Evaluation of Fermentation Products of Palm Wine Yeasts and Role of Sacoglottis gabonensis Supplement on Products Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Physico-Chemical Analysis
2.3. Yeast Identification
2.4. Atmospheric Pressure Chemical Ionisation-Mass Spectrometry
2.5. Gas Chromatography-Mass Spectrometry
2.6. Statistical Analysis
3. Results
3.1. PH
3.2. HPLC Analysis
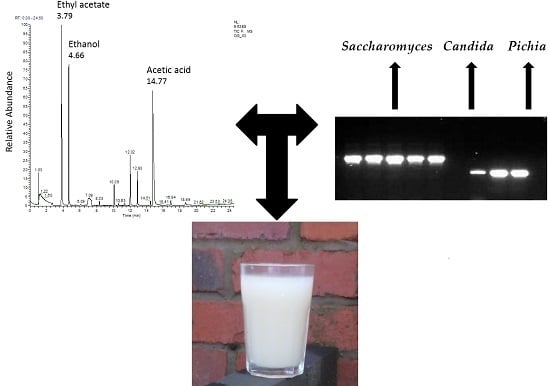
3.3. Molecular Identification
3.4. APCI-MS Analysis
3.5. GC-MS Evaluation
4. Discussion
4.1. Physico-Chemical Quantification
4.2. Diversity of Yeasts
4.3. Main Aroma Volatiles
4.4. Evaluation of Fermentation Compounds
4.4.1. Fermentation Products
4.4.2. Fermentation Products Abundance
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santiago-Urbina, J.A.; Ruíz-Terán, F. Microbiology and biochemistry of traditional palm wine produced around the world. Int. Food Res. J. 2014, 21, 1261–1269. [Google Scholar]
- Okafor, N. Preliminary microbiological studies on the preservation of palm wine. J. Appl. Bacteriol. 1975, 38, 1–7. [Google Scholar] [CrossRef]
- Ouoba, L.; Kando, C.; Parkouda, C.; Sawadogo-Lingani, H.; Diawara, B.; Sutherland, J.P. The microbiology of Bandji, palm wine of Borassus akeassii from Burkina Faso: Identification and genotypic diversity of yeasts, lactic acid and acetic acid bacteria. J. Appl. Microbiol. 2012, 113, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Dalibard, C. Overall View on the Tradition of Tapping Palm Trees and Prospects for Animal Production. Livestock Research for Rural Development 1999. Available online: http://www.lrrd.org/lrrd11/1/dali111.htm (assessed on 12 March 2016).
- Amoa-Awua, W.K.; Sampson, E.; Tano-Debrah, K. Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm (Elaeis guineensis) in Ghana. J. Appl. Microbiol. 2007, 102, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ezeronye, O.U.; Legras, J.-L. Genetic analysis of Saccharomyces cerevisiae strains isolated from palm wine in eastern Nigeria. Comparison with other African strains J. Appl. Microbiol. 2009, 106, 1569–1578. [Google Scholar] [PubMed]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.; Lewis, M.J.; Ngoddy, P.O. Volatile constituents of palm wine and palm sap. J. Sci. Food Agric. 1994, 64, 405–411. [Google Scholar] [CrossRef]
- Lasekan, O.; Buettner, A.; Christlbauer, M. Investigation of important odorant of palm wine (Elaeis guineensis). Food Chem. 2007, 105, 15–23. [Google Scholar] [CrossRef]
- Lasekan, O.; Buettner, M.; Christlbauer, M. Investigation of the retronasal perception of palm wine (Elaeis guineensis) aroma by application of sensory analysis and exhaled odorant measurement (exom). Afr. J. Food Agric. Nutr. Dev. 2009, 9, 793–813. [Google Scholar]
- Elijah, A.I.; Ojimelukwe, P.C.; Ekong, U.S.; Asamudo, N.U. Effect of Sacoglottis gabonensis and Alstonia boonei on the kinetics of Saccharomyces cerevisiae isolated from palm wine. Afr. J. Biotechnol. 2010, 9, 5730–5734. [Google Scholar]
