Abstract
A preliminary evaluation of yeast fermented palm wine sourced from Imo State in Nigeria was carried out to establish compounds that contribute to the distinct flavor of the beverage and to determine if the product abundance is affected when the drink is supplemented with Sacoglottis gabonensis. Palm wine samples from two different trees Elaeis sp. and Raphia sp. (pH less than 5) that contain Saccharomyces cerevisiae and other yeast species identified by sequencing the D1/D2 domain of the 26S rRNA genes were used. Evaluation was carried out using high performance liquid chromatography (HPLC), atmospheric pressure chemical ionization-mass spectrometry (APCI-MS) and gas chromatography-mass spectrometry (GC-MS). Samples contained 5.9–11.6, 2.2–7.1, 4.2–43.0, and 4.4–43.7 g/L of acetic acid, lactic acid, ethanol and glucose, respectively. Ethyl acetate, acetic acid and ethanol had the most aroma intensity and an assessment on the yeast metabolome database showed that 23 out of the 31 products detected were present in the database. Addition of Sacoglottis gabonensis supplement to a Raphia sp. palm wine sample showed lower abundance of acetoin, acetic acid, methylpropyl lactate, ethyl octanoate and propyl acetate. We conclude that Sacoglottis gabonensis supplementation could suppress specific compounds during palm wine fermentation. This knowledge could be applied in new product development for the beverage.
1. Introduction
Palm wine is a fermented traditional beverage consumed in many parts of the world and well known as a white colored alcoholic drink. The drink is known by different names around the world [1] and is regarded as a heavy suspension of yeasts in fermenting palm sap [2]. Even though palm wine is consumed around the world and plays an important role in the economic and social life of the people, palm wine has not been comprehensively evaluated for quality improvement and possible exploitation of the biological and chemical constituents or byproducts [3]. The methods for tapping palms have been reported [4]. According to the report, the techniques are numerous, varies drastically from one continent to another and sap yields may depend on the skills of the tapper. It was noted that in Ghana incision of stem apex of felled palm is preferred, whereas in Nigeria excision of male or female inflorescence is carried out to initiate sap flow, which is collected in a gourd or plastic container. The report pointed out that tapping the inflorescence is practiced throughout Southeast Asia and that the most advanced method of tapping is believed to be tapping applied to the inflorescence spadix, which guarantees a high yield of sap for long periods without affecting the well-being of the tree.
Yeasts, lactic acid bacteria (LAB) and acetic acid bacteria (AAB) are the most reported microbial constituent in literature and the species Saccharomyces cerevisiae is normally the main organism of interest because it is mainly responsible for converting the sugary sap to alcohol. Palm wine drinkers know that the drink tastes differently at different stages of fermentation as a result of yeast fermentation and accumulation of organic acids especially acetic acid from fermentation by AAB as fermentation progresses each day [5]. It is common knowledge that palm wine fermentation consists of an initial lactic acid fermentation by lactic acid bacteria, a middle alcoholic fermentation by yeasts followed by a final acetic fermentation by acetic acid bacteria and the general consensus among other investigators is that the nature of fermentation depends on the composition of sap, type of palm tree and location of the tree [1,3,6].
Most palm wine tappers in South East Nigeria get their palm wine from three types of palm trees namely, the palm oil tree Elaeis guineensis or Raphia palms, namely Raphia hookeri and Raphia vinifera [6], and the flavor or aroma of palm wine obtained from Elaeis sp. is somewhat different from that of Raphia sp. To the best of the knowledge of the authors, the drink sourced from trees in Imo State area have not been subjected to current analytical methods (HPLC, APCI-MS and GC-MS) to generate more information. However, volatiles [7] from the southwest region and odorants [8] of palm wine from E. guineensis sourced from other regions in Nigeria have been studied and it was found that no one compound is responsible for the characteristic palm wine odor. Odorants responsible for the intense aroma qualities perceived upon sample introduction into the mouth, while swallowing the drink and nasal aroma perception have been reported [9] but quantification of the intensity of the main compounds responsible for the intense odor of fermenting palm wine in the atmosphere is yet to be explored by many workers.
To improve taste of palm wine during fermentation in southeastern region of Nigeria, the bark of a local plant Sacoglottis gabonensis known locally as Nche can be added as a supplement to preserve Raphia sp. palm wine [10]. Reports of toxicity to humans on consumption of palm wine containing the supplement are rare, but it has been reported that intraperitoneal administration of doses ranging from 400–3200 mg/kg aqueous extracts in rats produced varying degrees of toxicity that included depression, drowsiness, unsteady gait, paralysis of hind limbs, coma and death [11]. A holistic mechanism of palm wine preservation with the supplement showing the exact compounds in palm wine reduced or biochemical pathways involved has not been reported. Preservation may be aided by reduction in pH which has been reported to be due to the production of lactic acid and acetic acid by LAB and AAB [3]. Reduction of pH for samples supplemented with S. gabonensis has been shown earlier by other investigators [12].
Current commercial production of bottled palm wine in Nigeria has failed to reproduce a drink with exactly the same flavor characteristics of the fresh local drink and in most labels on bottled palm wine, the nutritional composition showing quantities of other compounds apart from alcohol are not displayed. A better understanding of the palm wine fermentation products is needed to encourage the commercial exploitation of the numerous aroma found in the drink and enable quality and taste consistency across the various bottled commercial palm wines currently produced. Up to the time of this work the products of Nigerian palm wine have not been compared to the compounds listed by Jewison et al. [13] in the yeast metabolome database (YMDB).
Therefore, the aims of this study were to evaluate the products of yeasts fermentation that contributes to the flavor of palm wine, establish if the equivalent compound produced is held in the yeast metabolome database and ascertain the effect of S. gabonensis supplement on the fermentation products of palm wine.
2. Materials and Methods
2.1. Samples Collection
Palm wine samples sold in plastic containers were purchased from different locations in Imo State, South East Nigeria. Samples of palm wine from Elaeis sp. were purchased from Ohaji, Orlu and Orodo towns whereas samples from Raphia sp. were purchased from Ikeduru area. A pair of the same batch of palm wine samples from Raphia sp., one supplemented with S. gabonensis and the other containing no supplement was purchased from Mbaise town. Sellers advised that samples were in the first day after production. Samples were set up on the day of purchase for physico-chemical and microbiological analysis while the rest were stored at 4 °C for further use.
