Chemical and Sensory Attributes of Different Ethanol Reduction Methods in Muscadine Wine Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Fermentation
2.1.1. Control Wine (Saccharomyces cerevisiae Yeast)
2.1.2. Sampling
2.1.3. Distilled Wine
2.1.4. Stopped Fermentation
2.1.5. Non-Saccharomyces cerevisiae Yeast Wine
2.2. Chemical Analysis
2.2.1. Brix Analysis
2.2.2. Titrable Acidity (TA)/pH Analysis
2.2.3. Color Analysis
2.3. Gas Chromatography Analysis
2.3.1. Extraction of Volatile and Semi-Volatile Compounds
2.3.2. Compound Response
2.4. Fermentation Statistics
2.5. Consumer Panel and Analysis
2.6. Study Limitations
3. Results
3.1. Wine Properties
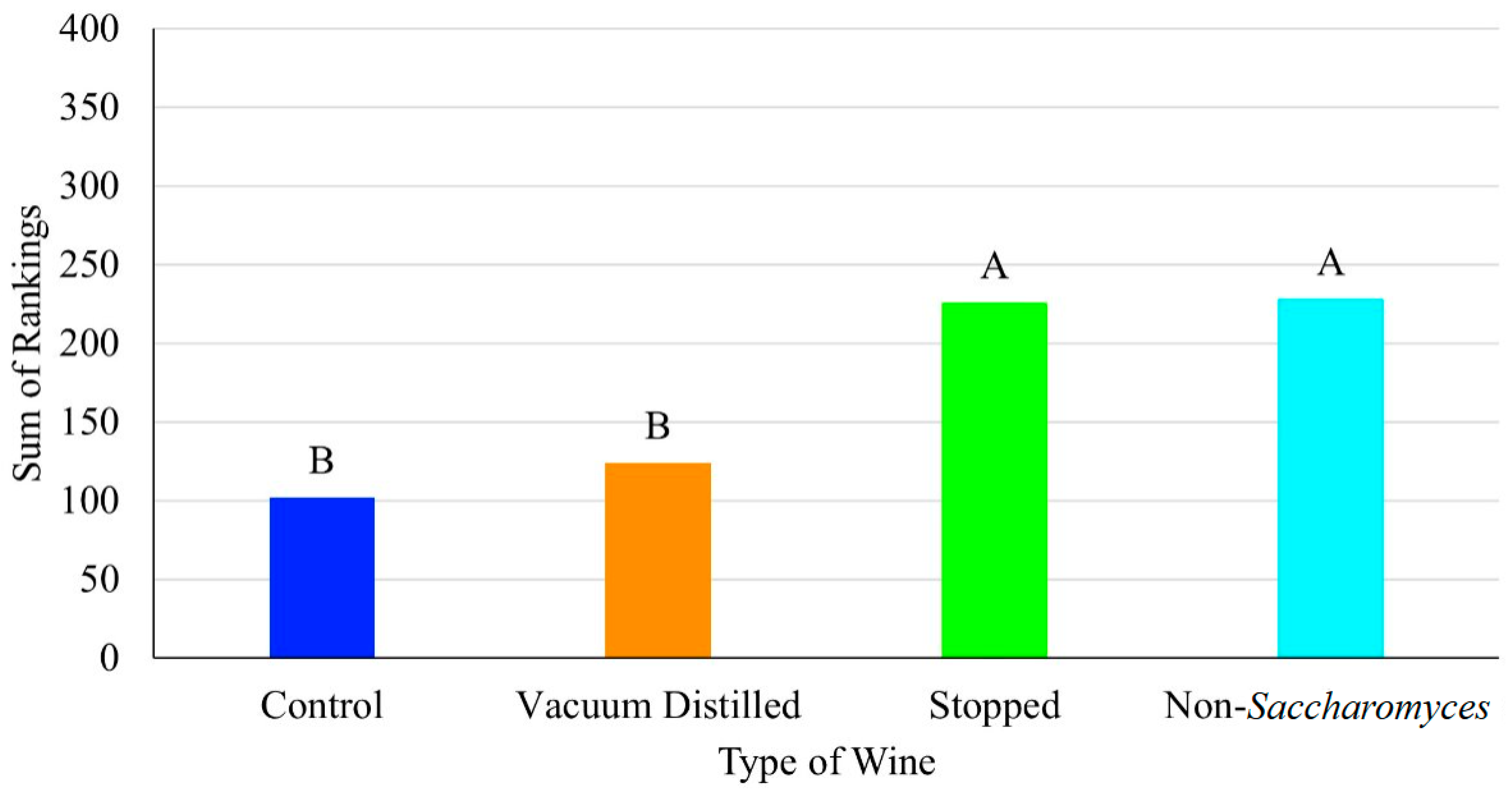
3.2. Consumer Panel Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NA | Non-alcoholic |
| TA | Titratable acidity |
| USD | United States Dollar |
| CAGR | Compound annual growth rate |
| pH | Potential of Hydrogen |
| EDTA | Ethylenediaminetetraacetic acid |
| i.d. | Inner diameter |
| ASBC | American Society of Brewing Chemists |
| ANOVA | Analysis of variance |
| DI | De-ionized |
| SPME | Solid-phase microextraction |
| MSD | Mass spectrometer detector |
| GC-MS | Gas chromatography–mass spectrometry |
| RI | Retention index |
| LRIs | Linear retention indices |
| SD | Significant difference |
Appendix A
| Wine Type | pH | TA (g/L Tartaric Acid) | Color Hue | Color Intensity |
|---|---|---|---|---|
| F value | 2.91 | 6.037 | 267.7 | 70.81 |
| Degrees of Freedom | 14 | 14 | 14 | 14 |
| p-value | 0.0777 | 0.0098 | <0.0001 | <0.0001 |

References
- 360 Market Updates. Market Research Reports, Industry Analysis, Business Overview & Trends. 2023. Available online: https://www.360marketupdates.com/TOC/24117297#Tables (accessed on 7 March 2024).
