Wine-Derived Phenolic Metabolites in the Digestive and Brain Function
Abstract
1. Introduction
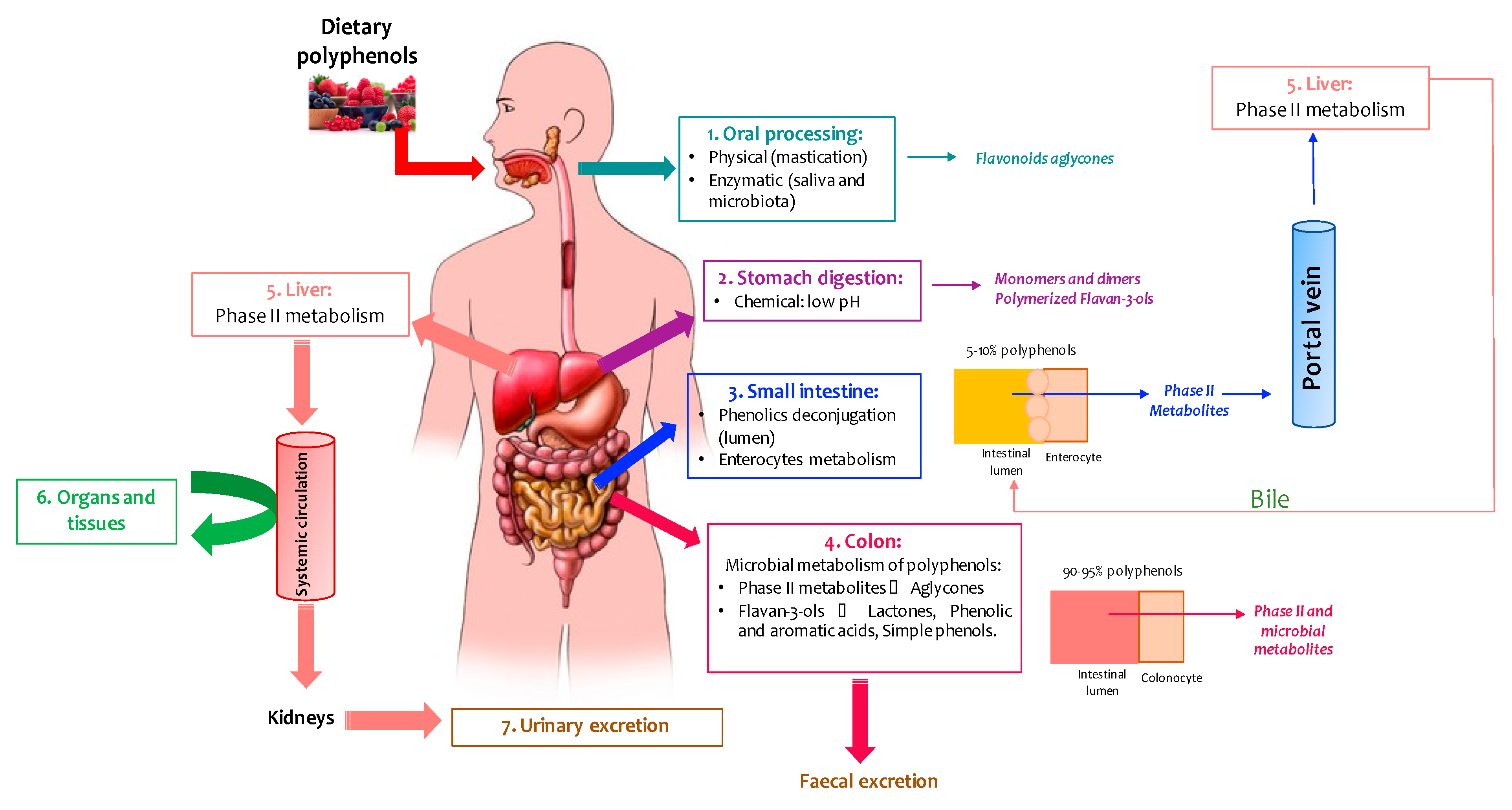
2. Metabolism and Bioavailability of Wine Polyphenols
3. Effects of Wine Polyphenols and Wine-Derived Metabolites at Oral and Intestinal Level
3.1. Implications in Oral Health
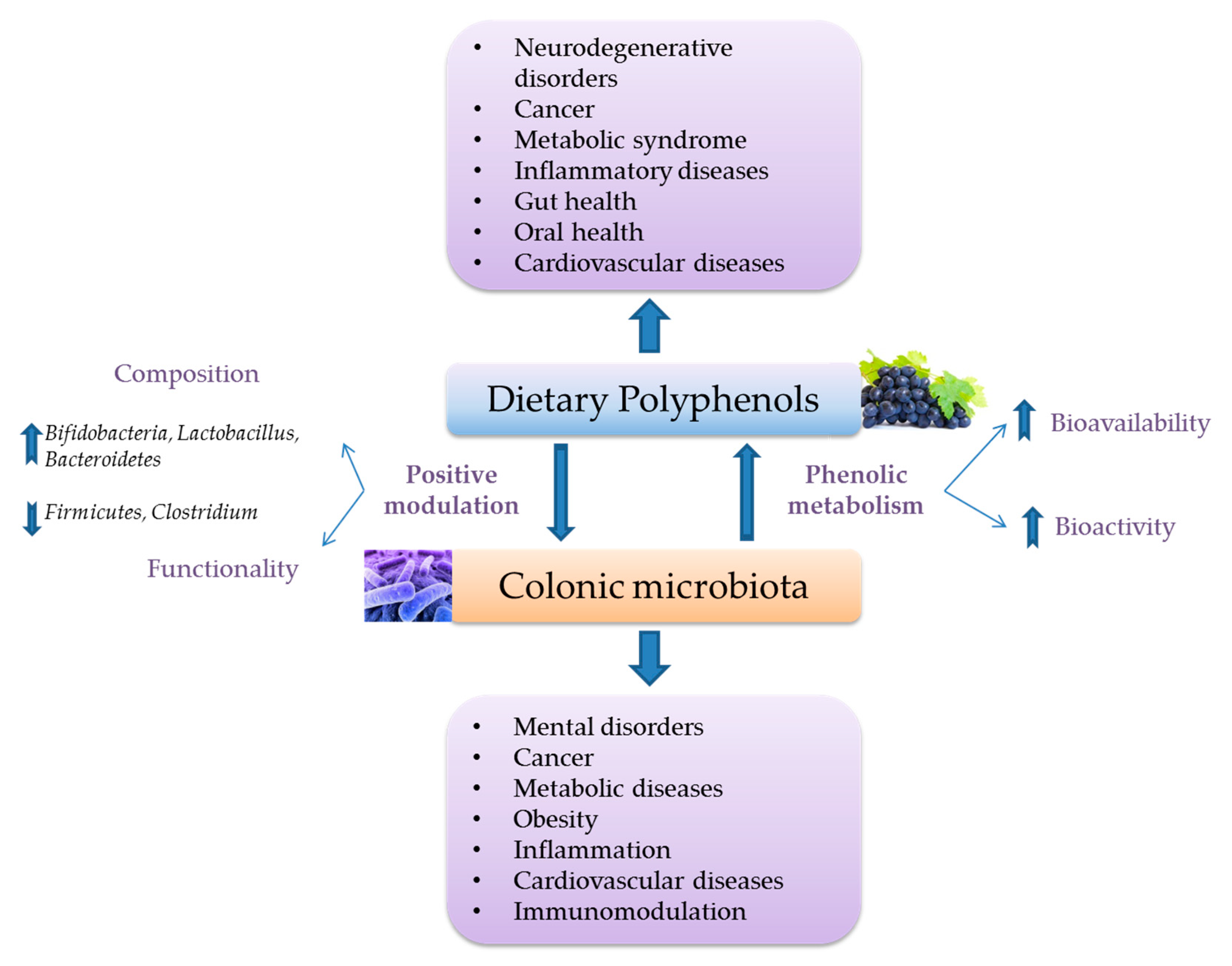
3.2. Interactions with Intestinal Microbiota and Its Functional Metabolic Activity
4. Role of Wine and Polyphenols in Brain Function
4.1. Neurodegenerative Disorders
4.2. Neuroticism as Indicator of Cognitive Disorders: Polyphenols as Genetic Modulators
4.3. Role of Inflammatory Processes in Stress-Induced Depression: Polyphenols as Preventive Agents
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Allison, R.L. Back to Basics: The Effect of Healthy Diet and Exercise on Chronic Disease Management. S. D. Med. 2017, Spec No, 10–18. [Google Scholar]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. The healthy effects of strawberry bioactive compounds on molecular pathways related to chronic diseases. Ann. N. Y. Acad. Sci. 2017, 1398, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillen, M.; Lamuela-Raventos, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Artero, A.; Tarin, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M.; Estruch, R. Mechanism of the Protective Effects of Wine Intake on Cardiovascular Disease. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, M.V., Bartolomé Suáldea, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 231–239. [Google Scholar]
- Cueva, C.; Gil-Sanchez, I.; Ayuda-Duran, B.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Bartolome, B.; Moreno-Arribas, M.V. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Fernandes, I.; Perez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Chapter 26-Wine A2-Frias, Juana. In Fermented Foods in Health and Disease Prevention; Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 593–621. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antiox. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health—A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Llorach, R.; Urpi-Sarda, M.; Khymenets, O.; Bulló, M.; Corella, D.; Fitó, M.; Martínez-González, M.A.; Estruch, R.; Andres-Lacueva, C. An NMR metabolomics approach reveals a combined-biomarkers model in a wine interventional trial with validation in free-living individuals of the PREDIMED study. Metabolomics 2015, 11, 797–806. [Google Scholar] [CrossRef]
- Fernandes, G.L.; Araujo, P.; Tufik, S.; Andersen, M.L. The role of IL-6 and STAT in sleep and neuroinflammation. Clin. Immunol. 2017, 180, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernandez, A.; Ibanez, C.; Simo, C.; Bartolome, B.; Moreno-Arribas, M.V. An Ultrahigh-Performance Liquid Chromatography-Time-of-Flight Mass Spectrometry Metabolomic Approach to Studying the Impact of Moderate Red-Wine Consumption on Urinary Metabolome. J. Proteome Res. 2018, 17, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Esteban-Fernández, A.; González de Llano, D.; Sanz-Buenhombre, M.; Guadarrana, A.