- Kuete, V. Physical, hematological and histopathological signs of toxicity induced by African medicinal plants. In Toxicological Survey of African Medicinal Plants, 1st ed.; Kuete, V., Ed.; Elsevier: Amsterdam, the Netherlands, 2014; pp. 648–649. [Google Scholar]
- Faparusi, S.I.; Bassir, O. Effect of extracts of the bark of Saccoglottis gabonensis on the microflora of palm wine. Appl. Microbiol. 1972, 24, 853–856. [Google Scholar] [PubMed]
- Jewison, T.; Knox, C.; Neveu, V.; Djoumbou, Y.; Guo, A.C.; Lee, J.; Liu, P.; Mandal, R.; Krishnamurthy, R.; Sinelnikov, I.; et al. YMDB: The Yeast Metabolome Database. Nucl. Acids Res. 2012, 40, D815–D820. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Fisk, I.D.; Linforth, R.; Brown, K.; Walsh, S.; Mooney, S.; Sturrock, C.; Hort, J. Impact of flavour solvent on biscuit micro-structure as measured by X-ray micro-computed tomography and the distribution of vanillin and HMF (HPLC). Eur. Food Res. Technol. 2012, 235, 1083–1091. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Urbina, J.A.; Arias-García, J.A.; Ruíz-Terán, F. Yeast species associated with spontaneous fermentation of taberna, a traditional palm wine from the southeast of Mexico. Ann. Microbiol. 2015, 65, 287–296. [Google Scholar] [CrossRef]
- Tsachaki, M.; Linforth, R.S.; Taylor, A.J. Aroma release from wines under dynamic conditions. J. Agric. Food Chem. 2009, 57, 6976–6981. [Google Scholar] [CrossRef] [PubMed]
- Khidr, S.K.; Linforth, R.S.T.; Hardy, I.C.W. Genetic and environmental influences on the cuticular hydrocarbon profiles of Goniozus wasps. Entomol. Exp. Appl. 2013, 147, 175–185. [Google Scholar] [CrossRef]
- Shepherd, L.V.T.; Fraser, P.; Stewart, D. Metabolomics: A second-generation platform for crop and food analysis. Bioanalysis 2011, 3, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Karamoko, D.; Djeni, N.T.; N’guessan, K.F.; Bouatenin, K.M.J.-P.; Dje, K.M. The biochemical and microbiological quality of palm wine samples produced at different periods during tapping and changes which occurred during their storage. Food Control 2012, 26, 504–551. [Google Scholar] [CrossRef]
- Santiago-Urbina, J.A.; Verdugo-Valdez, A.G.; Ruíz-Terán, F. Physicochemical and microbiological changes during tapping of palm sap to produce an alcoholic beverage called “Taberna”, which is produced in the south east of Mexico. Food Control 2013, 33, 58–62. [Google Scholar] [CrossRef]
- Maduka, H.C.C.; Okoye, Z.S.C. Elemental composition of S. gabonensis, a Nigerian alcoholic beverage additive. Pak. J. Biol. Sci. 2002, 5, 66–68. [Google Scholar]
- Okafor, N. Palm-wine yeasts from parts of Nigeria. J. Sci. Food Agric. 1972, 23, 1399–1407. [Google Scholar] [CrossRef]
- Faparusi, S.I. Origin of initial microflora of palm wine from oil palm trees (Elaeis guineensis). J. Appl. Bacteriol. 1973, 36, 559–565. [Google Scholar] [CrossRef]