- NielsenIQ. Non-Alcoholic Beverage Trends in the US. 2022. Available online: https://nielseniq.com/global/en/insights/education/2022/non-alcoholic-beverage-trends-in-the-us/ (accessed on 7 March 2024).
- Office of Communications. Wine Labeling: Overview of Labeling Requirements for Domestic Wines—Less than 7 Percent Alcohol by Volume|TTB: Alcohol and Tobacco Tax and Trade Bureau. Ttbgov. as Found on the Website. 2025. Available online: https://www.ttb.gov/regulated-commodities/beverage-alcohol/wine/7percentorless (accessed on 12 May 2025).
- Aguera, E.; Bes, M.; Roy, A.; Camarasa, C.; Sablayrolles, J.M. Partial Removal of Ethanol during Fermentation to Obtain Reduced-Alcohol Wines. Am. J. Enol. Vitic. 2010, 61, 53–60. [Google Scholar] [CrossRef]
- Australian Wine Research Institute. Predicting Alcohol Levels; Australian & New Zealand Grapegrower & Winemaker: Urrbrae, SA, Australia, 2016. [Google Scholar]
- Fleming, A.J.; Threlfall, R.T. Using non-Saccharomyces yeast to modify acidity during wine fermentations from VitisHybrid grapes grown in a warm region. Am. J. Enol. Vitic. 2024, 75, 0750004. [Google Scholar] [CrossRef]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; Liang, H.; Zhan, J.; Huang, W.; You, Y. Mechanisms and effects of non-Saccharomyces yeast fermentation on the aromatic profile of wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Esteves, M.; Barbosa, C.; Vasconcelos, I.; Tavares, M.J.; Mendes-Faia, A.; Mira, N.P.; Mendes-Ferreira, A. Characterizing the potential of the non-conventional yeast Saccharomycodes ludwigii UTAD17 in winemaking. Microorganisms 2019, 7, 478. [Google Scholar] [CrossRef]
- Andersen, P.C.; Sarkhosh, A.; Huff, D.; Breman, J.W. The Muscadine Grape (Vitis rotundifolia Michx). edisifasufledu. as Found on the Website. 2024. Available online: https://edis.ifas.ufl.edu/publication/HS100 (accessed on 9 May 2025).
