Effect of the Flash Vacuum Expansion (FVE) Process on the Response of Limosilactobacillus fermentum J24 to the Metabolism of Sugars and Organic Acids During the Development of a Papaya-Based Drink
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Papaya Purees
2.2. Papaya Beverages Formulation and Fermentation
2.3. Identification and Quantification of Free Sugars and Organic Acids Using HPLC-DAD
2.4. Functional Annotations and Gene Categorization
2.5. Identification of Active Enzymes in Carbohydrates
2.6. Statistical Analysis
3. Results and Discussion
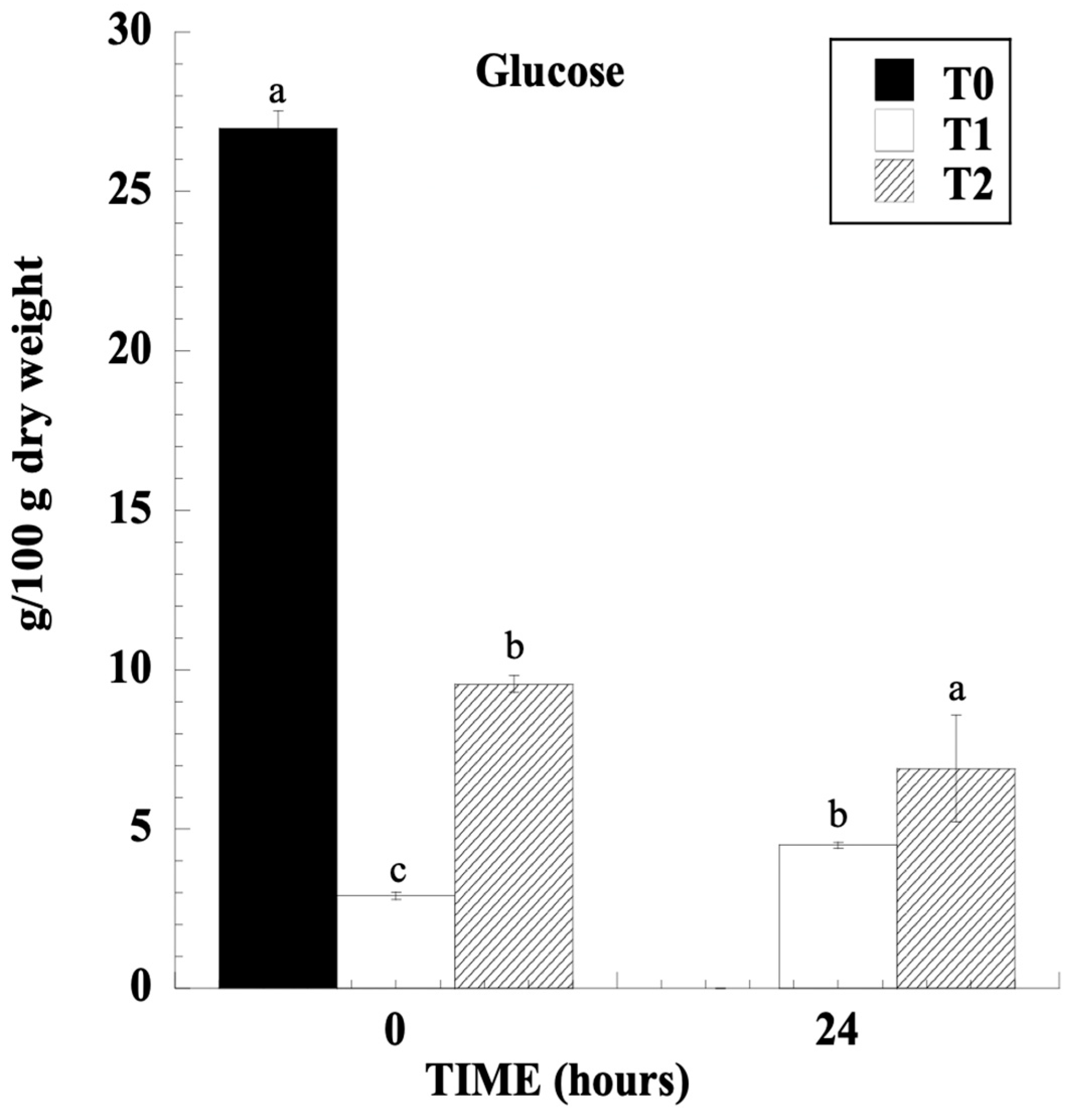
3.1. Free Sugars
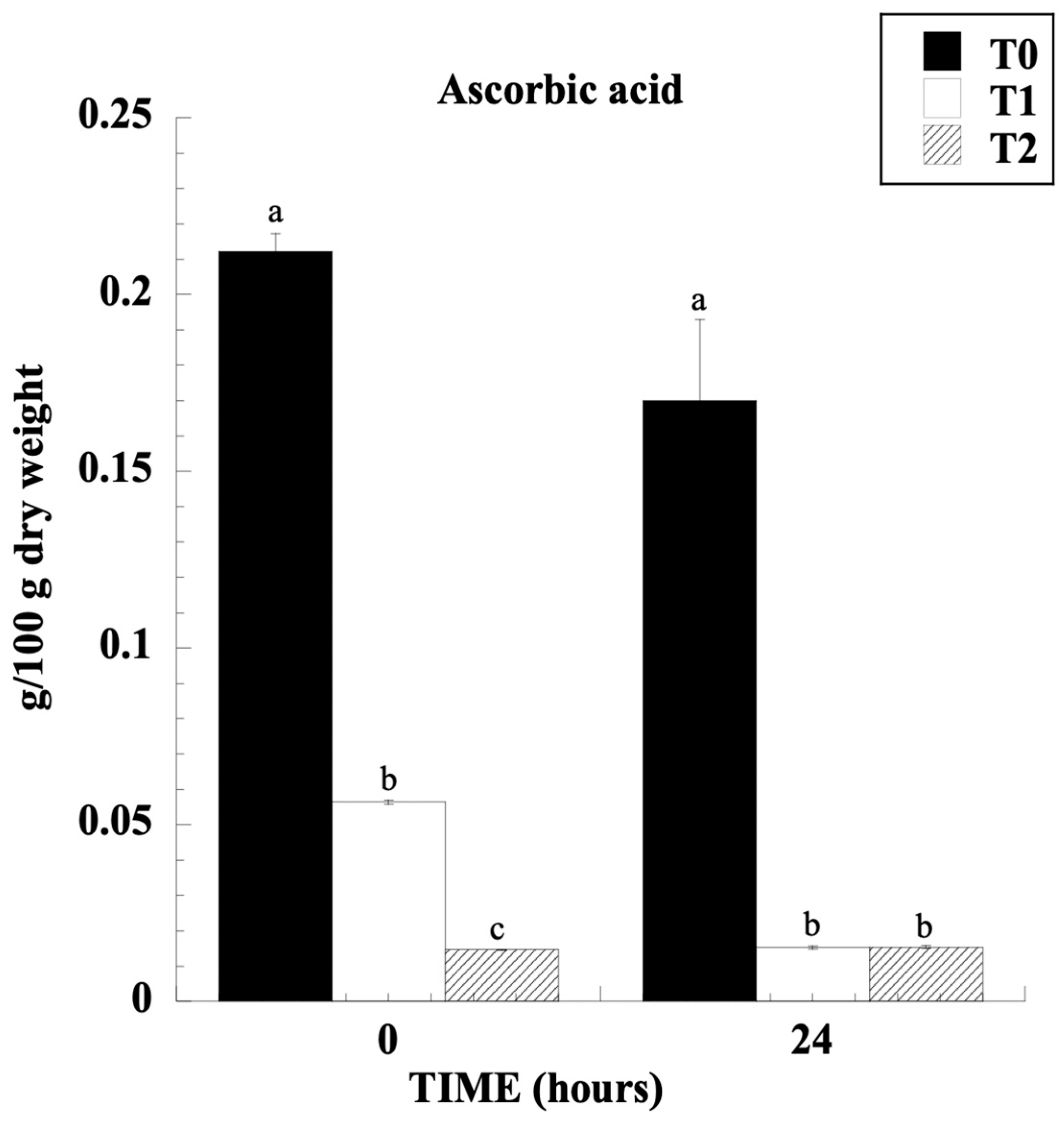
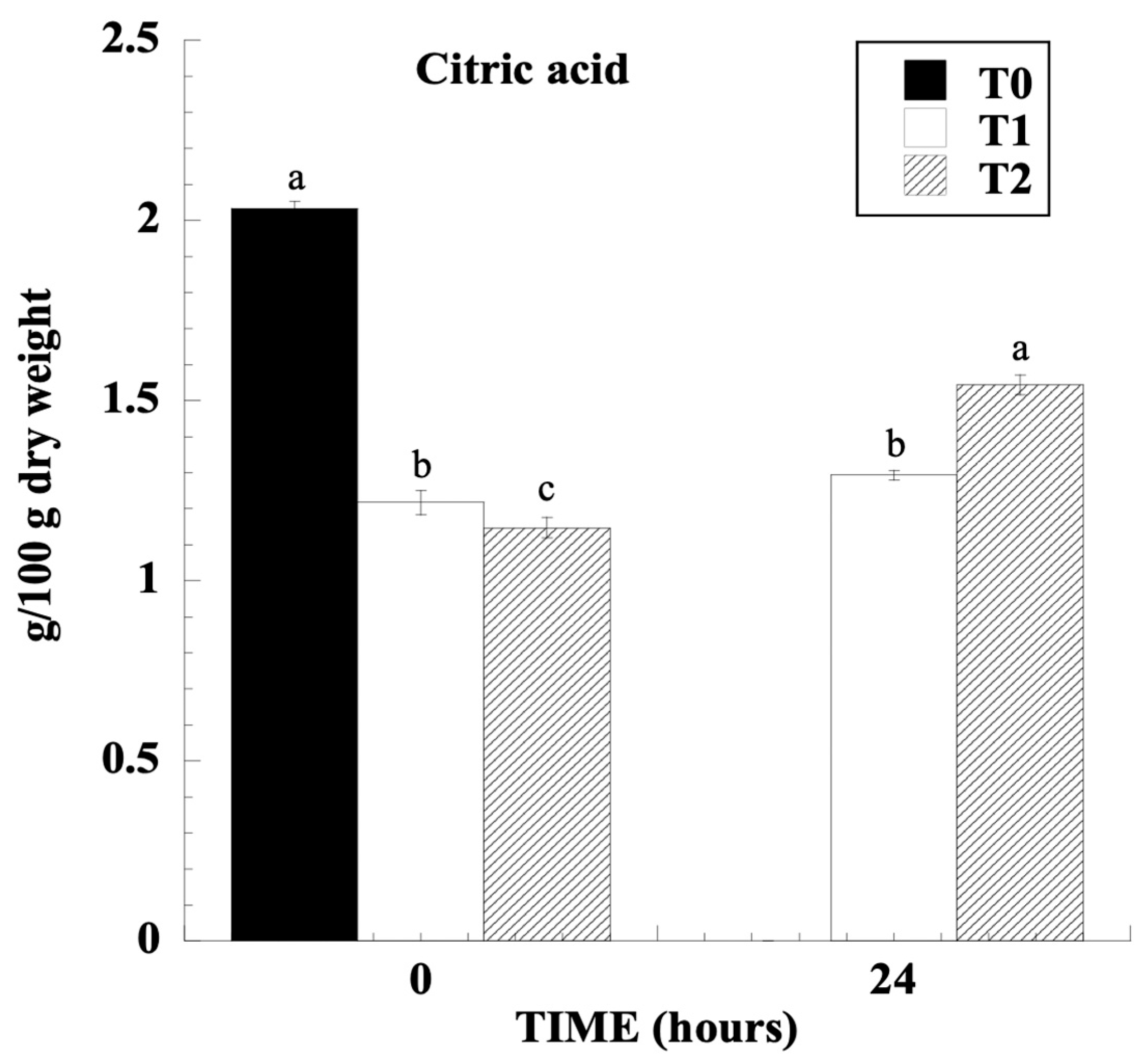