- Ezeronye, O.U.; Okerentugba, P.O. Genetic and physiological variants of yeast selected from palm wine. Mycopathologia 2001, 152, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Stringini, M.; Comitini, F.; Taccari, M.; Ciani, M. Yeast diversity during tapping and fermentation of palm wine from Cameroon. Food Microbiol. 2009, 26, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Ohkusu, M.; Kubo, T.; Kawamoto, S.; Sone, K.; Hata, K. Isolation of yeasts from palm tissues damaged by the red palm weevil and their possible effect on the weevil overwintering. Mycoscience 2010, 51, 215–223. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Del Mónaco, S.M.; Barda, N.B.; Rubio, N.C.; Caballero, A.C. Selection and characterization of a Patagonian Pichia. kudriavzevii for wine deacidification. J. Appl. Microbiol. 2014, 117, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Tarifa, M.C.; Lozano, J.E.; Brugnoni, L.I. Dual-species relations between Candida tropicalis isolated from apple juice ultrafiltration membranes, with Escherichia coli O157:H7 and Salmonella sp. J. Appl. Microbiol. 2015, 118, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M.; López-Romero, E.; Villagómez-Castro, J.C.; Ruiz-Baca, E. Candida species: New insights into biofilm formation. Futur. Microbiol. 2012, 7, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Rybárová, J.; Stros, F.; Kocková-Kratochvílová, A. Candida ethanolica n. sp. J. Basic Microbiol. 1980, 20, 579–581. [Google Scholar]
- Ashraf, N.; Linforth, R.S.T.; Bealin-Kelly, F.; Smart, K.; Taylor, A.J. Rapid analysis of selected beer volatiles by atmospheric pressure chemical ionisation-mass spectrometry. Int. J. Mass Spectrom. 2010, 294, 47–53. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Lasekan, O. A comparative analysis of the influence of human salivary enzymes on odorant concentration in three palm wines. Molecules 2013, 18, 11809–11823. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Linforth, R.S.T.; Harvey, B.A.; Blake, A. Atmospheric pressure chemical ionisation-mass spectrometry for in vivo analysis of volatile flavour release. Food Chem. 2000, 71, 327–338. [Google Scholar] [CrossRef]
- Rabe, S.; Linforth, R.S.T.; Krings, U.; Taylor, A.J.; Berger, R.G. Volatile release from liquids: A comparison of in vivo APCI-MS, in-mouth headspace trapping and in vitro mouth model data. Chem. Senses 2004, 29, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.; Lewis, M.J.; Ngoddy, P.O. Volatiles of palm wine using solvent extracts. J. Food Qual. 1997, 20, 483–494. [Google Scholar] [CrossRef]
- Uzochukwu, S.V.A.; Balogh, E.; Tucknott, O.G.; Lewis, M.J.; Ngoddy, P.O. Role of palm wine yeast and bacteria in palm wine aroma. J. Food Sci. Technol. 1999, 36, 301–304. [Google Scholar]
