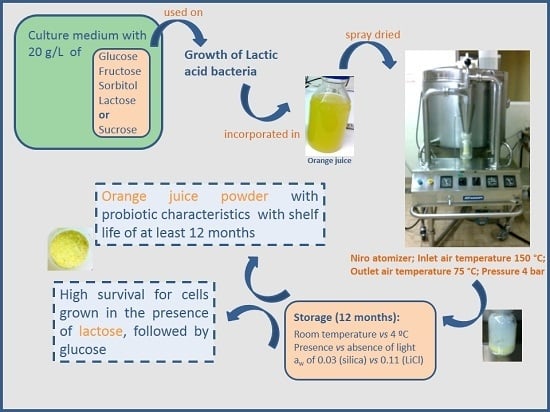
Effect of Different Conditions of Growth and Storage on the Cell Counts of Two Lactic Acid Bacteria after Spray Drying in Orange Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin, Growth and Storage Conditions of LAB Cultures
2.2. Spray Drying of Orange Juice Incorporated with LAB
2.2.1. Orange Juice Preparation
2.2.2. Preparation of LAB Cultures
2.2.3. Spray Drying
2.2.4. Analysis of Powders
- Drying yield (%)
- Water activity
2.2.5. Storage Conditions
2.2.6. Enumeration of LAB Cultures
2.3. Gastro-Intestinal Tract Simulation
2.3.1. Inoculum
2.3.2. Simulated Gastro-Intestinal Conditions
2.4. Statistical Analysis
3. Results
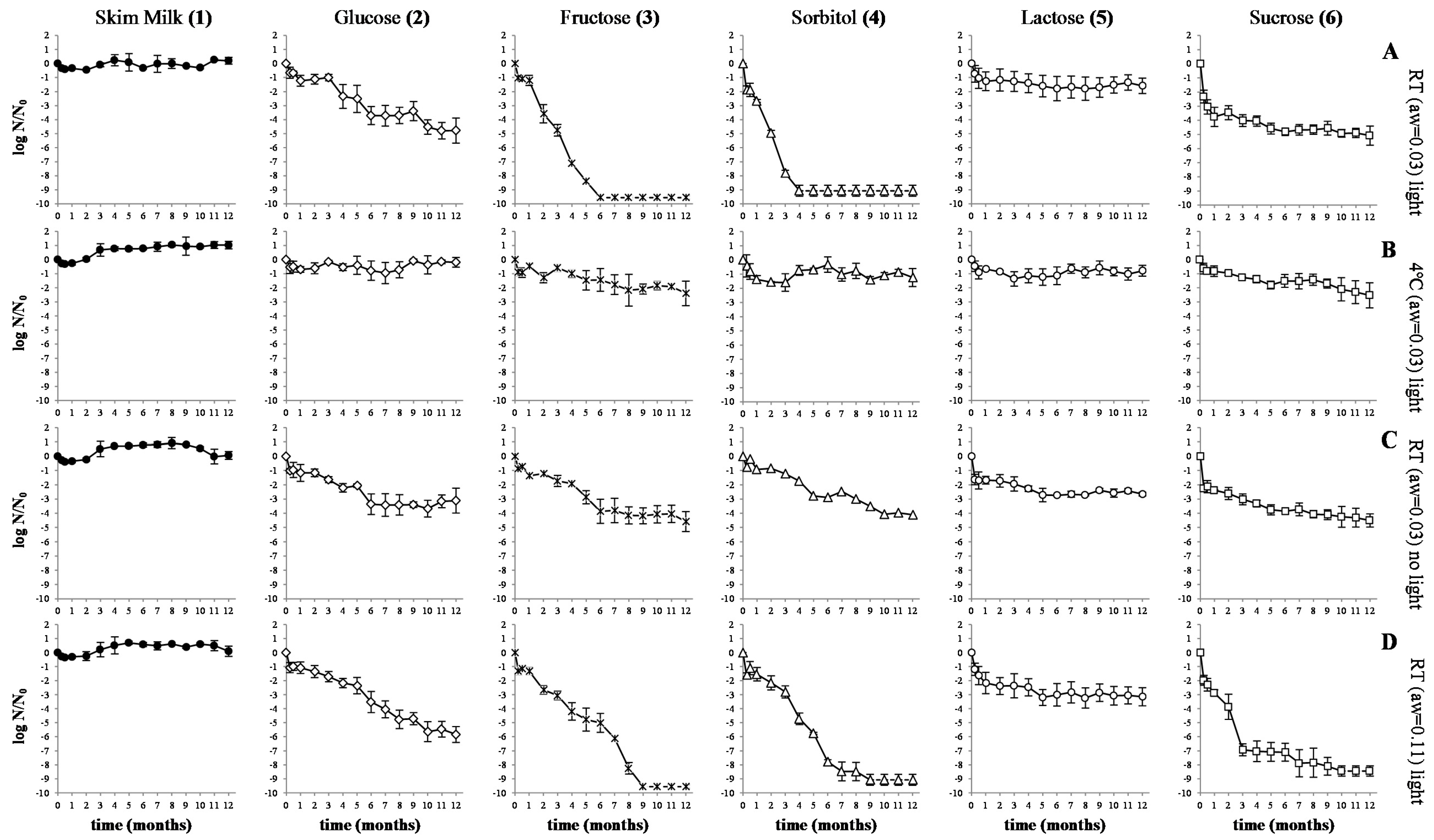
3.1. Spray Drying of Orange Juice Incorporated with LAB
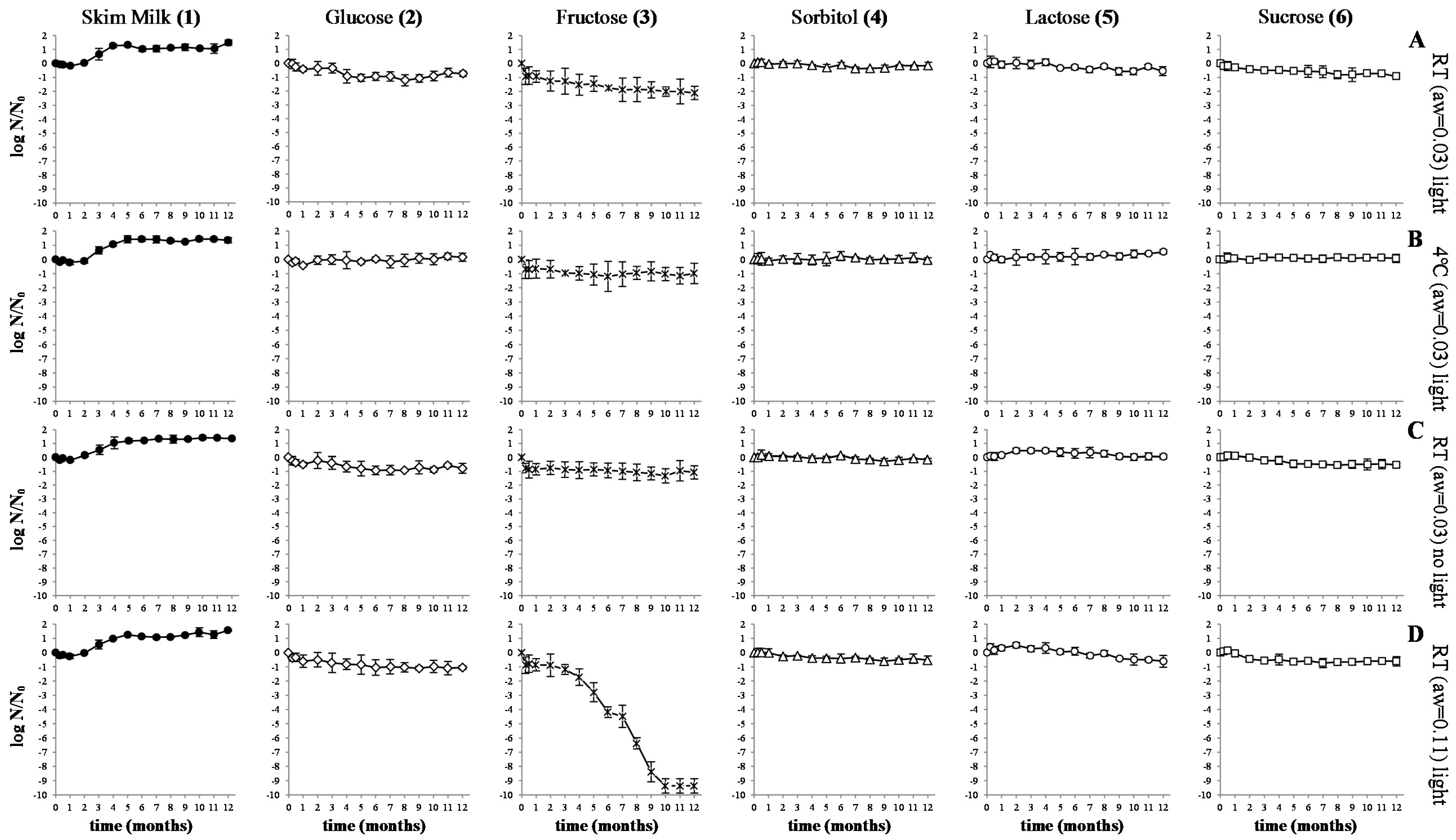
3.2. Gastro-Intestinal Tract Simulation
4. Discussion
4.1. Spray Drying of Orange Juice Incorporated with LAB
4.2. Gastro-Intestinal Tract Simulation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CFU | Colony forming unit |
| LAB | Lactic acid bacteria |
| MRS | Man, Rogosa & Sharpe |
| RSM | Reconstituted skim milk |
| aw | water activity |
| BPW | Buffered peptone water |
References
- Ying, D.; Schwander, S.; Weerakkody, R.; Sanguansri, L.; Gantenbein-Demarchi, C.; Augustin, M.A. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: Probiotic survival in fruit juice. J. Funct. Foods 2013, 5, 98–105. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and non-dairy probiotic product food development: Trends, concepts and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Silva, J.; Freixo, R.; Gibbs, P.; Teixeira, P. Spray-drying for the production of dried cultures. Int. J. Dairy Technol. 2011, 64, 321–335. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Senoussi, A.; Dumoulin, E.D.; Lebert, A. Spray drying of concentrated fruit juices. Dry. Technol. 