3.2. Organic Acids
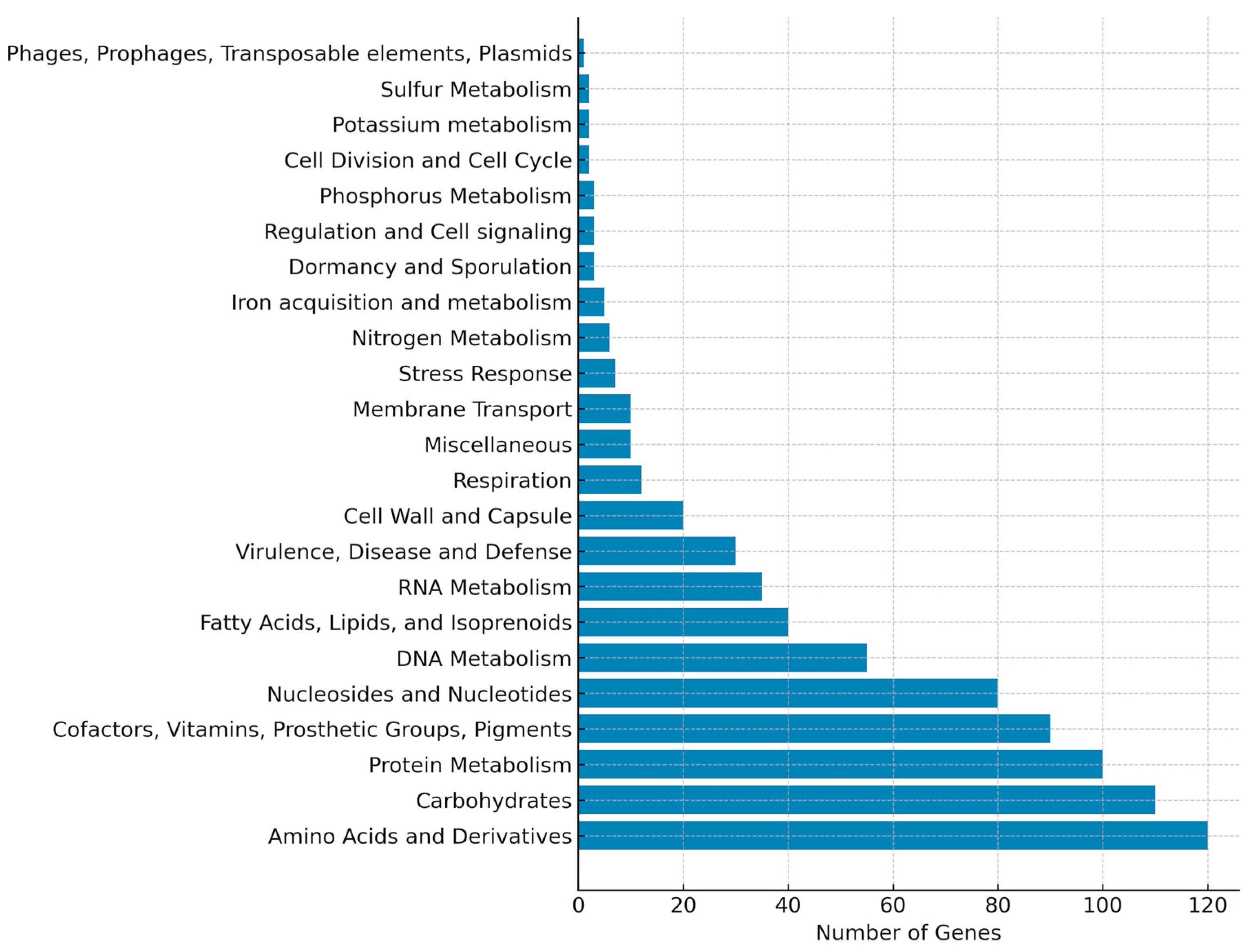
3.3. Genomic Data Associated with Limosilactobacillus fermentum J24
3.4. Sugar Metabolism
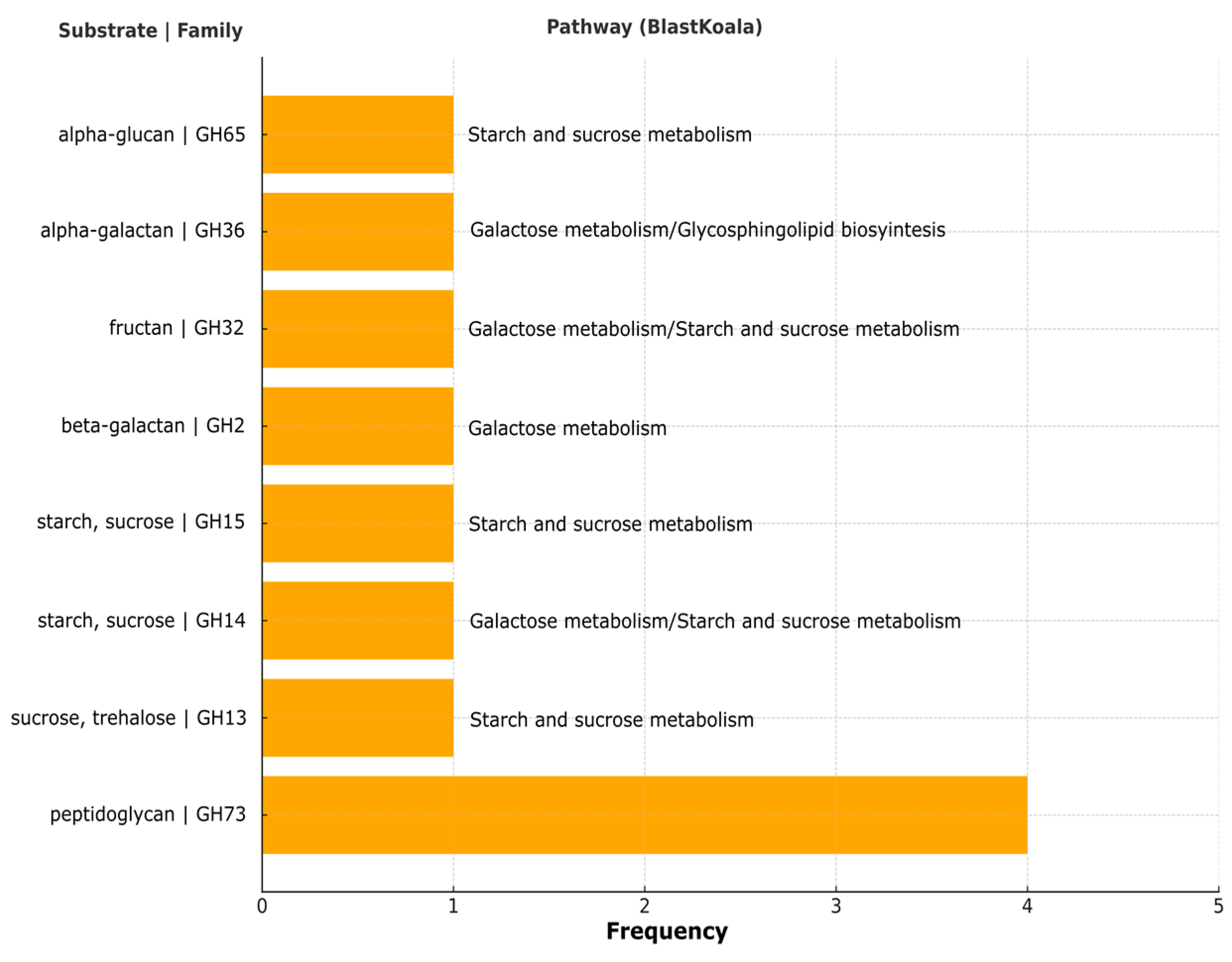
3.5. Carbohydrate-Active Enzymes
3.6. Acetoin and Butanediol Production
3.7. Stress Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayala-Zavala, J.; Castillo-Romero, T.D.J.; Salgado-Cervantes, M.; Marín-Castro, U.; Vargas-Ortiz, M.; Pallet, D.; Servent, A. Flash Vacuum Expansion: Effect on physicochemical, biochemical and sensory parameters in fruit processing. Food Rev. Int. 2024, 40, 833–866. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of fermentation of pasteurised papaya puree with different