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilánc, C.G.; Martín Gómez, L.; García Bermejo, M.L.; et al. Supplementation with grape pomace in healthy women: Changes in biochemical parameters, gut microbiota and related metabolic biomarkers. J. Funct. Foods 2018, 45, 34–46. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Profiling of microbial-derived phenolic metabolites in human feces after moderate red wine intake. J. Agric. Food Chem. 2013, 61, 9470–9479. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2018, 57, 1–15. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Zorraquín-Peña, I.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. The role of wine and food polyphenols in oral health. Trends Food Sci. Technol. 2017, 69, 118–130. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Corona, G.; Vauzour, D.; Spencer, J.P.E. Neuroprotective Effects Associated with Wine and Its Phenolic Constituents. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, V.M., Bartolomé Suáldea, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 279–292. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Martinez Huelamo, M.; Rotches Ribalta, M.; Estruch, R.; Ferrer, E.E.; Andres-Lacueva, C.; Illan, M.; Lamuela-Raventós, R.M. Oil matrix effects on plasma exposure and urinary excretion of phenolic compounds from tomato sauces: Evidence from a human pilot study. Food Chem. 2012, 130, 581–590. [Google Scholar] [CrossRef]
- Rubio, L.; Macia, A.; Motilva, M.J. Impact of various factors on pharmacokinetics of bioactive polyphenols: An overview. Curr. Drug Metab. 2014, 15, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Manach, C.; Faulks, R.M.; Kroon, P.A. Absorption and Metabolism of Dietary Plant Secondary Metabolites. In Plant Secondary Metabolites; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 303–351. [Google Scholar]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green Tea Flavan-3-ols: Colonic Degradation and Urinary Excretion of Catabolites by Humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Boto-Ordonez, M.; Rothwell, J.A.; Andres-Lacueva, C.; Manach, C.; Scalbert, A.; Urpi-Sarda, M. Prediction of the wine polyphenol metabolic space: An application of the Phenol-Explorer database. Mol. Nutr. Food Res. 2014, 58, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Giron, A.; Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Munoz-Gonzalez, I.; Sanchez-Patan, F.; Monagas, M.; Martin-Alvarez, P.J.; Murri, M.; Tinahones, F.J.; Andres-Lacueva, C.; et al. Comparative study of microbial-derived phenolic metabolites in human feces after intake of gin, red wine, and dealcoholized red wine. J. Agric. Food Chem. 2013, 61, 3909–3915. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharm. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Palmer, R.J., Jr. Composition and development of oral bacterial communities. Periodontol 2000 2014, 64, 20–39. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Ruby, J.; Barbeau, J. The buccale puzzle: The symbiotic nature of endogenous infections of the oral cavity. Can. J. Infect. Dis. 2002, 13, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A. The oral microbiome: Its role in health and in oral and systemic infections. Clin. Microbiol. Newsl. 2013, 35, 163–169. [Google Scholar] [CrossRef]