- Lasekan, O.; Otto, S. In vivo analysis of palm wine (Elaeis guineensis) volatile organic compounds (VOCs) by proton transfer reaction-mass spectrometry. Int. J. Mass Spectrom. 2009, 282, 45–49. [Google Scholar] [CrossRef]
- NTP-National Toxicology Program. Report on Carcinogens, 13th ed.Research Triangle Park: NC, USA, 2014; Department of Health and Human Services, Public Health Service. Available online: http://ntp.niehs.nih.gov/pubhealth/roc/roc13/ (assessed on 15 December 2015).
- FDA, Food and Drug Administration. Data on benzene in soft drinks and other beverages. Available online: http://www.fda.gov/Food/ (assessed on 12 December 2015).
- Okwu, D.E.; Nnamdi, F.U. Evaluation of the chemical composition of Dacryodes edulis and Raphia hookeri Mann and Wendl exudates used in herbal medicine in south eastern Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Sémon, E.; Martín-Álvarez, P.J.; Guichard, E.; Moreno-Arribas, M.V.; Feron, G.; Pozo-Bayón, M.A. Wine matrix composition affects temporal aroma release as measured by proton transfer reaction—time-of-flight—mass spectrometry. Aust. J. Grape Wine Res. 2015, 21, 367–375. [Google Scholar]
- Kawase, N.; Tsugawa, H.; Bamba, T.; Fukusaki, E. Different-batch metabolome analysis of Saccharomyces cerevisiae based on gas chromatography/mass spectrometry. J. Biosci. Bioeng. 2014, 117, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Shibamoto, T. Effect of different strains of Saccharomyces cerevisiae on production of volatiles in Napa Gamay wine and Petite Sirah wine. J. Agric. Food Chem. 2002, 50, 5649–5653. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [PubMed]
 ) and glucose (
) and glucose (  ) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
 ) and glucose (
) and glucose (  ) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) in samples (S) 1–7, obtained from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).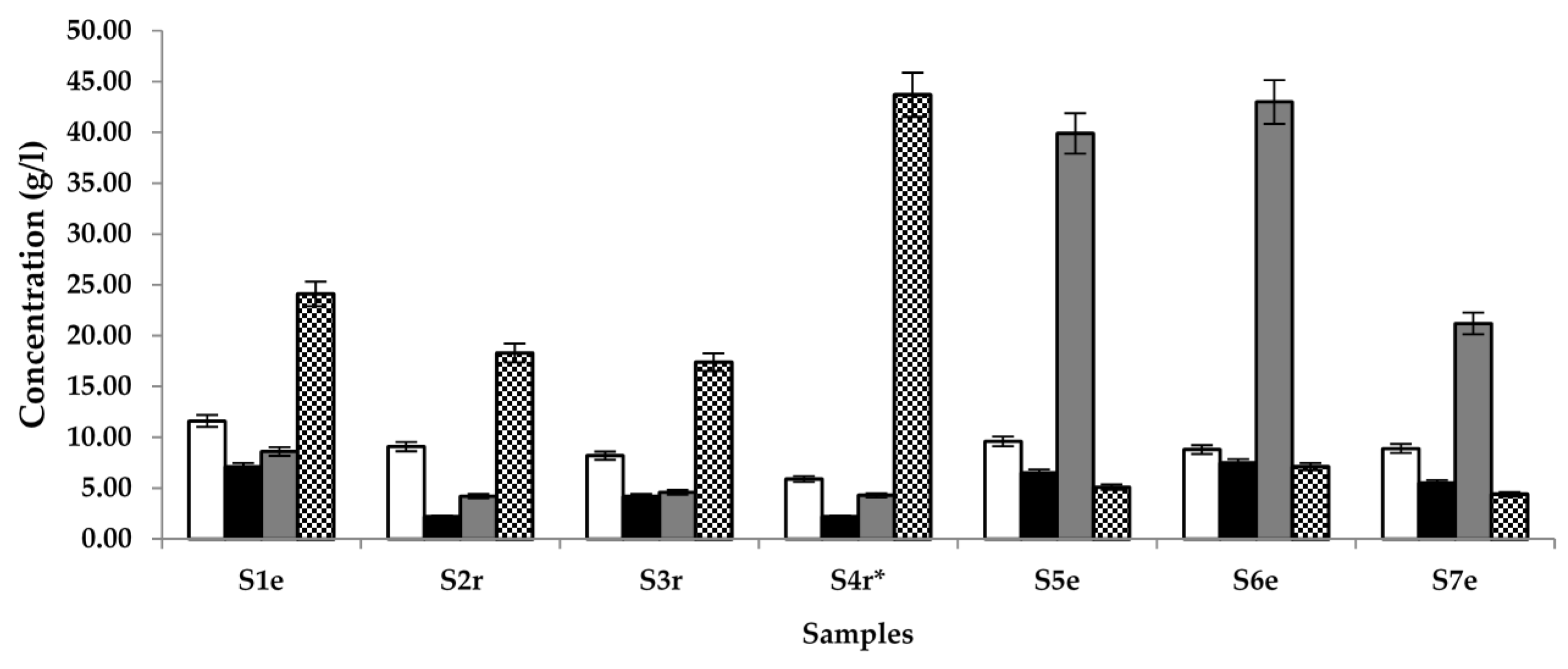
 ) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
 ) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
) ethyl acetate in samples (S) 1–7, sourced from different locations (r = Raphia sp.; e = Elaeis sp.; * = sample supplemented with S. gabonensis).
| S/n | Strain | ITS Amplicon | HaeIII Digest Fragments | NCBI Closest Relative | Assigned Accession No | Identification | Identity Match (%) |
|---|---|---|---|---|---|---|---|
| 1 | YN1D e | 520 | 380 + 100 | KC454395.1 | HG425325 | P. kudriavzevii | 100 |
| 2 | YN2B r | 880 | 320 + 230 + 180 + 150 | JX867131.1 | HG425326 | S. cerevisiae | 100 |
| 3 | YN7F e | 880 | 320 + 230 + 180 + 150 | JX867131.1 | HG425327 | S. cerevisiae | 100 |
| 4 | YN1A e | 880 | 320 + 230 + 180 + 150 | JX867131.1 | HG425328 | S. cerevisiae | 99 |
| 5 | YN2E r | 880 | 320 + 230 + 180 + 150 | JX141339.1 | HG425329 | S. cerevisiae | 99 |
| 6 | YN3A r | 880 | 320 + 230 + 180 + 150 | JX867131.1 | HG425330 | S. cerevisiae | 99 |
| 7 | YN3B r | 880 | 320 + 230 + 180 + 150 | JX867131.1 | HG425331 | S. cerevisiae | 99 |