1993, 11, 1081–1092. [Google Scholar] [CrossRef]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried acai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Food and Agriculture Organization/World Health Organization. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol. Progr. 2004, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Soares, V.; Santos, C.; Silva, J.; Gibbs, P.A.; Teixeira, P. Survival of L. sakei during heating, drying and storage in the dried state when growth has occurred in the presence of sucrose or monosodium glutamate. Biotechnol. Lett. 2005, 27, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S.; Neureiter, M.; Geppl, M.; Braun, R.; Danner, H. Influence of lyophilization, fluidized bed drying, addition of protectants, and storage on the viability of lactic acid bacteria. J. Appl. Microbiol. 2009, 107, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.C.; Castro, M.H.; Malcata, F.X.; Kirby, R.M. Survival of Lactobacillus delbrueckii ssp. bulgaricus following spray-drying. J. Dairy Sci. 1995, 78, 1025–1031. [Google Scholar]
- Barbosa, J.; Borges, S.; Teixeira, P. Pediococcus acidilactici as a potential probiotic to be used in food industry. Int. J. Food Sci. Technol. 2015, 50, 1151–1157. [Google Scholar]
- Miles, A.A.; Misra, S.S. The estimation of the bactericidal power of blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Magalhães, R.; Ferreira, V.; Santos, I.; Silva, J.; Almeida, G.; Gibbs, P.; Teixeira, P. Behaviour of Listeria monocytogenes isolates through gastro-intestinal tract passage simulation, before and after two sub-lethal stresses. Food Microbiol. 2012, 30, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Teixeira, P. Spray drying conditions for orange juice incorporated with lactic acid bacteria. Int. Food Res. J. 2016, in press. [Google Scholar]