lactic acid bacterial strains on quality and bioaccessibility of phenolic compounds during in vitro digestion. Foods 2021, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, W.; Chen, H.; Zhang, G.; Chen, W. Comparative evaluation of the antioxidant capacities, organic acids, and volatiles of papaya juices fermented by Lactobacillus acidophilus and Lactobacillus plantarum. J. Food Qual. 2018, 2018, 9490435. [Google Scholar] [CrossRef]
- Arias, C.; Rodríguez, P.; Soto, I.; Vaillant, R.; Cortés, M.; Vaillant, F. Flash vacuum expansion, a low-cost and energy-efficient alternative process to produce high-quality fruit puree: Application to Physalis peruviana. Heliyon 2023, 9, e16969. [Google Scholar] [CrossRef]
- Ortega-Villalba, K.J.; Velez-Pasos, C.; Rodriguez-Fonseca, P.E.; Vaillant-Barka, F. Unleashing the potential of flash vacuum expansion: An innovative approach for andean blackberry (Rubus glaucus Benth) processing. Ing. Compet. 2023, 25, e-20213132. [Google Scholar] [CrossRef]
- Paranjpe, S.S.; Morgan, M.T. Improving grape juice yield and quality using flash vacuum expansion. In Proceedings of the 2007 ASAE Annual Meeting, Chicago, IL, USA, 11–14 August 2007; p. 1. [Google Scholar]
- Valencia-García, F.E. Patrimonios culinarios fermentados como herramientas para mejorar la seguridad alimentaria. Rev. Colomb. Biotecnol. 2023, 25, 3–4. [Google Scholar]