- Okada, H.; Murakami, S. Cytokine expression in periodontal health and disease. Crit. Rev. Oral Biol. Med. 1998, 9, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Al-Haroni, M.; Skaug, N.; Bakken, V.; Cash, P. Proteomic analysis of ampicillin-resistant oral Fusobacterium nucleatum. Oral Microbiol. Immunol. 2008, 23, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, I.; Thurnheer, T.; Bartolomé, B.; Moreno-Arribas, M.V. Red Wine and Oenological Extracts Display Antimicrobial Effects in an Oral Bacteria Biofilm Model. J. Agric. Food Chem. 2014, 62, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- Aureli, A.; Sebastiani, P.; Del Beato, T.; Marimpietri, A.E.; Graziani, A.; Sechi, E.; Di Loreto, S. Involvement of IL-6 and IL-1 receptor antagonist on intellectual disability. Immunol. Lett. 2014, 162, 124–131. [Google Scholar] [CrossRef]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Esteban-Fernandez, A.; Zorraquin-Pena, I.; Ferrer, M.D.; Mira, A.; Bartolome, B.; Gonzalez de Llano, D.; Moreno-Arribas, M.V. Inhibition of Oral Pathogens Adhesion to Human Gingival Fibroblasts by Wine Polyphenols Alone and in Combination with an Oral Probiotic. J. Agric. Food Chem. 2018, 66, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Nieuwdorp, M. Genomics: A gut prediction. Nature 2013, 498, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Claesson, M.J.; O’Toole, P.W.; Shanahan, F. Categorization of the gut microbiota: Enterotypes or gradients? Nat. Rev. Microbiol. 2012, 10, 591. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In Vitro Bioconversion of Polyphenols from Black Tea and Red Wine/Grape Juice by Human Intestinal Microbiota Displays Strong Interindividual Variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Coin-Araguez, L.; Roca-Rodriguez, M.M.; Delgado-Lista, J.; Cardona, F.; Andres-Lacueva, C.; Tinahones, F.J. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am. J. Clin. Nutr. 2013, 97, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.; Munoz-Gonzalez, I.; Jimenez, E.; Bartolome, B.; Moreno-Arribas, M.V.; Pelaez, C.; Del Carmen Martinez-Cuesta, M.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2016, 61, 1600620. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Reyes-Gavilan, C.G.; Ruas-Madiedo, P.; Lopez, P.; Suarez, A.; Gueimonde, M.; Gonzalez, S. Red wine consumption is associated with fecal microbiota and malondialdehyde in a human population. J. Am. Coll. Nutr. 2015, 34, 135–141. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Espin, J.C.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Gieger, C.; Hauner, H.; Daniel, H.; Linseisen, J. Metabotyping and its application in targeted nutrition: An overview. Br. J. Nutr. 2017, 117, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, C.B.; Walsh, M.C.; Gibney, M.J.; Gibney, E.R.; Brennan, L. Can metabotyping help deliver the promise of personalised nutrition? Proc. Nutr. Soc. 2016, 75, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Koverech, G.; Luca, M.; Calandra, C.; Calabrese, V. Chapter 19—Neuroprotective Mechanisms of Dietary Phytochemicals: Implications for Successful Brain Aging A2—Malavolta, Marco. In Molecular Basis of Nutrition and Aging; Mocchegiani, E., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 251–261. [Google Scholar]