| 8 | YN3D r | 450 | 400 + 90 | EF550225.1 | HG425332 | C. ethanolica | 100 |
| 9 | YN6B e | 520 | 380 + 100 | EF126358.1 | HG425333 | P. kudriavzevii | 99 |
| 10 | YN7B r | 550 | 450 + 90 | EU585758.1 | HG425334 | C. tropicalis | 100 |
| 11 | YN7E r | 520 | 380 + 100 | KC616319.1 | HG425335 | P. kudriavzevii | 99 |
| 12 | YN6C e | 450 | 400 + 90 | DQ466540.1 | HG425336 | C. ethanolica | 99 |
| 13 | YN6A e | 880 | 320 + 230 + 180 + 150 | JX141339.1 | HG425337 | S. cerevisiae | 99 |
| 14 | YN4Br | 880 | 320 + 230 + 180 + 150 | JX141339.1 | HG425338 | S. cerevisiae | 100 |
| 15 | * YN4D r | 880 | 320 + 230 + 180 + 150 | JX423567.1 | HG425339 | S. cerevisiae | 100 |
| 16 | YN5A e | 880 | 320 + 230 + 180 + 150 | JX867131.1 | HG425340 | S. cerevisiae | 100 |
| 17 | YN5B e | 880 | 320 + 230 + 180 + 150 | HM165257.1 | HG425341 | S. cerevisiae | 99 |
| 18 | YN5D e | 880 | 320 + 230 + 180 + 150 | JQ964228.1 | HG425342 | S. cerevisiae | 100 |
| 19 | NCYC 1406 | 880 | 320 + 230 + 180 + 150 | JX423566.1 | control | S. cerevisiae | 100 |
| 20 | S288c | 880 | 320 + 230 + 180 + 150 | JX867131.1 | control | S. cerevisiae | 100 |
| S/N | Compound | RT | m/z | Aroma |
|---|---|---|---|---|
| 1 | Ethyl acetate | 3.78 | 70 | Sweet smell |
| 2 | Ethanol | 4.63 | 31 | alcohol |
| 3 | Trimethyldioxolane | 4.76 | 101 | ND |
| 4 | Propionic acid | 5.02 | 57 | Pungent |
| 5 | Propyl acetate | 5.36 | 61 | Pear odour |
| 6 | Methyl cinnamate | 5.98 | 69 | Strawberry |
| 7 | Butyl acetate | 6.09 | 56 | Fruity |
| 8 | Dichloro methane | 6.31 | 83 | Absent |
| 9 | 1-Propanol | 6.61 | 56 | ND |
| 10 | Water | 7.10 | 18 | Plain |
| 11 | Methylene fluoride | 8.33 | 52 | Absent |
| 12 | Isopentyl alcohol | 8.35 | 70 | Malty |
| 13 | Butanol | 8.81 | 73 | Potato like |
| 14 | Benzene | 9.52 | 105 | Absent |
| 15 | 3-Methyl butanol | 10.07 | 70 | ND |
| 16 | Hexanoic acid | 10.63 | 88 | Unpleasant |
| 17 | Ethyl hexanoate | 10.64 | 99 | Flowery/fruity |
| 18 | Styrene | 11.35 | 104 | Absent |
| 19 | Acetoin | 12.00 | 88 | Pleasant/Buttery |
| 20 | Ethyl lactate | 12.92 | 45 | Buttery |
| 21 | Methylpropylacetate | 13.68 | 87 | ND |
| 22 | Ethyl octanoate | 14.51 | 127 | Pleasant/sweet |
| 23 | Acetic acid | 14.77 | 60 | Vinegar |
| 24 | Methyl-2-methyl propanoate | 16.97 | 88 | Light floral |
| 25 | Dichloroethanol | 17.8 | 31 | Absent |
| 26 | Ethyl dodecanoate | 18.64 | 43 | Pleasant/sweet |
| 27 | Dimethylhydrazine | 18.7 | 60 | Absent |
| 28 | 3-Methyl-6,7-benzylisoquinoline | 20.04 | 193 | Absent |
| 29 | Tetraacetyl-d-xylonic nitrile | 20.56 | 44 | Absent |
| 30 | Oleic acid | 22.7 | 91 | ND |
| 31 | 2-phenylethanol | 22.66 | 91 | pleasant floral |
| Compounds | S1 e | S2 r | S3 r | * S4 r | S5 e | S6 e | S7 e |
|---|---|---|---|---|---|---|---|
| Esters | |||||||
| Ethyl acetate | 88 | 54 | 33 | 25 | 77 | 100 | 68 |
| Propyl acetate | 100 | 36 | 27 | 17 | 98 | 54 | 93 |
| Methyl cinnamate | 36 | 65 | 100 | 46 | 48 | 49 | 94 |