- Zhang, Y.; Chang, S.K.C.; Stringer, S.J.; Zhang, Y. Characterization of titratable acids, phenolic compounds, and antioxidant activities of wines made from eight mississippi-grown muscadine varieties during fermentation. LWT 2017, 86, 302–311. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production Technologies for Reduced Alcoholic Wines. J. Food Sci. 2012, 77, R25–R41. [Google Scholar] [CrossRef]
- Silva, P. Low-Alcohol and Nonalcoholic Wines: From Production to Cardiovascular Health, along with Their Economic Effects. Beverages 2024, 10, 49. [Google Scholar] [CrossRef]
- American Society of Brewing Chemists. Beer Method 4. Beer and Distillate Measured Gravimetrically. Approved (1958), rev. (1975), rev. (2018); American Society of Brewing Chemists: St. Paul, MN, USA. [CrossRef]
- Cutaia, A.J.; Reid, A.J.; Speers, R.A. Examination of the relationships between original, real and apparent extracts, and alcohol in pilot plant and commercially produced beers. J. Inst. Brew. 2009, 115, 318–327. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Titratable Acidity of Red Wine by Manual Titration (Potentiometric); Thermo Fisher Scientific: Waltham, MA, USA, 2014. [Google Scholar]
- Wendrick, N. The Influence of Packaging Material on the Properties of Carbonated Muscadine Wine Under Accelerated and Ambient Storage Conditions. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2022. [Google Scholar]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Budner, D.; Carr, J.; Serafini, B.; Tucker, S.; Dieckman-Meyer, E.; Bell, L.; Thompson-Witrick, K.A. Statistical Significant Differences between Aroma Profiles of Beer Brewed from Sorghum. Beverages 2021, 7, 56. [Google Scholar] [CrossRef]
- Thompson-Witrick, K.A.; Rouseff, R.L.; Cadwallader, K.R.; Duncan, S.E.; Eigel, W.N.; Tanko, J.M.; O’Keefe, S.F. Comparison of two extraction techniques, solid-phase microextraction versus continuous liquid-liquid extraction/solvent-assisted flavor evaporation, for the analysis of flavor compounds in Gueuze lambic beer. J. Food Sci. 2015, 80, C571–C576. [Google Scholar] [CrossRef]
- American Society of Brewing Chemists. ASBC Methods of Analysis Online Yeast Method 14 Miniature Fermentation Assay Approved; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar] [CrossRef]
- MacIntosh, A.J.; Adler, J.; Eck, E.; Speers, R.A. Suitability of the miniature fermentability method to monitor industrial fermentations. J. Am. Soc. Brew. Chem. 2012, 70, 205–211. [Google Scholar] [CrossRef]
- Reid, S.J.; Josey, M.; MacIntosh, A.J.; Maskell, D.L.; Speers, R.A. Predicting Fermentation Rates in Ale, Lager and Whisky. Fementation 2021, 7, 13. [Google Scholar] [CrossRef]
- Rudolph, A.; MacIntosh, A.J.; Speers, R.A.; St Mary, C. Modeling Yeast in Suspension during Laboratory and Commercial Fermentations to Detect Aberrant Fermentation Processes. J. Am. Soc. Brew. Chem. 2019, 78, 63–73. [Google Scholar] [CrossRef]
- Vion, C.; Yeramian, N.; Hranilovic, A.; Masneuf-Pomarède, I.; Marullo, P. Influence of yeasts on wine acidity: New insights into Saccharomyces cerevisiae. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Rasines-Perea, Z.; Prieto-Perea, N.; Asensio-Regalado, C.; Alonso-Salces, R.M.; Sánchez-Ilárduya, M.B.; Gallo, B. Formation and evolution profiles of anthocyanin derivatives and tannins during fermentations and aging of red wines. Eur. Food Res. Technol. 2019, 246, 149–165. [Google Scholar] [CrossRef]
- Chanprasartsuk, O.; Prakitchaiwattana, C. Growth kinetics and fermentation properties of autochthonous yeasts in pineapple juice fermentation for starter culture development. Int. J. Food Microbiol. 2022, 371, 109636. [Google Scholar] [CrossRef]
- Moreno, S.R.; Curtis, S.J.; Sarkhosh, A.; Sarnoski, P.J.; Sims, C.A.; Dreyer, E.; Rudolph, A.B.; Thompson-Witrick, K.A.; MacIntosh, A.J. Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches. Fermentation 2022, 8, 567. [Google Scholar] [CrossRef]
- Serviss, M.T.; Wendrick, N.A.; MacIntosh, A.J.; Thompson-Witrick, K.A. A Holistic View of the Fate of Berry-Derived Adjuncts throughout Fermentation. Beverages 2024, 10, 38. [Google Scholar] [CrossRef]
- ASBC. ASBC Beer Flavor Database. 2012. Available online: https://www.asbcnet.org/Methods/SensoryAnalysis/Pages/default.aspx (accessed on 1 May 2024).
