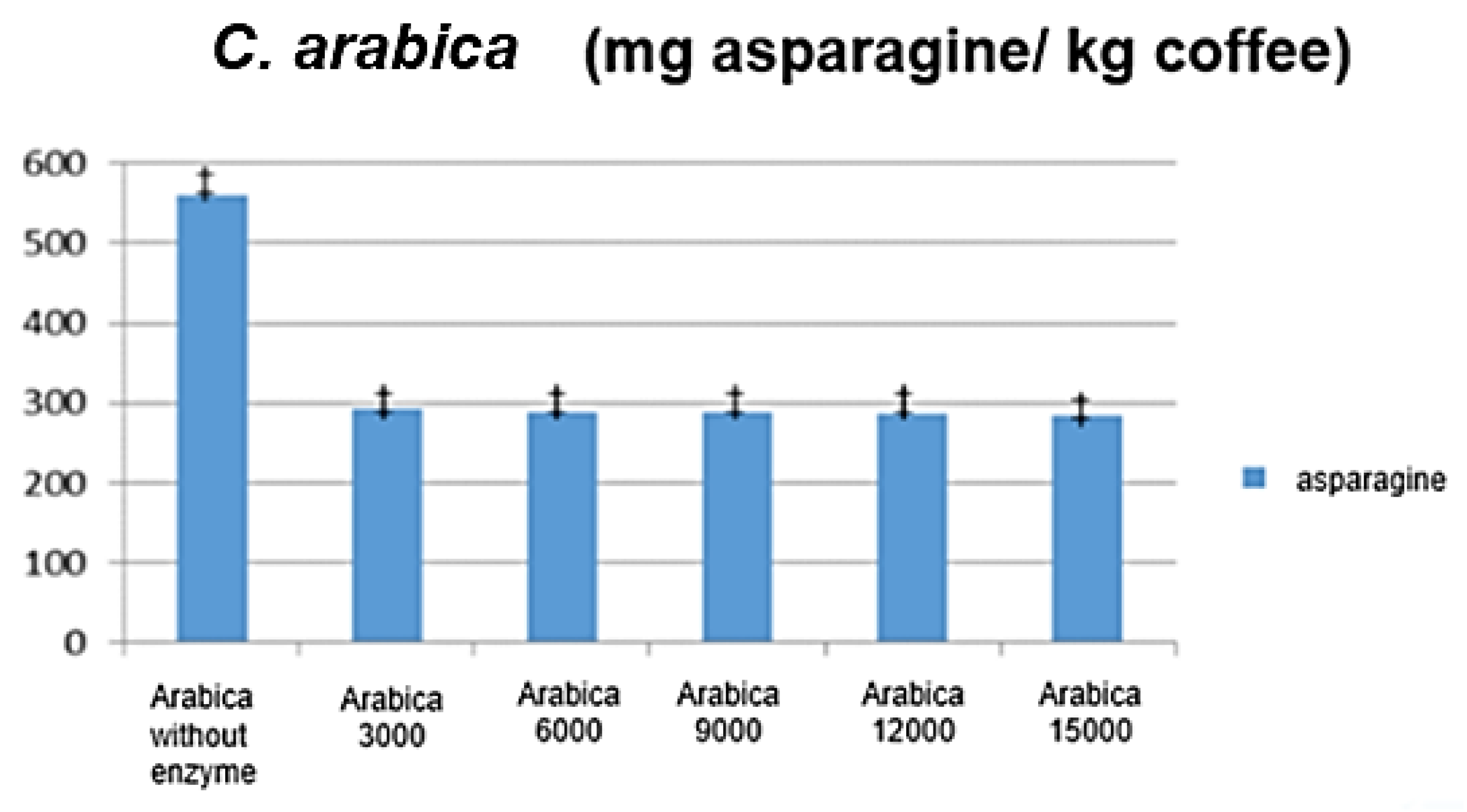
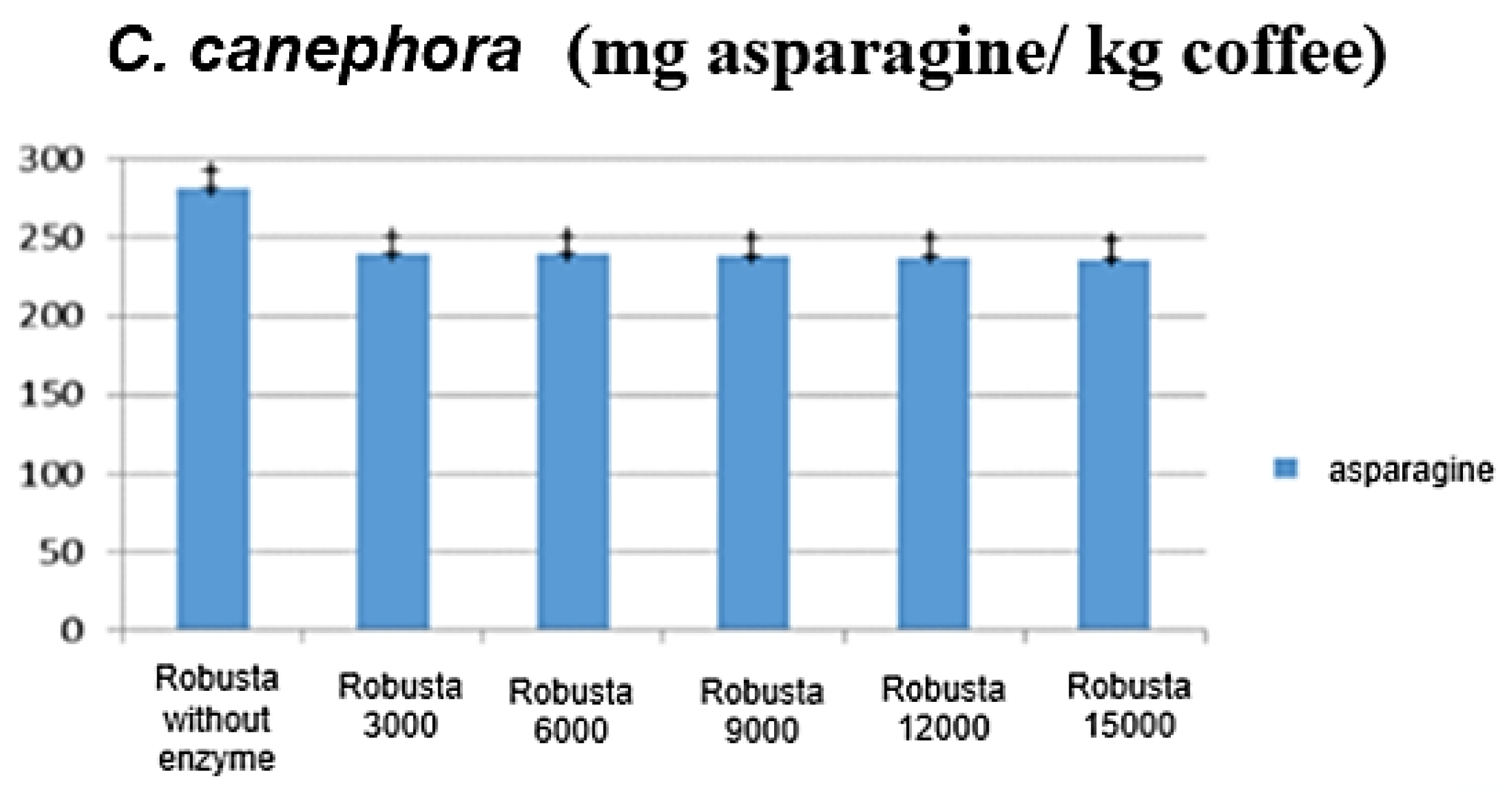
3.2. Analysis of Different Enzymatic Loads
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naidu, M.M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar] [CrossRef]
- Dybing, E.; Farmer, P.B.; Andersen, M.; Fennell, T.R.; Lalljie, S.P.; Müller, D.J. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef]
- Dias, E.D.; Borém, F.M.; Pereira, R.G.; Soares, C.; Cunha, S.; Fernandes, J.O. Determinação dos níveis de acrilamida em cafés verdes obtidos por diferentes processamentos. In Proceedings of the VI Simpósio de Pesquisa dos Cafés do Brasil, Vitória, ES, Brazil, June 2009. [Google Scholar]
- Claeys, W.L.; De Vleeschouer, K.; Hendrickx, M.E. Quantifying the formation of carcinogens during food processing: Acrylamide. Trends Food Sci. Technol. 2005, 16, 181–193. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Review of methods for the reduction of dietary content and toxicity of acrylamide. Food Chem. 2008, 56, 6113–6140. [Google Scholar] [CrossRef] [PubMed]
- Guenther, H.; Anklam, E.; Wenzl, T.; Stadler, R.H. Acrylamide in coffee: Review of progress in analysis, formation and level reduction. Food Addit. Contam. 2007, 24, 60–70. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.G.; Breitling-Utzmann, C.M. Potential antagonistic effects of acrylamide mitigation during coffee roasting on furfuryl alcohol, furan and 5-hydroxymethylfurfural. Toxics 2018, 7, 1. [Google Scholar] [CrossRef]
- IARC. Acrylamide. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans: Some Industrial Chemicals; International Agency for Research on Cancer: Lyon, France, 1994; pp. 389–433. [Google Scholar]