- Corcoran, B.M.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J. Appl. Microbiol. 2004, 96, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Páez, R.; Lavari, L.; Vinderola, G.; Audero, G.; Cuatrin, A.; Zaritzky, N.; Reinheimer, J. Effect of heat-treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Res. Int. 2012, 48, 748–754. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudencio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrao-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Gerez, C.L.; Silva, J.; Abraham, A.G.; De Antoni, G.L.; Teixeira, P. Survival of spray-dried Lactobacillus kefir is affected by different protectants and storage conditions. Biotechnol. Lett. 2011, 33, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B.P.K.; Madhu, A.N.; Prapulla, S.G. Comparative survival and evaluation of functional probiotic properties of spray-dried lactic acid bacteria. Int. J. Dairy Technol. 2009, 62, 240–248. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Gomez-Zavaglia, A.; Disalvo, E.A. Effect of sugars and growth media on the dehydration of Lactobacillus delbrueckii ssp. bulgaricus. J. Appl. Microbiol. 2007, 102, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.P.; Teixeira, P.M.; Kirby, R. Storage of lyophilized cultures of Lactobacillus bulgaricus under different relative humidities and atmospheres. Appl. Microbiol. Biotechnol. 1995, 44, 172–176. [Google Scholar] [CrossRef]
- Abe, F.; Miyauchi, H.; Uchijima, A.; Yaeshima, T.; Iwatsuki, K. Effects of storage temperature and water activity on the survival of bifidobacteria in powder form. Int. J. Dairy Technol. 2009, 62, 234–239. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of micro-organisms by drying; a review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Chávez, B.E.; Ledeboer, A.M. Drying of probiotics: optimization of formulation and process to enhance storage survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Panoff, J.M.; Thammavongs, B.; Gueguen, M. Cryoprotective lead to phenotypic adaptation to freeze-thaw stress in Lactobacillus delbrueckii ssp. bulgaricus. Cryobiology 2000, 40, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Effect of additives on survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage. Biotechnol. Lett. 2002, 24, 1587–1591. [Google Scholar] [CrossRef]