2.2. Physico-Chemical Analysis
Palm wine samples for pH monitoring were set up in aliquots of 100 mL and three replicates in glass conical flasks at room temperature (26 °C) and measured daily for 10 days with a pH meter (Hannah HI 98171, Woonsocket RI, USA).
Quantification of glucose, lactic acid, ethanol and acetic acid was carried out using standard high performance liquid chromatography (HPLC) performed according to Yang et al. [14]. Aliquots (5 mL) of palm wine in duplicate were placed in 30 mL universal bottles and centrifuged at 3000 × g for 1 min before filtering the supernatant with 0.45-µm filter membrane (Millipore; Bedford, MA, USA). After 1 mL of the filtered supernatant was placed into duplicate 2 mL amber vials (Chromacol 11573690; Fisher Scientific, Loughborough, UK), the samples were passed through Rezex ion-exclusion ROA organic acid H+, 300 × 7.8 mm column (Phenomenex, Cheshire, UK). Quantities of glucose, lactic acid, ethanol and acetic acid were calculated by reference to chromatographic peak areas of standards with known concentrations.
2.3. Yeast Identification
Yeasts were recovered from palm wine after purchase using standard methods by spreading 100 µL of fermented palm wine on Rose-Bengal chloramphenicol agar (CM0549; Oxoid, Basingstoke, UK) prepared with the supplement (SR0078; Oxoid). Forty-two single colonies (six per sample) in total were selected from colonies that emerged after 48 h incubation at 28 °C, following which they were examined with light microscope to confirm typical yeast morphology. Standard baker’s yeast (S288c) and wine yeast (NCYC 1406) sourced from Bioenergy and Brewery research group, University of Nottingham, United Kingdom were used as controls.
Strains were grown in Yeast Peptone Dextrose (YPD) medium (Sigma-Aldrich) before DNA templates were extracted from each strain using a Yeast DNA Extraction Kit (Thermo Scientific, Waltham, IL, USA) following manufacturer’s instructions. Species identification was performed after PCR amplification of the ITS1-5.8S rDNA-ITS2 regions using HaeIII restriction endonuclease (Promega, Madison, WI, USA) for restriction fragment length polymorphism (RFLP) analysis according to Esteve-Zarzoso et al. [15]. The PCR reaction was carried out using 0.5 µM of primers ITS1 (5′ TCCGTAGGTGAACCTGCGG 3′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′) under the following conditions: initial denaturation at 95 °C for 5 min; 35 cycles of denaturing at 94 °C for 1 min, annealing at 55.5 °C for 2 min and extension at 72 °C for 2 min; and a final extension at 72 °C for 10 min. To 25 µL of unpurified amplified PCR fragments, 0.5 µL of HaeIII enzyme was added to make a final volume of 25.5 µL, after which the mixture was incubated for 3 h at 37 °C and then viewed in 3% agarose gel.
For selected strains, identities were confirmed by sequencing (MWG Eurofins, Ebersberg, Germany) of the D1/D2 domain of the nuclear 26S rRNA previously performed by others [16,17]. PCR amplicons were generated using the primers NL1 (5′-GCATATCAATAAGCG GAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACG G-3′) under the following conditions: initial denaturation at 94 °C for 5 min, then 30 cycles at 94 °C for 90 s, 53 °C for 30 s and 72 °C for 90 s before carrying out the final extension at 72 °C for 7 min. Species confirmation was carried out by subjecting sequences obtained to a basic local alignment search tool (BLAST) activity on the NCBI database (http://blast. ncbi.nlm.nih.gov/Blast), after which identification was carried out based on sequences of closest relatives. Sequenced strains were stored at −85 °C for reference purposes and the sequences obtained were submitted to EMBL nucleotide sequence data library.
2.4. Atmospheric Pressure Chemical Ionisation-Mass Spectrometry
Aroma intensity from palm wine was measured directly in real time by static head space quantification using atmospheric pressure chemical ionization-mass spectrometry (APCI-MS) analysis as performed by Tsachaki et al. [18]. Duplicate samples (20 mL) were placed in a 200 mL Duran bottle (Sigma-Aldrich, Poole, U.K.) and left overnight (approximately 18 h) to equilibrate at room temperature (22 °C). The Duran bottle containing the palm wine was fitted with a one port lid connected to the APCI-MS equipment operating in full scan mode. The dominant ion species were noted and signal intensities of distinct peaks in the head space of the Duran bottle were recorded and areas representing volatile release within 10 s were captured.
2.5. Gas Chromatography-Mass Spectrometry
Gas chromatography-mass spectrometry (GC-MS) was carried out as previously described [19]. Compound identification was based on comparison of GC-MS spectra with those of a standard library of mass spectra (NIST MS Search Version 2.0; National Institute Standards and Technology, Gaithersburg, MD, USA). A search was also carried out on the yeast metabolome database (YMDB) to verify if compounds identified are associated with yeast fermentation [13]. Compound or product abundance was estimated by comparing peak areas and expressing each compound peak area as a percentage of the maximum peak area obtained for the same compound across all samples analyzed. Samples in duplicate were stored at 4 °C for up to 2 weeks after purchase before analysis was carried out to determine volatiles.
2.6. Statistical Analysis
Analysis of variance and T-tests for samples were determined using Minitab 17 software (Minitab Inc., PA, USA). Analysis was based on 95% confidence limit.
3. Results
3.1. PH
In other to measure the freshness of the palm wine samples, pH of samples were monitored and there was a decrease in pH for all samples after which a stable pH (between 3 and 4) was achieved after seven-day storage. Overall, the pH decrease was from 4.50 to 3.0 after 10-day storage. There was no significant difference (p > 0.05) in pH of samples supplemented with S. gabonensis and pH of samples without the supplement but samples with the supplement had lower pH in the first four days before all samples averaged pH of 3.5 after five days.