- Anese, M.; Suman, M.; Nicoli, M.C. Acrylamide removal from heated foods. Food Chem. 2010, 119, 791–794. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, M.S.; Granby, K. Current issues in dietary acrylamide: Formation, mitigation and risk assessment. J. Sci. Food Agric. 2014, 94, 9–20. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008, 109, 386–392. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, S.; Granby, K.; Risum, J. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. Food Sci. Technol. 2011, 44, 1473–1476. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Guidance for Industry Acrylamide in Foods. Center for Food Safety and Applied Nutrition. Available online: https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/UCM374534.pdf (accessed on 20 November 2018).
- FDE (Food Drink Europe). Acrylamide Toolbox Document. Available online: http://www.fooddrinkeurope.eu/uploads/publications_documents/Toolboxfinal26 0911.pdf (accessed on 20 August 2018).
- Medeiros, V.R.; Mestdagh, F.; Van Poucke, C.; Kerkaert, B.; De Muer, N.; Denon, Q.; Van Peteghem, C.; De Meulenaer, B. Implementation of acrylamide mitigation strategies on industrial production of french fries: Challenges and pitfalls. J. Agric. Food Chem. 2011, 59, 898–906. [Google Scholar] [CrossRef]
- Elmore, J.S.; Briddon, A.; Dodson, A.T.; Muttucumaru, N.; Halford, N.G.; Mottram, D.S. Acrylamide in potato crisps prepared from 20 UK-grown varieties: Effects of variety and tuber storage time. Food Chem. 2015, 182, 1–8. [Google Scholar] [CrossRef]
- Vinci, R.M.; Mestdagh, F.; De Meulenaer, B. Acrylamide formation in fried potato products: Present and future, a critical review on mitigation strategies. Food Chem. 2012, 133, 1138–1154. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Powers, S.J.; Balagiannis, D.; Elmore, J.S.; Mottram, D.S.; Parry, M.A.; Halford, N.G. Free amino acids and sugars in rye grain: Implications for acrylamide formation. J. Agric. Food Chem. 2010, 58, 1959–1969. [Google Scholar] [CrossRef]