- Stockley, C.S. Role of wine components on inflammation and chronic diseases. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, M.V., Bartolomé Suáldea, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 240–258. [Google Scholar]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.B. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. 1997, 153, 185–192. [Google Scholar] [PubMed]
- Weyerer, S.; Schaufele, M.; Wiese, B.; Maier, W.; Tebarth, F.; van den Bussche, H.; Pentzek, M.; Bickel, H.; Luppa, M.; Riedel-Heller, S.G. Current alcohol consumption and its relationship to incident dementia: Results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing 2011, 40, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Frisardi, V.; Lorusso, M.; Santamato, A.; Seripa, D.; Pilotto, A.; Scafato, E.; et al. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. J. Alzheimers Dis. 2009, 17, 7–31. [Google Scholar] [CrossRef]
- Arntzen, K.A.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B. Moderate wine consumption is associated with better cognitive test results: A 7 year follow up of 5033 subjects in the Tromso Study. Acta Neurol. Scand. Suppl. 2010, 122, 23–29. [Google Scholar] [CrossRef]
- Truelsen, T.; Thudium, D.; Gronbaek, M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of Moderate Alcohol Consumption on Cognitive Function in Women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef]
- Assuncao, M.; Santos-Marques, M.J.; de Freitas, V.; Carvalho, F.; Andrade, J.P.; Lukoyanov, N.V.; Paula-Barbosa, M.M. Red wine antioxidants protect hippocampal neurons against ethanol-induced damage: A biochemical, morphological and behavioral study. Neuroscience 2007, 146, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimers Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef]
- Hurley, L.L.; Akinfiresoye, L.; Kalejaiye, O.; Tizabi, Y. Antidepressant effects of resveratrol in an animal model of depression. Behav. Brain Res. 2014, 268, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Santa-Maria, I.; Ho, L.; Ksiezak-Reding, H.; Ono, K.; Teplow, D.B.; Pasinetti, G.M. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Zhao, J.; Kurihara, H.; Nakagata, N.; Okajima, K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J. Nutr. Biochem. 2011, 22, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Vauzour, D.; Spencer, J.P. Neuroinflammation and its modulation by flavonoids. Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 211–224. [Google Scholar] [CrossRef]