3.2. HPLC Analysis
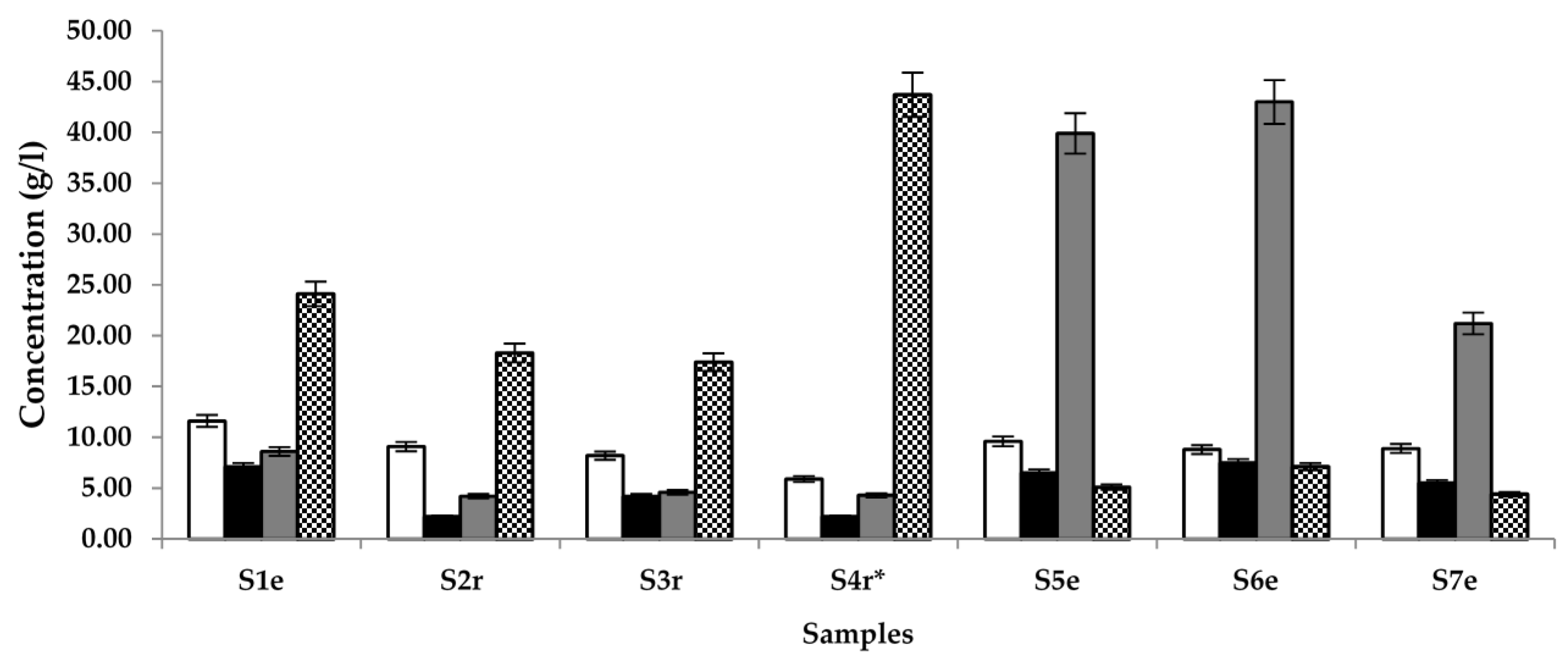
The overall quality of the palm wine samples under study was determined by measuring the quantity of compounds that are generally known to be in abundance in the early (Lactic acid and glucose), middle (ethanol) and later stages (acetic acid) of palm wine fermentation using HPLC. Results (Figure 1) show that the samples contained 5.9–11.6, 2.2–7.1, 4.2–43.0, and 4.4–43.7 g/L of acetic acid, lactic acid, ethanol and glucose, respectively. The highlight of this assay is that the sample with S. gabonensis supplement showed the highest glucose content and the least lactic or acetic acid content. Most samples from Raphia sp. tree had less alcohol but more glucose than samples from Elaeis sp.
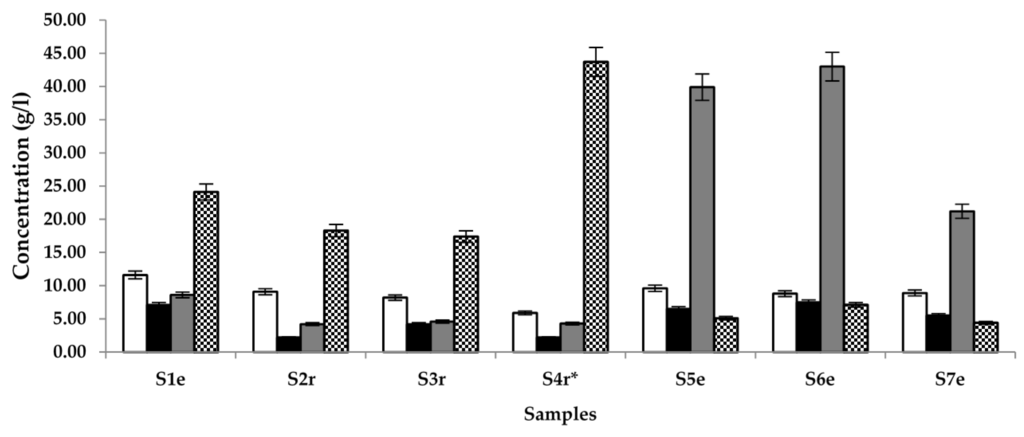
Figure 1.
Quantification of the main compounds of palm wine fermentation, namely acetic acid (□), lactic acid (■), ethanol (  ) and glucose (
) and glucose (  ) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
 ) and glucose (
) and glucose (  ) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
3.3. Molecular Identification
Since the samples were undergoing natural fermentation, it was fundamental to determine the yeast diversity of the samples. The molecular characterization of yeast isolates from the fermented palm wine showed narrow yeast diversity since only four different yeast species namely, Pichia kudriavzevii, Candida tropicalis and Candida ethanolica were identified. The species P. kudriavzevii, C. tropicalis and C. ethanolica were not recovered in all samples but occurred in palm wine from both Elaeis sp. and Raphia sp. palm trees. The sequenced isolates from palm wine supplemented with S. gabonensis did not yield other genera except S. cerevisiae.