- Halford, N.G.; Curtis, T.Y.; Muttucumaru, N.; Postles, J.; Elmore, J.S.; Mottram, D.S. The acrylamide problem: A plant and agronomic science issue. J. Exp. Bot. 2012, 63, 2841–2851. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Contribution of lipid oxidation products to acrylamide formation in model systems. J. Agric. Food Chem. 2008, 56, 6075–6080. [Google Scholar] [CrossRef]
- Shu, C.K. Pyrazine formation from serine and threonine. J. Agric. Food Chem. 1999, 47, 4332–4335. [Google Scholar] [CrossRef]
- Hamzalioglu, A.; Gökmen, V. Role of bioactive carbonyl compounds on the conversion of asparagine into acrylamide during heating. Eur. Food Res. Technol. 2012, 235, 1093–1099. [Google Scholar] [CrossRef]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Strecker aldehydes and alpha-keto acids, produced by carbonyl-amine reactions, contribute to the formation of acrylamide. Food Chem. 2011, 128, 465–470. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A. Acrylamide from maillard reaction products. Nature 2002, 419, 449. [Google Scholar] [CrossRef]
- Svensson, K.; Abramsson, L.; Becker, W.; Glynn, A.; Hellenäs, K.E.; Lind, Y.; Rose’n, J. Dietary intake of acrylamide in Sweden. Food Chem. Toxicol. 2003, 41, 1581–1586. [Google Scholar] [CrossRef]
- Spiller, G.A. Caffeine; Taylor & Francis: London, UK, 1997. [Google Scholar]
- Schenker, S.; Handschin, S.; Frey, B.; Perren, R.; Eschere, E. Pore structure of coffee beansaffected by roasting conditions. J. Food Sci. 2000, 65, 452–457. [Google Scholar] [CrossRef]
- Whitehurst, R.J.; Van Oort, M. Enzymes in food technology. Int. J. Food Sci. Technol. 2009, 53, 248–256. [Google Scholar]
- Hendriksen, H.V.; Kornbrust, B.A.; Ostergaard, P.R.; Stringer, M.A. Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from aspergillus oryzae. J. Agric. Food Chem. 2013, 57, 4168–4176. [Google Scholar] [CrossRef]
- Association of applied biologists. Acrylamide, furans and other food-borne contaminants: From. Plant Sci. Food Chem. 2013, 8–9. [Google Scholar]