- Basu Mallik, S.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Dukie, S.A.; Grant, G.; Rao, C.M.; Arora, D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Solanki, I.; Parihar, P.; Parihar, M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 2016, 95, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE Pathway by Food Polyphenols: A Nutritional Neuroprotective Strategy for Cognitive and Neurodegenerative Disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Münch, G. Plant polyphenols as inhibitors of NF-κB induced cytokine production—A potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 2014, 1842, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Palavra, F.; Ambrósio, A.F.; Reis, F. Chapter 19—mTOR and Neuroinflammation A2—Maiese, Kenneth. In Molecules to Medicine with mTOR; Academic Press: Boston, MA, USA, 2016; pp. 317–329. [Google Scholar]
- Ormel, J.; Jeronimus, B.F.; Kotov, R.; Riese, H.; Bos, E.H.; Hankin, B.; Rosmalen, J.G.M.; Oldehinkel, A.J. Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clin. Psychol. Rev. 2013, 33, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Ohi, K.; Shimada, T.; Yasuyama, T.; Kimura, K.; Uehara, T.; Kawasaki, Y. Spatial and temporal expression patterns of genes around nine neuroticism-associated loci. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 164–171. [Google Scholar] [CrossRef]
- De Moor, M.H.; van den Berg, S.M.; Verweij, K.J.; Krueger, R.F.; Luciano, M.; Arias Vasquez, A.; Matteson, L.K.; Derringer, J.; Esko, T.; Amin, N.; et al. Meta-analysis of Genome-wide Association Studies for Neuroticism, and the Polygenic Association with Major Depressive Disorder. JAMA Psychiatry 2015, 72, 642–650. [Google Scholar] [CrossRef]
- Kim, Y.K.; Won, E. The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behav. Brain Res. 2017, 329, 6–11. [Google Scholar] [CrossRef]
- Hodes, G.E.; Ménard, C.; Russo, S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress 2016, 4, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.P.; Cavegn, N.; Nix, A.; do Nascimento Bevilaqua, M.C.; Stangl, D.; Zainuddin, M.S.A.; Nardi, A.E.; Gardino, P.F.; Thuret, S. The Role of Dietary Polyphenols on Adult Hippocampal Neurogenesis: Molecular Mechanisms and Behavioural Effects on Depression and Anxiety. Oxid. Med. Cell. Longev. 2012, 2012, 541971. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Vignes, M.; Maurice, T.; Lante, F.; Nedjar, M.; Thethi, K.; Guiramand, J.; Recasens, M. Anxiolytic properties of green tea polyphenol (-)-epigallocatechin gallate (EGCG). Brain Res. 2006, 1110, 102–115. [Google Scholar] [CrossRef]
- Barros, D.; Amaral, O.B.; Izquierdo, I.; Geracitano, L.; do Carmo Bassols Raseira, M.; Henriques, A.T.; Ramirez, M.R. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol. Biochem. Behav. 2006, 84, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; You, W.; Zhang, X.; Li, S.; Barish, P.A.; Vernon, M.M.; Du, X.; Li, G.; Pan, J.; et al. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. 2010, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.-L.; Manaye, K.; Khan, I.; Luo, Y. Antidepressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 2010, 58, 911–920. [Google Scholar] [CrossRef]