The approximate band produced by S. cerevisiae, P. kudriavzevii, C. tropicalis and C. ethanolica after PCR amplification of ITS1-5.8S rDNA-ITS2 region is shown in Table 1 and the distinct bands (bp) after restriction with HaeIII endonuclease for S. cerevisiae, P. kudriavzevii, C. tropicalis and C. ethanolica was as previously described [14]. Overall, the distribution was S. cerevisiae (70.40%), P. kudriavzevii (15.14%), C. ethanolica (9.76%) and C. tropicalis (4.70%). The identities of selected strains confirmed by 26S rRNA sequencing were assigned accession numbers HG425325-42 and six of the S. cerevisiae strains had an identity match of 100% with their closest relative on the NCBI database. Sequence details can be consulted at European nucleotide archives website (www.ebi.ac.uk/ena)

Table 1.
Identification of yeasts strains from palm wine sourced from two different palm tree species.
3.4. APCI-MS Analysis
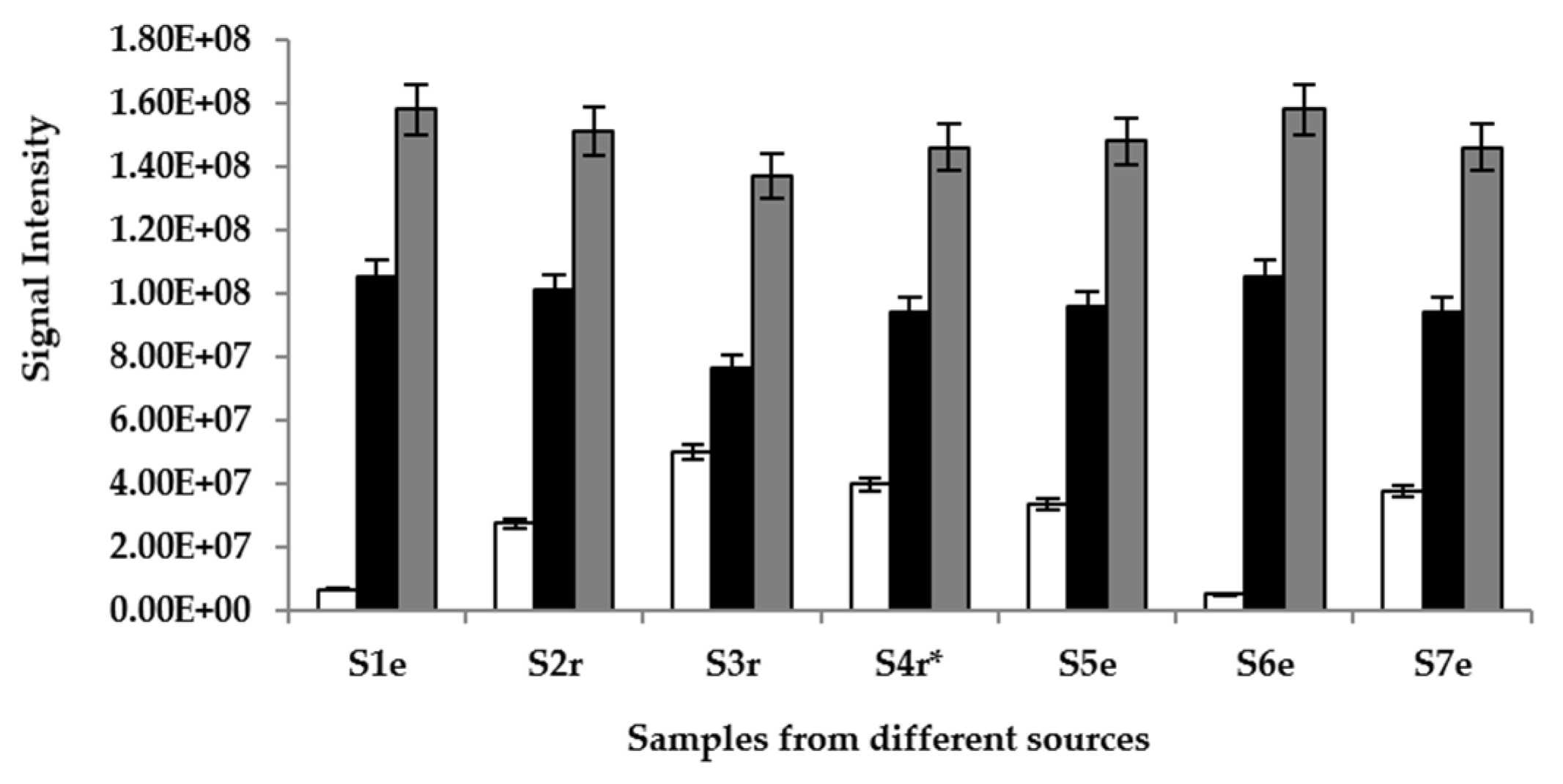
In order to establish and quantify the intensity of the main volatiles that are responsible for the drink’s environmental distinct aroma, the equilibrium headspace above the palm wine samples were analyzed in full scan mode by APCI-MS and it was found that ions m/z 47, 61 and 89 were the most abundant species and the intensities of all other ions were about 10 times less. The ions corresponded to ethanol, acetic acid and ethyl acetate, respectively, and were detected within 10 s of connecting the samples to the equipment. Ethyl acetate showed the most intensity (Figure 2). There was no significant difference (p > 0.05) in the intensity of samples supplemented with S. gabonensis and un-supplemented samples for each compound detected.