- Xu, F.; Khalid, P.; Oruna-Concha, M.; Elmore, S. Effect of asparaginase on flavour formation in roasted coffee. In Flavour Science, Proceedings of the XIV Weurman Flavour Research Symposium, Queen’s College, Cambridge, UK, 15–19 September 2014; 2015; pp. 563–566. [Google Scholar]
- Xu, F.; Oruna-Concha, M.J.; Elmore, J.S. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016, 210, 163–171. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Oracz, J. Correlation between the stability of chlorogenic acids, antioxidant activity and acrylamide content in coffee beans roasted in different conditions. Int. J. Food Prop. 2015, 2, 290–302. [Google Scholar] [CrossRef]
| Parameter | Reaction Conditions |
|---|---|
| Temperature | 37.0 ± 0.5 °C |
| pH | 7.00 ± 0.05 (at room temperature) |
| L-asparagine | 9.2 mg/mL |
| Enzyme working range | 0.0207–0.0775 ASNU/mL |
| Interval kinetic measuring time | 2 min Incubate 1.5 min before measuring |
| Wavelength | 340 nm |
| NADH | 0.405 mg/mL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, A.C.V.; Freitas-Silva, O.; Souza, E.F.d.; Gottschalk, L.M.F. Effect of Asparaginase Enzyme in the Reduction of Asparagine in Green Coffee. Beverages 2019, 5, 32. https://doi.org/10.3390/beverages5020032
Porto ACV, Freitas-Silva O, Souza EFd, Gottschalk LMF. Effect of Asparaginase Enzyme in the Reduction of Asparagine in Green Coffee. Beverages. 2019; 5(2):32. https://doi.org/10.3390/beverages5020032
Chicago/Turabian StylePorto, Ana Carolina Vieira, Otniel Freitas-Silva, Erika Fraga de Souza, and Leda Maria Fortes Gottschalk. 2019. "Effect of Asparaginase Enzyme in the Reduction of Asparagine in Green Coffee" Beverages 5, no. 2: 32. https://doi.org/10.3390/beverages5020032
APA StylePorto, A. C. V., Freitas-Silva, O., Souza, E. F. d., & Gottschalk, L. M. F. (2019). Effect of Asparaginase Enzyme in the Reduction of Asparagine in Green Coffee. Beverages, 5(2), 32. https://doi.org/10.3390/beverages5020032