- Tomic, M.; Ignjatovic, D.; Tovilovic-Kovacevic, G.; Krstic-Milosevic, D.; Rankovic, S.; Popovic, T.; Glibetic, M. Reduction of anxiety-like and depression-like behaviors in rats after one month of drinking Aronia melanocarpa berry juice. Food Funct. 2016, 7, 3111–3120. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Liu, H.; Wang, X.; Bo, S.; Xie, Y.; Bai, X.; Wu, L.; Wang, Z.; Liu, D. Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortex. Behav. Brain Res. 2016, 302, 191–199. [Google Scholar] [CrossRef]
- Jernigan, C.S.; Goswami, D.B.; Austin, M.C.; Iyo, A.H.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1774–1779. [Google Scholar] [CrossRef]
- Gold, P.W.; Machado-Vieira, R.; Pavlatou, M.G. Clinical and Biochemical Manifestations of Depression: Relation to the Neurobiology of Stress. Neural. Plast. 2015, 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Y.; Jia, X.; Wang, Q.; Li, Y.; Hu, M.; Tian, L.; Yang, J.; Xing, W.; Zhang, W.; et al. Neuroprotection by IFN-γ via astrocyte-secreted IL-6 in acute neuroinflammation. Oncotarget 2017, 8, 40065–40078. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. The pathological and physiological roles of IL-6 amplifier activation. Int. J. Biol. Sci. 2012, 8, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry 2014, 71, 1121–1128. [Google Scholar] [CrossRef]
- Luo, Y.; He, H.; Zhang, M.; Huang, X.; Fan, N. Altered serum levels of TNF-alpha, IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. 2016, 244, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Alberts, I.; Li, X. Brain IL-6 and autism. Neuroscience 2013, 252, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2013, 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-kappaB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Martyn, D.; Jacquier, J.; O’Riordan, D.; Gibney, E.R. Inhibition of proinflammatory biomarkers in THP1 macrophages by polyphenols derived from chamomile, meadowsweet and willow bark. Phytother. Res. 2013, 27, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.B.; Sung, N.Y.; Byun, E.H.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Byun, M.W.; et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-kappaB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2013, 15, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Joo, Y.-E. Theaflavin Inhibits LPS-Induced IL-6, MCP-1, and ICAM-1 Expression in Bone Marrow-Derived Macrophages Through the Blockade of NF-κB and MAPK Signaling Pathways. Chonnam Med. J. 2011, 47, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Capiralla, H.; Vingtdeux, V.; Venkatesh, J.; Dreses-Werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Identification of potent small-molecule inhibitors of STAT3 with anti-inflammatory properties in RAW 264.7 macrophages. FEBS J. 2012, 279, 3791–3799. [Google Scholar] [CrossRef]
- Dinicola, S.; Santiago-Reyes, M.; Canipari, R.; Cucina, A.; Bizzarri, M.; Fuso, A. Alpha-lipoic acid represses IL-1B and IL-6 through DNA methylation in ovarian cells. PharmaNutrition 2017, 5, 77–83. [Google Scholar] [CrossRef]