| Butyl acetate | 100 | 14 | 13 | 6 | 17 | 55 | 2 |
| Ethyl hexanoate | 69 | 100 | 25 | 16 | 51 | 68 | 32 |
| Ethyl lactate | 76 | 97 | 12 | 25 | 100 | 75 | 58 |
| Methylpropylacetate | 100 | 36 | 25 | 1 | 8 | 28 | 8 |
| Methyl-2-methyl propanoate | 49 | 78 | 100 | 78 | 24 | 18 | 25 |
| Ethyl dodecanoate | 20 | 75 | 100 | 72 | 4 | 11 | 9 |
| Ethyl octanoate | 40 | 100 | 67 | 8 | 39 | 29 | 61 |
| Alcohols | |||||||
| Ethanol | 83 | 85 | 52 | 56 | 100 | 94 | 77 |
| 1-Propanol | 100 | 24 | 3 | 4 | 13 | 34 | 72 |
| Isopentyl alcohol | 100 | 18 | 25 | 6 | 29 | 84 | 24 |
| Butanol | 100 | 3 | 8 | 5 | 10 | 52 | 8 |
| 3-Methyl butanol | 95 | 53 | 100 | 77 | 82 | 93 | 65 |
| 2-Phenylethanol | 9 | 43 | 71 | 100 | 81 | 11 | 12 |
| Carboxylic acids | |||||||
| Propionic acid | 83 | 25 | 10 | 48 | 60 | 100 | 63 |
| Acetic acid | 100 | 86 | 77 | 15 | 33 | 44 | 35 |
| Fatty acids | |||||||
| Hexanoic acid | 77 | 100 | 28 | 19 | 52 | 70 | 34 |
| Oleic acid | 100 | 10 | 13 | 10 | 8 | 95 | 8 |
| Acyloin | |||||||
| Acetoin | 51 | 100 | 82 | 3 | 29 | 30 | 20 |
| Others | |||||||
| Trimethyldioxolane | 100 | 6 | 2 | 8 | 2 | 47 | 11 |
| Dichloro methane | 26 | 35 | 57 | 100 | 30 | 28 | 48 |
| Water | 85 | 96 | 100 | 94 | 78 | 78 | 67 |
| Methylene fluoride | 100 | 20 | 25 | 9 | 31 | 99 | 31 |
| Benzene | 100 | 72 | 34 | 13 | 31 | 15 | 71 |
| Styrene | 100 | 85 | 46 | 22 | 35 | 30 | 85 |
| Dichloroethanol | 100 | 15 | 13 | 12 | 8 | 50 | 12 |
| Dimethylhydrazine | 15 | 73 | 100 | 61 | 5 | 11 | 6 |
| 3-Methyl-6,7-benzylisoquinoline | 53 | 89 | 100 | 62 | 77 | 34 | 58 |
| Tetraacetyl-d-xylonic nitrile | 83 | 59 | 84 | 47 | 94 | 75 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwaiwu, O.; Ibekwe, V.I.; Amadi, E.S.; Udebuani, A.C.; Nwanebu, F.C.; Oguoma, O.I.; Nnokwe, J.C. Evaluation of Fermentation Products of Palm Wine Yeasts and Role of Sacoglottis gabonensis Supplement on Products Abundance. Beverages 2016, 2, 9. https://doi.org/10.3390/beverages2020009
Nwaiwu O, Ibekwe VI, Amadi ES, Udebuani AC, Nwanebu FC, Oguoma OI, Nnokwe JC. Evaluation of Fermentation Products of Palm Wine Yeasts and Role of Sacoglottis gabonensis Supplement on Products Abundance. Beverages. 2016; 2(2):9. https://doi.org/10.3390/beverages2020009
Chicago/Turabian StyleNwaiwu, Ogueri, Vincent I. Ibekwe, Ekperechi S. Amadi, Angela C. Udebuani, Ferdinand C. Nwanebu, Okechukwu I. Oguoma, and Justin C. Nnokwe. 2016. "Evaluation of Fermentation Products of Palm Wine Yeasts and Role of Sacoglottis gabonensis Supplement on Products Abundance" Beverages 2, no. 2: 9. https://doi.org/10.3390/beverages2020009
APA StyleNwaiwu, O., Ibekwe, V. I., Amadi, E. S., Udebuani, A. C., Nwanebu, F. C., Oguoma, O. I., & Nnokwe, J. C. (2016). Evaluation of Fermentation Products of Palm Wine Yeasts and Role of Sacoglottis gabonensis Supplement on Products Abundance. Beverages, 2(2), 9. https://doi.org/10.3390/beverages2020009