- Xiong, S.; Xu, X.; Du, T.; Liu, Q.; Huang, T.; Ren, H.; Xiong, T.; Xie, M. Organic acids drove the microbiota succession and consequently altered the flavor quality of Laotan Suancai across fermentation rounds: Insights from the microbiome and metabolome. Food Chem. 2024, 450, 139335. [Google Scholar] [CrossRef]
- Okoye, C.O.; Dong, K.; Wang, Y.; Gao, L.; Li, X.; Wu, Y.; Jiang, J. Comparative genomics reveals the organic acid biosynthesis metabolic pathways among five lactic acid bacterial species isolated from fermented vegetables. New Biotechnol. 2022, 70, 73–83. [Google Scholar] [CrossRef]
- Castillo-Romero, T.d.J.; Ayala-Zavala, J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Méndez-Romero, J.I.; Salgado-Cervantes, M.; Vargas-Ortiz, M. Flash vacuum expansion process promotes increased viability of Limosilactobacillus fermentum J24 in papaya-based beverages under simulated digestion. Food Biosci. 2024, 62, 105219. [Google Scholar] [CrossRef]
- Castillo-Romero, T.d.J.; López-Martínez, L.X.; Salgado-Cervantes, M.A.; Quintana-Obregón, E.A.; González-Aguilar, G.A.; Vargas-Ortiz, M. The flash vacuum expansion process increases the bioaccessibility and stability of antioxidant compounds in papaya puree during digestion. Resources 2024, 13, 175. [Google Scholar] [CrossRef]
- CODEX STAN 247-2005; General Standard for Fruit Juices and Nectars. Codex Alimentarius Commission: Rome, Italy, 2005.