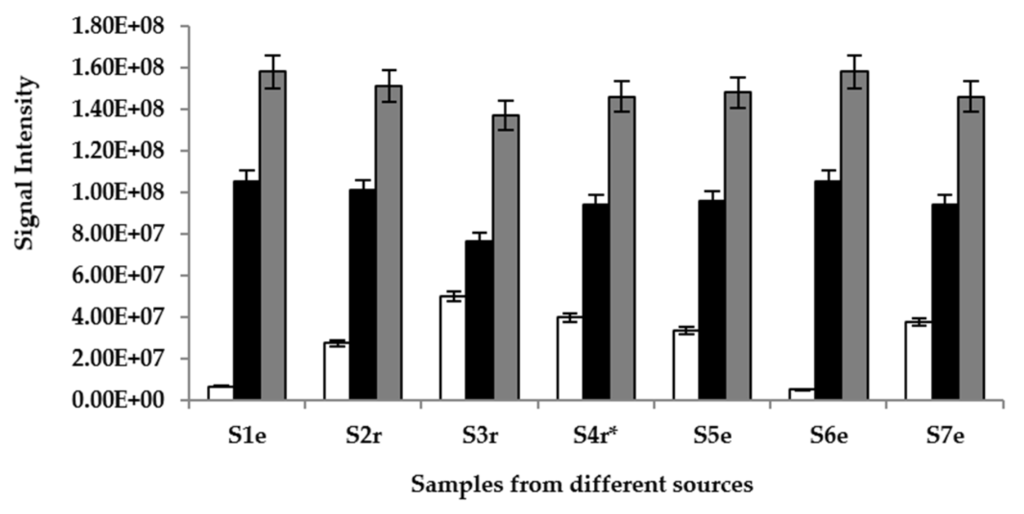
Figure 2.
Signal intensities recorded for three main palm wine aroma volatiles after APCI-MS analysis: (□) ethanol, (■) acetic acid and (  ) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
 ) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
3.5. GC-MS Evaluation
Yeast palm wine metabolic products were determined in order to identify compounds that could be optimized for the distinct palm wine flavor. Volatiles of compounds in the fermented palm wine were determined using GC-MS because it is one of the mainstream analytical tools that underpin the concept of metabolomics [20]. Compounds found were checked on YMDB, which contains information on the secondary metabolites of yeast fermentation. Results in order of elution are detailed in Table 2 and the 31 volatiles detected were found present in all samples. Compounds were confirmed by two different parameters namely retention time and mass to charge ratio. There were no differences (p > 0.05) in total compound peak areas between samples and after a search in YMDB, 23 out of the 31 compounds detected were found, whereas eight compounds were found absent. Aroma description for the compounds detected is shown Table 2 and full description of the compounds can be consulted on the YMDB.

Table 2.
Retention time (RT), mass to charge ratio (m/z) and the aroma described for detected products (ND = Aroma not described).
The compounds found in the samples consisted mainly of esters, alcohols, carboxylic acids, fatty acids and an acyloin (Table 3). The dominant compound group that was volatilized from the fermented palm wine were esters and it included the major esters associated with wine production especially methylpropylacetate (YMDB01589) described on the YMDB as a constituent in fruits, brandies and fortified wines. When compound abundance was estimated by comparing peak areas and expressing each compound peak area as a percentage of the maximum peak area obtained for the same compound across all samples analyzed (Table 3), it was found that the relative amount of each of these compounds were significantly different (p < 0.05).

Table 3.
Relative abundance (%) of fermentation products across all samples.
Samples from Raphia sp. had higher water content and some compounds were more abundant in samples from Elaeis sp. than samples from Raphia sp. and vice versa. All the acetate esters had the highest percentage maximum in samples from Elaeis sp. and the non-acetate esters had the maximum percentage abundance in samples from Raphia sp. For samples (S4, Table 3) supplemented with S. gabonensis, there was decreased abundance for 77% (42% overall) of the compounds detected when compared with samples that had no supplement (S3, Table 3). The highest suppression by virtue of percentage difference across all samples was acetoin followed by acetic acid and then ethyl octanoate.
4. Discussion
4.1. Physico-Chemical Quantification
Palm wine pH can be used to ascertain the freshness of a palm wine sample and a pH value of over 5 indicates that the palm wine was harvested on the first day of tapping [4,21]. The fermented palm wine samples under study did not show pH in that range and the pH decreases or the acidic nature of the product observed were in the range previously obtained for fermented palm wine by other workers [22]. The measurement of physico-chemical constituents of palm wine quantified by HPLC is within the range reported by other investigators [21] and shows that the palm wine at the time of analysis was a well-fermented palm wine. The highest sugar concentration observed for sample with S. gabonensis supplement indicates a lower rate of yeast fermentation possibly because the supplement has a major active compound bergenin, which has been found to significantly inhibit the glucose-depleting action of ethanol [23]. As shown by Ouoba et al. [3], it was observed that samples with lower percentages of alcohol contained higher concentrations of sugar and vice versa.
4.2. Diversity of Yeasts
Early microbiological characterizations of palm wine in Nigeria were mainly classical or conventional [24,25] and much later, traditional genetics studies were carried out [26]. With the advent of molecular biology, the first molecular characterization of an autochthonous palm wine S. cerevisiae population from Nigeria [6] was carried out and it was shown that Nigerian palm wine yeast represents a local specific yeast flora, whereas a European origin or hybrid was suspected for several isolates from other African countries. Results of this study suggest that in addition to the local specific S. cerevisiae flora of Nigerian palm wine, there exists S. cerevisiae similar to well characterized reference strains used around the world since six of the yeast strains sequenced had the same closest relative (100% match) as the standard laboratory strain S. cerevisiae S288c.
The high carbon dioxide environment among other conditions during palm wine fermentation favours the proliferation of Saccharomyces [27] and that may be why overall, the dominant yeast isolated was S. cerevisiae. Although Santiago-Urbina et al. [17] failed to find any S. cerevisiae in one out of three palm saps analyzed in Mexico, the dominance of S. cerevisiae among yeast species in palm wine is widely reported.