- Ying, D.; Sun, J.; Sanguansri, L.; Weerakkody, R.; Augustin, M.A. Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. J. Food Eng. 2012, 109, 597–602. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Ramanan, R.N.; Vakhshiteh, F.; Mustafa, S.; Ling, T.C.; Rahim, R.A.; Ariff, A.B. Isolation of Pediococcus acidilactici Kp10 with ability to secrete bacteriocin-like inhibitory substance from milk products for applications in food industry. BMC Microbiol. 2012, 12, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Mirlohi, M.; Soleimanian-Zad, S.; Dokhani, S.; Sheikh-Zeinodin, M.; Abghary, A. Investigation of acid and bile tolerance of native lactobacilli isolated from fecal samples and commercial probiotics by growth and survival studies. Iran. J. Biotech. 2009, 7, 233–240. [Google Scholar]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.F.; Gigante, M.L. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
| LAB | Condition | log cfu/mL a | Powder | ||
|---|---|---|---|---|---|
| Before SD | After SD | aw b | Yield (%) c | ||
| L. plantarum 299v | MRS-Glucose + RSM | 9.9 ± 0.12 | 10.4 ± 0.12 | 0.383 ± 0.125 | 62.8 ± 5.4 |
| MRS-Glucose + OJM | 9.4 ± 0.03 | 9.5 ± 0.30 | 0.421 ± 0.076 | 53.7 ± 5.5 | |
| MRS-Fructose + OJM | 9.2 ± 0.04 | 9.6 ± 0.12 | 0.398 ± 0.032 | 43.8 ± 5.7 | |
| MRS-Sorbitol + OJM | 9.4 ± 0.11 | 9.1 ± 0.39 | 0.386 ± 0.015 | 38.9 ± 6.0 | |
| MRS-Lactose + OJM | 8.4 ± 0.12 | 7.9 ± 0.57 | 0.379 ± 0.116 | 48.4 ± 6.6 | |
| MRS-Sucrose + OJM | 8.2 ± 0.33 | 8.4 ± 0.37 | 0.374 ± 0.054 | 56.0 ± 8.9 | |
| P. acidilactici HA-6111-2 | MRS-Glucose + RSM | 9.2 ± 0.02 | 10.3 ± 0.03 | 0.358 ± 0.122 | 61.5 ± 1.2 |
| MRS-Glucose + OJM | 9.0 ± 0.06 | 9.4 ± 0.23 | 0.357 ± 0.038 | 53.8 ± 8.6 | |
| MRS-Fructose + OJM | 9.4 ± 0.12 | 9.4 ± 0.50 | 0.488 ± 0.095 | 58.4 ± 7.7 | |
| MRS-Sorbitol + OJM | 8.6 ± 0.24 | 8.3 ± 0.43 | 0.372 ± 0.052 | 41.1 ± 5.1 | |