- Matt, S.M.; Lawson, M.A.; Johnson, R.W. Aging and peripheral lipopolysaccharide can modulate epigenetic regulators and decrease IL-1beta promoter DNA methylation in microglia. Neurobiol. Aging 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Poplutz, M.K.; Wessels, I.; Rink, L.; Uciechowski, P. Regulation of the Interleukin-6 gene expression during monocytic differentiation of HL-60 cells by chromatin remodeling and methylation. Immunobiology 2014, 219, 619–626. [Google Scholar] [CrossRef]
- Klengel, T.; Pape, J.; Binder, E.B.; Mehta, D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology 2014, 80, 115–132. [Google Scholar] [CrossRef]
- Nicolia, V.; Cavallaro, R.A.; López-González, I.; Maccarrone, M.; Scarpa, S.; Ferrer, I.; Fuso, A. DNA Methylation Profiles of Selected Pro-Inflammatory Cytokines in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2017, 76, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xu, X. Chapter 8—The epigenetics of brain aging and psychiatric disorders A2—Yasui, Dag H. In Neuropsychiatric Disorders and Epigenetics; Peedicayil, J., Grayson, D.R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 141–162. [Google Scholar]
- Cuevas, A.; Saavedra, N.; Salazar, L.A.; Abdalla, D.S.P. Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased Expression of Synapse-Related Genes and Loss of Synapses in Major Depressive Disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef]
- Aston, C.; Jiang, L.; Sokolov, B.P. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol. Psychiatry 2005, 10, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.A.; Christoffel, D.J.; Heshmati, M.; Hodes, G.E.; Magida, J.; Davis, K.; Cahill, M.E.; Dias, C.; Ribeiro, E.; Ables, J.L.; et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 2013, 19, 337–344. [Google Scholar] [CrossRef]
- Christoffel, D.J.; Golden, S.A.; Heshmati, M.; Graham, A.; Birnbaum, S.; Neve, R.L.; Hodes, G.E.; Russo, S.J. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology 2012, 37, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.L.; Russo, S.J. Neuroinflammation regulates cognitive impairment in socially defeated mice. Trends Neurosci. 2016, 39, 353–355. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorraquín-Peña, I.; Esteban-Fernández, A.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. Wine-Derived Phenolic Metabolites in the Digestive and Brain Function. Beverages 2019, 5, 7. https://doi.org/10.3390/beverages5010007
Zorraquín-Peña I, Esteban-Fernández A, González de Llano D, Bartolomé B, Moreno-Arribas MV. Wine-Derived Phenolic Metabolites in the Digestive and Brain Function. Beverages. 2019; 5(1):7. https://doi.org/10.3390/beverages5010007
Chicago/Turabian StyleZorraquín-Peña, Irene, Adelaida Esteban-Fernández, Dolores González de Llano, Begoña Bartolomé, and M. Victoria Moreno-Arribas. 2019. "Wine-Derived Phenolic Metabolites in the Digestive and Brain Function" Beverages 5, no. 1: 7. https://doi.org/10.3390/beverages5010007
APA StyleZorraquín-Peña, I., Esteban-Fernández, A., González de Llano, D., Bartolomé, B., & Moreno-Arribas, M. V. (2019). Wine-Derived Phenolic Metabolites in the Digestive and Brain Function. Beverages, 5(1), 7. https://doi.org/10.3390/beverages5010007