The other species isolated namely P. kudriavzevii and C. tropicalis have been isolated from palm wine by other workers [3,16,17]. Previous isolation of C. ethanolica and C. tropicalis have been reported from damaged plant tissue of oil palm tree [28] and this could be the source of the organism in the drink. Although their role in palm wine fermentation has not been well defined, Pichia species are associated with wine production [29] and can produce important levels of glycerol, which increases the fruity aroma of wines [30]. It has been reported that C. tropicalis is a common spoilage yeast [31] and a biofilm former [32] which may enable it to persist in the palm wine production environment. Proliferation of C. ethanolica probably occurred at stage two of palm wine fermentation [1] where mostly ethanol is fermented due to sugar depletion. The species has been shown to differ from other Candida species in not fermenting sugars and can utilize ethanol as the only source of carbon [33].
According to Esteve-Zarzoso et al. [15], restriction analysis of the 5.8S-ITS region for some genera analyzed in their study exhibited the same patterns with different endonucleases, not only with three general CfoI, HaeIII or HinfI restriction enzymes, but also with AluI, DdeI, ScrFI and TaqI and, for the genus Hanseniaspora, species the use of only one enzyme (CfoI, HaeIII or HinfI) was sufficient to obtain a pattern representative of each species. Against this background and considering the fact that molecular identification by sequencing is now the gold standard, the restriction analysis of the 42 isolates served as an initial species identification step in this study, after which only 18 strains were selected for sequencing to reduce duplication.
4.3. Main Aroma Volatiles
The APCI-MS analysis was used because spectral information on the key aroma compounds can be obtained and the method has the advantage of direct quantification and rapid acquisition of signals [34]. Ethyl acetate was the most intense aroma observed. It has been noted that at each stage of palm wine fermentation members of the microbial consortium trade metabolites and the product of one organism becomes the substrate of the next such that yeasts ferment sugar to produce ethanol and ethanol is fermented by acetic acid bacteria to yield acetic acid [1]. Ethanol and acetic acid can combine to form ethyl acetate [35], which possibly caused depletion of ethanol and acetic acid and probably resulted in an increase in ethyl acetate aroma intensity.
The five aroma compounds out of 13 potent odorants identified as important contributors to palm wine aroma in another study [36] were not among the three distinct peaks recorded and this may be because they were suppressed. Taylor et al. [37] have shown that in an APCI-MS analysis of a fermented mixture, if a particular volatile is more abundant than another volatile, the major volatile may suppress ionization of the minor component. It is also known that the released quantity of a volatile determines, among other variables, the intensity of aroma sensation [38].
4.4. Evaluation of Fermentation Compounds
4.4.1. Fermentation Products
The palm wine industry is now a flourishing sector in Nigeria because many food companies bottle the product, sell it locally and export internationally for consumption by Africans in diaspora. Previous studies by Uzochukwu et al. [7,39,40] heralded the identification of compounds with the use of GC-MS analytical tool and it was hoped that many studies on palm wine constituents will follow and translate into a higher quality drink. Much later, Lasekan et al. [9] detected more compounds and carried out studies on volatilized palm wine flavor molecules that interact with the human olfactory receptors through the ortho-nasal route [10]. In vivo studies [41] have also been carried out but more investigations are required to characterize palm wine from various regions in Nigeria because tastes vary according to region.
Volatile profiles of the samples studied were similar to volatiles found previously by Uzochukwu et al. [7] in palm wine headspace after full fermentation but varied slightly. In comparison, 14 esters, three alcohols, four acids and one unknown ketone was detected in their study, whereas 10 esters, six alcohols, and one ketone compound (acetoin) was found in this study. It is possible that the unknown ketone found in their study is acetoin because it is described as an organic compound containing an alpha hydroxy ketone on the YMDB.
Overall, it is possible that the S. gabonensis reduced the occurrence of the plastic monomer styrene since its abundance in the supplemented samples was half of the amount observed for the sample without the supplement. It has been previously reported that the reaction of benzoic acid and ascorbic acid can induce benzene formation [42], the benzene found in palm wine could also be due to reaction of benzoic acid from plant material [43] and the ascorbic acid (vitamin C) inherent in the fresh alcohol free drink [44]. It would be beneficial if levels of benzene and other artifacts found in this study were determined to establish if the levels are within known safety limits after which steps should be taken to eliminate risks if any. A starting point might be to discontinue the use of plastic containers right from the start of the tapping process up to the point the drink is consumed.
4.4.2. Fermentation Products Abundance
Fermentation abundance can be affected by a number of factors. It has been pointed out that the physicochemical characteristics of aroma compounds and wine matrix composition play a significant role on the temporal aroma release from wines [45]. In addition, fermentation abundance may be strain dependent because it has been demonstrated [46] that S. cerevisiae yeast strains used to ferment grapes produced the same major components, with certain variations in formation level as seen in this study. This has been confirmed by application of a selective ion monitoring (SIM) method to metabolome analysis of single transcription factor deletion mutants which obtained clusters that were independent of cultivation day and analysis day but were strain-dependent [47]. The level of compound abundance that translates to significant physical increase or reduction will need to be investigated.
To the best of knowledge of the authors, the specific compounds in palm wine suppressed by the addition of S. gabonensis supplement have not been reported but the antimicrobial properties is well known and more investigators seem to agree that bacteria is inhibited more than yeasts. Direct addition of S. gabonensis supplement to fresh palm juice at 10% (w/v) [12] showed that the supplement inhibited growth of S. cerevisiae, Leuconostoc mesenteroides and Lactobacillus plantarum isolated from the drink but only the reduction in counts of bacteria isolates were significant. Previous work [2] later found that water extracts from S. gabonensis failed to inhibit several yeasts and bacteria from palm wine and other researchers [10] found that yeast growth was not inhibited by adding supplement to palm wine but sedimentation rate was affected. More investigations are required to identify all the bioactive constituents and mode of action of the supplement at different stages of palm wine fermentation.
Yeasts are known to produce acetoin in small amounts and according to the pathway of acetoin biosynthesis in yeasts shown by Romano et al. [48], glycolysis precedes pyruvate decarboxylation. Considering the fact that the sample containing S. gabonensis supplement had the highest glucose concentration when HPLC quantification of compounds was carried out in this study and the growth inhibition of LAB after addition of S. gabonensis supplement demonstrated earlier [12], we propose that the lower abundance of acetoin observed was most likely as a result of microbial inhibition that caused a delay in breakdown of glucose to pyruvate by the consortium of microorganisms involved in fermentation of palm wine.