- Sigala-Robles, R.; Estrada-Montoya, M.D.C.; Torres-Llanez, M.J.; Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Tracking the metabolite footprint of four lactic acid bacteria in semiskimmed milk: A chemometric analysis. Int. J. Dairy Technol. 2024, 77, 1171–1179. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ge, Q.; Yan, Y.; Zhang, X.; Huang, L.; Yin, Y. dbCAN3: Automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 2023, 51, W115–W121. [Google Scholar] [CrossRef]
- Barabote, R.D.; Saier, M.H., Jr. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 2005, 69, 608–634. [Google Scholar] [CrossRef]
- Hossain, T.J. Functional genomics of the lactic acid bacterium Limosilactobacillus fermentum LAB-1: Metabolic, probiotic and biotechnological perspectives. Heliyon 2022, 8, e11412. [Google Scholar] [CrossRef]
- López, D.S.Y. Ingeniería de las Proteínas del Sistema PTS en Escherichia coli. Ph.D. Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2020. [Google Scholar]
- Ge, Y.; Li, D.; Wang, N.; Shi, Y.; Guo, G.; Fang, L.; Zou, Q.; Liu, Q. Unveiling the fructose metabolism system in Staphylococcus aureus: Insights into the regulatory role of FruR and the FruRKT operon in bacterial fitness. BMC Microbiol. 2024, 24, 13. [Google Scholar] [CrossRef]
- He, Y.; Mok, K.; Chumnanpuen, P.; Nakphaichit, M.; Vongsangnak, W. Dissecting metabolic functions and sugar transporters using genome and transportome of probiotic Limosilactobacillus fermentum KUB-D18. Genes 2025, 16, 348. [Google Scholar] [CrossRef]
- Lugo-Zarate, L.; Cruz-Cansino, N.D.S.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Arias-Rico, J.; Cervantes-Elizarrarás, A. Evaluation of physicochemical, microbiological, and antioxidant properties of a drinkable yogurt added with ultrasonicated purple cactus pear (Opuntia ficus-indica) juice powder. J. Food Process. Preserv. 2021, 45, e15720. [Google Scholar] [CrossRef]
- Valencia, T.G.; Páez, M.I.; Hoyos, O.L. Seguimiento de la degradación térmica y lumínica del ácido ascórbico en uchuva (Physalis peruviana L.). Sci. Tech. 2007, 1, 211–215. [Google Scholar]
- Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Fu, M.; Wen, J. Slight fermentation with Lactobacillus fermentium improves the taste (sugar: Acid ratio) of citrus (Citrus reticulata cv. chachiensis) juice. J. Food Sci. 2015, 80, M2543–M2547. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef]
- Muñoz-Villa, A.; Sáenz-Galindo, A.; López-López, L.; Cantú-Sifuentes, L.; Barajas-Bermúdez, L. Ácido Cítrico: Compuesto Interesante Citric Acid: Interesting Compound. Rev. Científica Univ. Autónoma Coahuila 2014, 6, 18–23. [Google Scholar]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Iskandar, C.F.; Cailliez-Grimal, C.; Borges, F.; Revol-Junelles, A.-M. Review of lactose and galactose metabolism in Lactic Acid Bacteria dedicated to expert genomic annotation. Trends Food Sci. Technol. 2019, 88, 121–132. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Janeček, Š.; Brumer, H.; Henrissat, B. News, trends, and challenges in carbohydrate-active enzymes. Biologia 2023, 78, 1739–1740. [Google Scholar] [CrossRef]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Wei, G.; Wang, D.; Wang, T.; Wang, G.; Chai, Y.; Li, Y.; Mei, M.; Wang, H.; Huang, A. Probiotic potential and safety properties of Limosilactobacillus fermentum A51 with high exopolysaccharide production. Front. Microbiol. 2025, 16, 1498352. [Google Scholar] [CrossRef]
- Meng, W.; Ma, C.; Xu, P.; Gao, C. Biotechnological production of chiral acetoin. Trends Biotechnol. 2022, 40, 958–973. [Google Scholar] [CrossRef]