| MRS-Lactose + OJM | 8.7 ± 0.05 | 8.7 ± 0.12 | 0.392 ± 0.045 | 59.4 ± 7.6 | |
| MRS-Sucrose + OJM | 8.8 ± 0.06 | 8.9 ± 0.19 | 0.359 ± 0.022 | 65.8 ± 6.4 | |
| log (N/N0)a | ||||
|---|---|---|---|---|
| Quick Digestion Simulation | ||||
| LAB | Condition | 0 min | 60 min b | 120 min c |
| L. plantarum 299v | SD in RSM | 0.00 ± 0.00 | −0.24 ± 0.18 | −1.38 ± 0.15 |
| SD in orange juice | 0.00 ± 0.00 | −0.51 ± 0.29 | −2.02 ± 0.45 | |
| P. acidilactici HA-6111-2 | SD in RSM | 0.00 ± 0.00 | −0.09 ± 0.01 | −0.66 ± 0.16 |
| SD in orange juice | 0.00 ± 0.00 | −0.83 ± 0.18 | −1.96 ± 0.43 | |
| Slow digestion simulation | ||||
| 0 min | 120 min b | 240 min c | ||
| L. plantarum 299v | SD in RSM | 0.00 ± 0.00 | −0.15 ± 0.10 | −1.35 ± 0.45 |
| SD in orange juice | 0.00 ± 0.00 | −0.76 ± 0.28 | −2.31 ± 0.27 | |
| P. acidilactici HA-6111-2 | SD in RSM | 0.00 ± 0.00 | −0.10 ± 0.07 | −1.16 ± 0.29 |
| SD in orange juice | 0.00 ± 0.00 | −1.03 ± 0.35 | −2.08 ± 0.37 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, J.; Borges, S.; Teixeira, P. Effect of Different Conditions of Growth and Storage on the Cell Counts of Two Lactic Acid Bacteria after Spray Drying in Orange Juice. Beverages 2016, 2, 8. https://doi.org/10.3390/beverages2020008
Barbosa J, Borges S, Teixeira P. Effect of Different Conditions of Growth and Storage on the Cell Counts of Two Lactic Acid Bacteria after Spray Drying in Orange Juice. Beverages. 2016; 2(2):8. https://doi.org/10.3390/beverages2020008
Chicago/Turabian StyleBarbosa, Joana, Sandra Borges, and Paula Teixeira. 2016. "Effect of Different Conditions of Growth and Storage on the Cell Counts of Two Lactic Acid Bacteria after Spray Drying in Orange Juice" Beverages 2, no. 2: 8. https://doi.org/10.3390/beverages2020008
APA StyleBarbosa, J., Borges, S., & Teixeira, P. (2016). Effect of Different Conditions of Growth and Storage on the Cell Counts of Two Lactic Acid Bacteria after Spray Drying in Orange Juice. Beverages, 2(2), 8. https://doi.org/10.3390/beverages2020008