5. Conclusions
The fermented palm wine from trees of Elaeis sp. and Raphia sp. showed the same constituents and the location of purchase did not have any influence on the pH, microorganisms or chemical constituents. There is variation in the abundance of compounds in palm wine from the two tree species analyzed and it appears that addition of S. gabonensis supplement resulted in suppression of several fermentation products. For the first time, the specific compounds in palm wine suppressed with the addition of S. gabonensis supplement are shown. A possibility of developing a new quality and safety index for palm wine based on the exploitation of the fermentation and non-fermentation compounds abundance knowledge looks promising and would be beneficial to public health.
Acknowledgments
Authors wish to thank Chris Dodd and Catherine Rees, of University of Nottingham (United Kingdom) for their support, encouragement and being UK hosts of the research. Appreciation extends to Robert Linforth for help with compound analysis and Chris Powell who supplied reference strains. The work was supported by the International Development Fund grant to O.N. from Society for General Microbiology (now Microbiology Society) UK. Grant No IDF 2012/12/3.
Author Contributions
Ogueri Nwaiwu conceived the research and all authors contributed in the design of the experiments. All authors contributed to paper plan. Ogueri Nwaiwu performed the experiments; Vincent I. Ibekwe and Ogueri Nwaiwu analyzed the data; Sample 1 was contributed by Ogueri Nwaiwu, Sample 2 by Angela C. Udebuani., Samples 3 and 4 by Okechukwu I. Oguoma , Sample 5 by Ekperechi S. Amadi , Sample 6 by Justin C. Nnokwe . and Sample 7 by Ferdinand C. Nwanebu.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Santiago-Urbina, J.A.; Ruíz-Terán, F. Microbiology and biochemistry of traditional palm wine produced around the world. Int. Food Res. J. 2014, 21, 1261–1269. [Google Scholar]
- Okafor, N. Preliminary microbiological studies on the preservation of palm wine. J. Appl. Bacteriol. 1975, 38, 1–7. [Google Scholar] [CrossRef]
- Ouoba, L.; Kando, C.; Parkouda, C.; Sawadogo-Lingani, H.; Diawara, B.; Sutherland, J.P. The microbiology of Bandji, palm wine of Borassus akeassii from Burkina Faso: Identification and genotypic diversity of yeasts, lactic acid and acetic acid bacteria. J. Appl. Microbiol. 2012, 113, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Dalibard, C. Overall View on the Tradition of Tapping Palm Trees and Prospects for Animal Production. Livestock Research for Rural Development 1999. Available online: http://www.lrrd.org/lrrd11/1/dali111.htm (assessed on 12 March 2016).
- Amoa-Awua, W.K.; Sampson, E.; Tano-Debrah, K. Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm (Elaeis guineensis) in Ghana. J. Appl. Microbiol. 2007, 102, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ezeronye, O.U.; Legras, J.-L. Genetic analysis of Saccharomyces cerevisiae strains isolated from palm wine in eastern Nigeria. Comparison with other African strains J. Appl. Microbiol. 2009, 106, 1569–1578. [Google Scholar] [PubMed]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.; Lewis, M.J.; Ngoddy, P.O. Volatile constituents of palm wine and palm sap. J. Sci. Food Agric. 1994, 64, 405–411. [Google Scholar] [CrossRef]
- Lasekan, O.; Buettner, A.; Christlbauer, M. Investigation of important odorant of palm wine (Elaeis guineensis). Food Chem. 2007, 105, 15–23. [Google Scholar] [CrossRef]
- Lasekan, O.; Buettner, M.; Christlbauer, M. Investigation of the retronasal perception of palm wine (Elaeis guineensis) aroma by application of sensory analysis and exhaled odorant measurement (exom). Afr. J. Food Agric. Nutr. Dev. 2009, 9, 793–813. [Google Scholar]
- Elijah, A.I.; Ojimelukwe, P.C.; Ekong, U.S.; Asamudo, N.U. Effect of Sacoglottis gabonensis and Alstonia boonei on the kinetics of Saccharomyces cerevisiae isolated from palm wine. Afr. J. Biotechnol. 2010, 9, 5730–5734. [Google Scholar]
- Kuete, V. Physical, hematological and histopathological signs of toxicity induced by African medicinal plants. In Toxicological Survey of African Medicinal Plants, 1st ed.; Kuete, V., Ed.; Elsevier: Amsterdam, the Netherlands, 2014; pp. 648–649. [Google Scholar]
- Faparusi, S.I.; Bassir, O. Effect of extracts of the bark of Saccoglottis gabonensis on the microflora of palm wine. Appl. Microbiol. 1972, 24, 853–856. [Google Scholar] [PubMed]
- Jewison, T.; Knox, C.; Neveu, V.; Djoumbou, Y.; Guo, A.C.; Lee, J.; Liu, P.; Mandal, R.; Krishnamurthy, R.; Sinelnikov, I.; et al. YMDB: The Yeast Metabolome Database. Nucl. Acids Res. 2012, 40, D815–D820. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Fisk, I.D.; Linforth, R.; Brown, K.; Walsh, S.; Mooney, S.; Sturrock, C.; Hort, J. Impact of flavour solvent on biscuit micro-structure as measured by X-ray micro-computed tomography and the distribution of vanillin and HMF (HPLC). Eur. Food Res. Technol. 2012, 235, 1083–1091. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Urbina, J.A.; Arias-García, J.A.; Ruíz-Terán, F. Yeast species associated with spontaneous fermentation of taberna, a traditional palm wine from the southeast of Mexico. Ann. Microbiol. 2015, 65, 287–296. [Google Scholar] [CrossRef]
- Tsachaki, M.; Linforth, R.S.; Taylor, A.J. Aroma release from wines under dynamic conditions. J. Agric. Food Chem. 2009, 57, 6976–6981. [Google Scholar] [CrossRef] [PubMed]
- Khidr, S.K.; Linforth, R.S.T.; Hardy, I.C.W. Genetic and environmental influences on the cuticular hydrocarbon profiles of Goniozus wasps. Entomol. Exp. Appl. 2013, 147, 175–185. [Google Scholar] [CrossRef]