- Bai, Y.; Feng, H.; Liu, N.; Zhao, X. Biomass-derived 2, 3-butanediol and its application in biofuels production. Energies 2023, 16, 5802. [Google Scholar] [CrossRef]
- Keo-Oudone, C.; Phommachan, K.; Suliya, O.; Nurcholis, M.; Bounphanmy, S.; Kosaka, T.; Yamada, M. Highly efficient production of 2, 3-butanediol from xylose and glucose by newly isolated thermotolerant Cronobacter sakazakii. BMC Microbiol. 2022, 22, 164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, Z.; Zhao, Y.; Peng, M. Unveiling the vital role of soil microorganisms in selenium cycling: A review. Front. Microbiol. 2024, 15, 1448539. [Google Scholar] [CrossRef] [PubMed]
- Ledgham, F.; Quest, B.; Vallaeys, T.; Mergeay, M.; Covès, J. A probable link between the DedA protein and resistance to selenite. Res. Microbiol. 2005, 156, 367–374. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, X.; Song, X.; Chang, J.; Zeng, X.; Zhu, L.; Xu, C. Selenite bioremediation by food-grade probiotic Lactobacillus casei ATCC 393: Insights from proteomics analysis. Microbiol. Spectr. 2023, 11, e00659-23. [Google Scholar] [CrossRef]
- Ozen, M.; Piloquet, H.; Schaubeck, M. Limosilactobacillus fermentum CECT5716: Clinical potential of a probiotic strain isolated from human milk. Nutrients 2023, 15, 2207. [Google Scholar] [CrossRef]
- Dos Santos, C.I.; Campos, C.D.; Nunes-Neto, W.R.; do Carmo, M.S.; Nogueira, F.A.; Ferreira, R.M.; Costa, E.P.S.; Gonzaga, L.F.; Araújo, J.M.M.; Monteiro, J.M.; et al. Genomic analysis of Limosilactobacillus fermentum ATCC 23271, a potential probiotic strain with anti-Candida activity. J. Fungi 2021, 7, 794. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Llanez, M.d.J.; Méndez-Romero, J.I.; Ayala-Zavala, J.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Salgado-Cervantes, M.A.; Vargas-Ortiz, M.; Castillo-Romero, T.d.J. Effect of the Flash Vacuum Expansion (FVE) Process on the Response of Limosilactobacillus fermentum J24 to the Metabolism of Sugars and Organic Acids During the Development of a Papaya-Based Drink. Beverages 2025, 11, 150. https://doi.org/10.3390/beverages11050150
Torres-Llanez MdJ, Méndez-Romero JI, Ayala-Zavala J, González-Córdova AF, Vallejo-Cordoba B, Salgado-Cervantes MA, Vargas-Ortiz M, Castillo-Romero TdJ. Effect of the Flash Vacuum Expansion (FVE) Process on the Response of Limosilactobacillus fermentum J24 to the Metabolism of Sugars and Organic Acids During the Development of a Papaya-Based Drink. Beverages. 2025; 11(5):150. https://doi.org/10.3390/beverages11050150
Chicago/Turabian StyleTorres-Llanez, María de Jesús, José Isidro Méndez-Romero, Jesús Ayala-Zavala, Aarón Fernando González-Córdova, Belinda Vallejo-Cordoba, Marco Antonio Salgado-Cervantes, Manuel Vargas-Ortiz, and Teresita de Jesús Castillo-Romero. 2025. "Effect of the Flash Vacuum Expansion (FVE) Process on the Response of Limosilactobacillus fermentum J24 to the Metabolism of Sugars and Organic Acids During the Development of a Papaya-Based Drink" Beverages 11, no. 5: 150. https://doi.org/10.3390/beverages11050150
APA StyleTorres-Llanez, M. d. J., Méndez-Romero, J. I., Ayala-Zavala, J., González-Córdova, A. F., Vallejo-Cordoba, B., Salgado-Cervantes, M. A., Vargas-Ortiz, M., & Castillo-Romero, T. d. J. (2025). Effect of the Flash Vacuum Expansion (FVE) Process on the Response of Limosilactobacillus fermentum J24 to the Metabolism of Sugars and Organic Acids During the Development of a Papaya-Based Drink. Beverages, 11(5), 150. https://doi.org/10.3390/beverages11050150