- Shepherd, L.V.T.; Fraser, P.; Stewart, D. Metabolomics: A second-generation platform for crop and food analysis. Bioanalysis 2011, 3, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Karamoko, D.; Djeni, N.T.; N’guessan, K.F.; Bouatenin, K.M.J.-P.; Dje, K.M. The biochemical and microbiological quality of palm wine samples produced at different periods during tapping and changes which occurred during their storage. Food Control 2012, 26, 504–551. [Google Scholar] [CrossRef]
- Santiago-Urbina, J.A.; Verdugo-Valdez, A.G.; Ruíz-Terán, F. Physicochemical and microbiological changes during tapping of palm sap to produce an alcoholic beverage called “Taberna”, which is produced in the south east of Mexico. Food Control 2013, 33, 58–62. [Google Scholar] [CrossRef]
- Maduka, H.C.C.; Okoye, Z.S.C. Elemental composition of S. gabonensis, a Nigerian alcoholic beverage additive. Pak. J. Biol. Sci. 2002, 5, 66–68. [Google Scholar]
- Okafor, N. Palm-wine yeasts from parts of Nigeria. J. Sci. Food Agric. 1972, 23, 1399–1407. [Google Scholar] [CrossRef]
- Faparusi, S.I. Origin of initial microflora of palm wine from oil palm trees (Elaeis guineensis). J. Appl. Bacteriol. 1973, 36, 559–565. [Google Scholar] [CrossRef]
- Ezeronye, O.U.; Okerentugba, P.O. Genetic and physiological variants of yeast selected from palm wine. Mycopathologia 2001, 152, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Stringini, M.; Comitini, F.; Taccari, M.; Ciani, M. Yeast diversity during tapping and fermentation of palm wine from Cameroon. Food Microbiol. 2009, 26, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Ohkusu, M.; Kubo, T.; Kawamoto, S.; Sone, K.; Hata, K. Isolation of yeasts from palm tissues damaged by the red palm weevil and their possible effect on the weevil overwintering. Mycoscience 2010, 51, 215–223. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Del Mónaco, S.M.; Barda, N.B.; Rubio, N.C.; Caballero, A.C. Selection and characterization of a Patagonian Pichia. kudriavzevii for wine deacidification. J. Appl. Microbiol. 2014, 117, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Tarifa, M.C.; Lozano, J.E.; Brugnoni, L.I. Dual-species relations between Candida tropicalis isolated from apple juice ultrafiltration membranes, with Escherichia coli O157:H7 and Salmonella sp. J. Appl. Microbiol. 2015, 118, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M.; López-Romero, E.; Villagómez-Castro, J.C.; Ruiz-Baca, E. Candida species: New insights into biofilm formation. Futur. Microbiol. 2012, 7, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Rybárová, J.; Stros, F.; Kocková-Kratochvílová, A. Candida ethanolica n. sp. J. Basic Microbiol. 1980, 20, 579–581. [Google Scholar]
- Ashraf, N.; Linforth, R.S.T.; Bealin-Kelly, F.; Smart, K.; Taylor, A.J. Rapid analysis of selected beer volatiles by atmospheric pressure chemical ionisation-mass spectrometry. Int. J. Mass Spectrom. 2010, 294, 47–53. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Lasekan, O. A comparative analysis of the influence of human salivary enzymes on odorant concentration in three palm wines. Molecules 2013, 18, 11809–11823. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Linforth, R.S.T.; Harvey, B.A.; Blake, A. Atmospheric pressure chemical ionisation-mass spectrometry for in vivo analysis of volatile flavour release. Food Chem. 2000, 71, 327–338. [Google Scholar] [CrossRef]
- Rabe, S.; Linforth, R.S.T.; Krings, U.; Taylor, A.J.; Berger, R.G. Volatile release from liquids: A comparison of in vivo APCI-MS, in-mouth headspace trapping and in vitro mouth model data. Chem. Senses 2004, 29, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.; Lewis, M.J.; Ngoddy, P.O. Volatiles of palm wine using solvent extracts. J. Food Qual. 1997, 20, 483–494. [Google Scholar] [CrossRef]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.G.; Lewis, M.J.; Ngoddy, P.O. Role of palm wine yeast and bacteria in palm wine aroma. J. Food Sci. Technol. 1999, 36, 301–304. [Google Scholar]
- Lasekan, O.; Otto, S. In vivo analysis of palm wine (Elaeis guineensis) volatile organic compounds (VOCs) by proton transfer reaction-mass spectrometry. Int. J. Mass Spectrom. 2009, 282, 45–49. [Google Scholar] [CrossRef]
- NTP-National Toxicology Program. Report on Carcinogens, 13th ed.Research Triangle Park: NC, USA, 2014; Department of Health and Human Services, Public Health Service. Available online: http://ntp.niehs.nih.gov/pubhealth/roc/roc13/ (assessed on 15 December 2015).
- FDA, Food and Drug Administration. Data on benzene in soft drinks and other beverages. Available online: http://www.fda.gov/Food/ (assessed on 12 December 2015).
- Okwu, D.E.; Nnamdi, F.U. Evaluation of the chemical composition of Dacryodes edulis and Raphia hookeri Mann and Wendl exudates used in herbal medicine in south eastern Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Sémon, E.; Martín-Álvarez, P.J.; Guichard, E.; Moreno-Arribas, M.V.; Feron, G.; Pozo-Bayón, M.A. Wine matrix composition affects temporal aroma release as measured by proton transfer reaction—time-of-flight—mass spectrometry. Aust. J. Grape Wine Res. 2015, 21, 367–375. [Google Scholar]
- Kawase, N.; Tsugawa, H.; Bamba, T.; Fukusaki, E. Different-batch metabolome analysis of Saccharomyces cerevisiae based on gas chromatography/mass spectrometry. J. Biosci. Bioeng. 2014, 117, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Shibamoto, T. Effect of different strains of Saccharomyces cerevisiae on production of volatiles in Napa Gamay wine and Petite Sirah wine. J. Agric. Food Chem. 2002, 50, 5649–5653. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).