- PubChem. PubChem Nihgov. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 May 2025).
- de Rosa, T.; Margheri, G.; Moret, I.; Scarponi, G.; Versini, G. Sorbic acid as a preservative in sparkling wine. Its efficacy and adverse flavor effect associated with ethyl sorbate formation. Am. J. Enol. Vitic. 1983, 34, 98–102. [Google Scholar] [CrossRef]
- Noordeloos, S.; Nagel, C.W. Effect of sugar on acid perception in wine. Am. J. Enol. Vitic. 1972, 23, 139–143. [Google Scholar] [CrossRef]
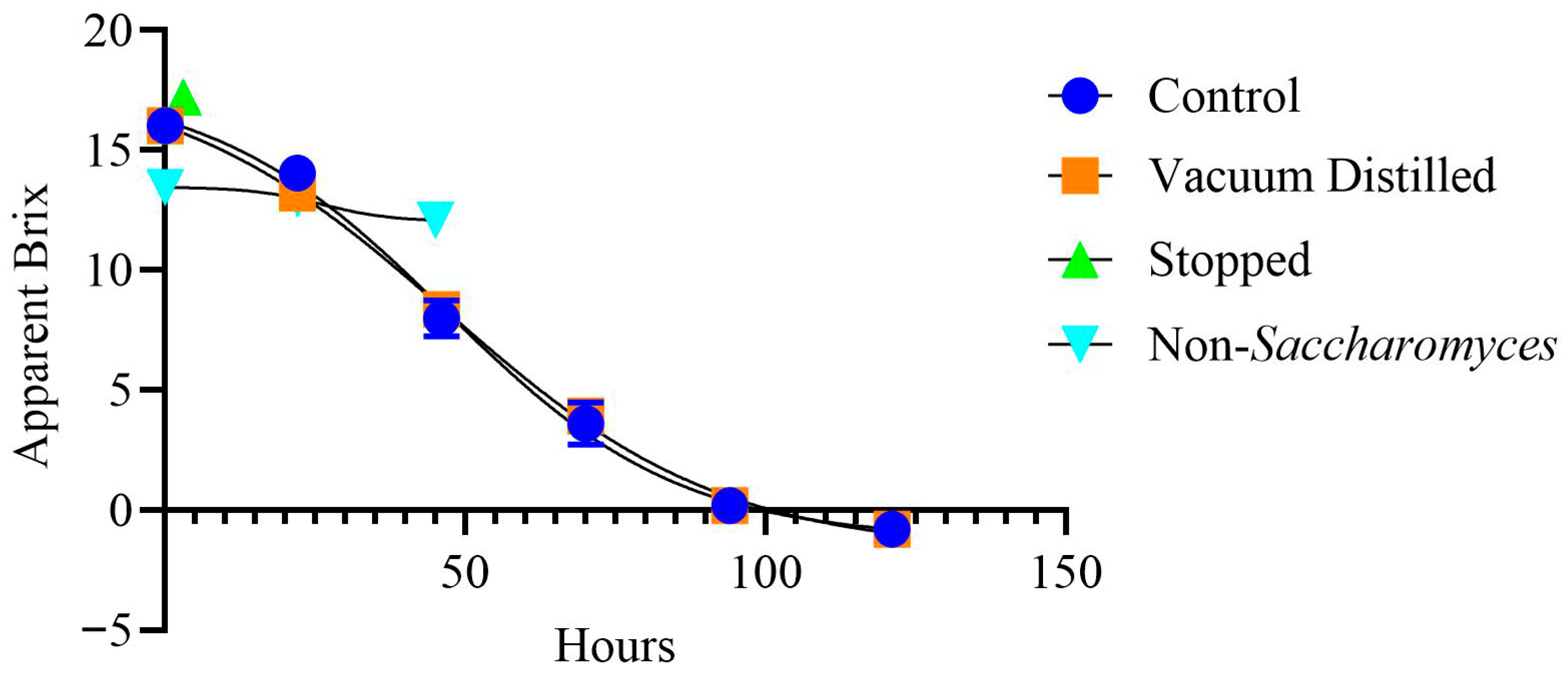
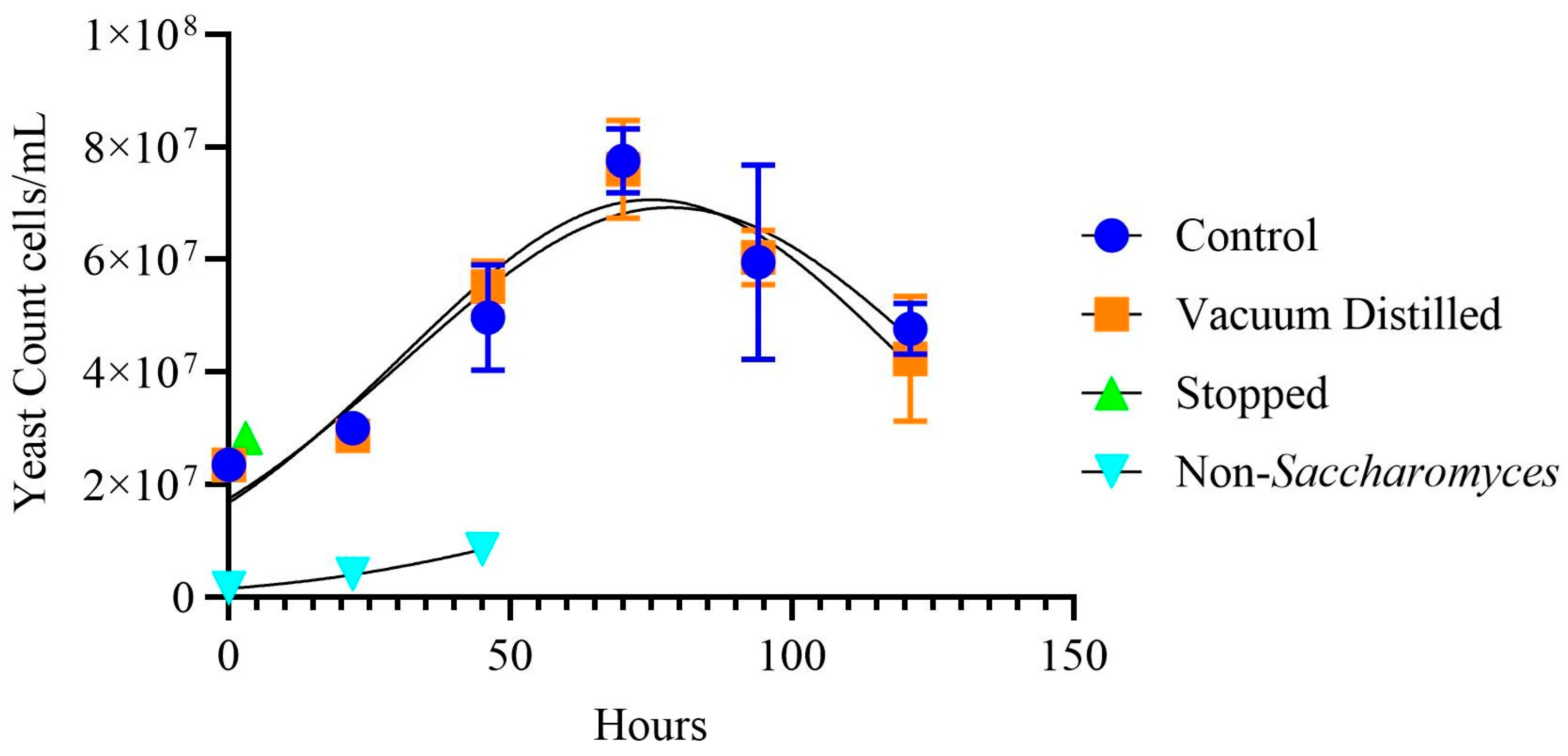

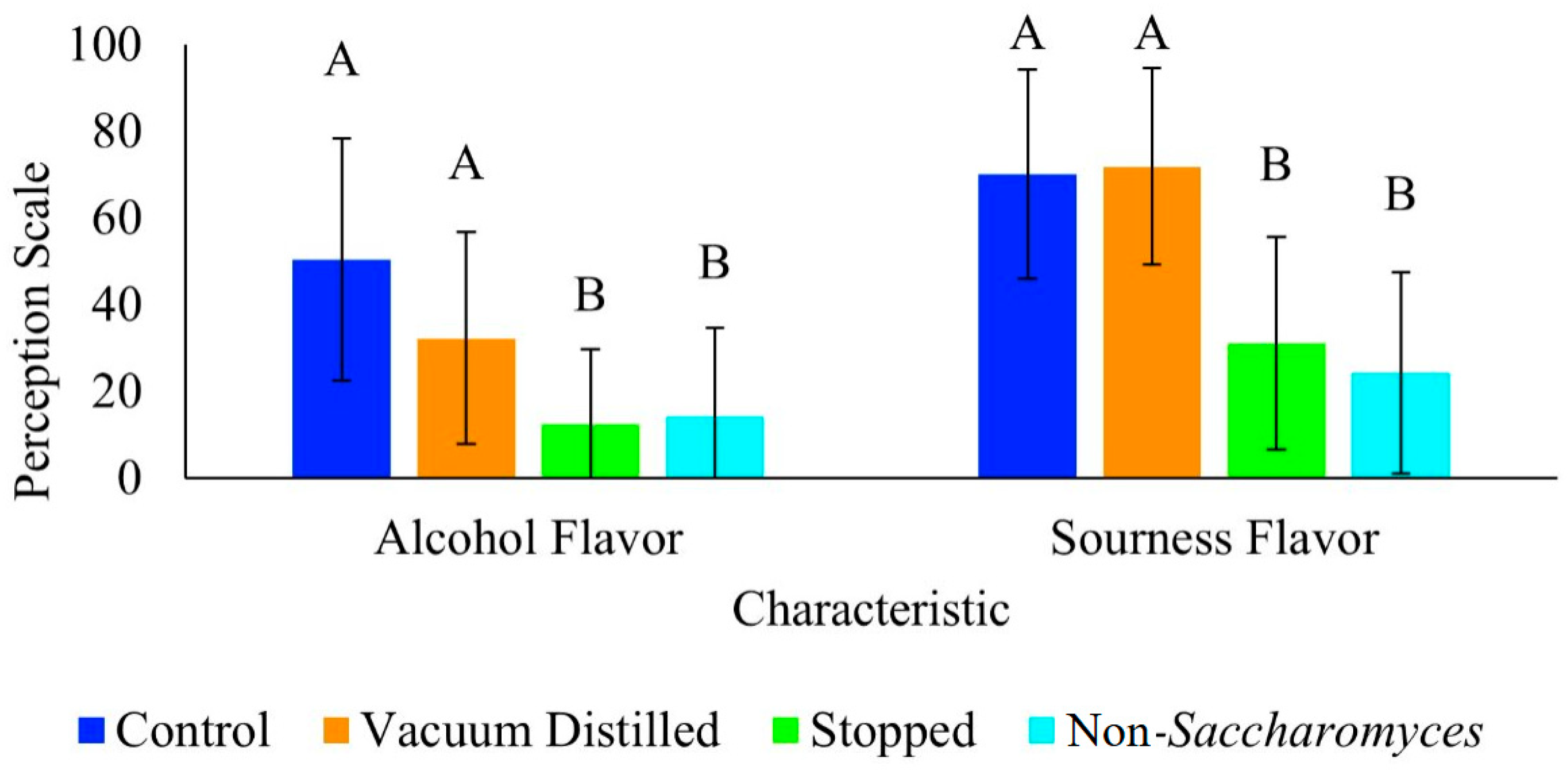
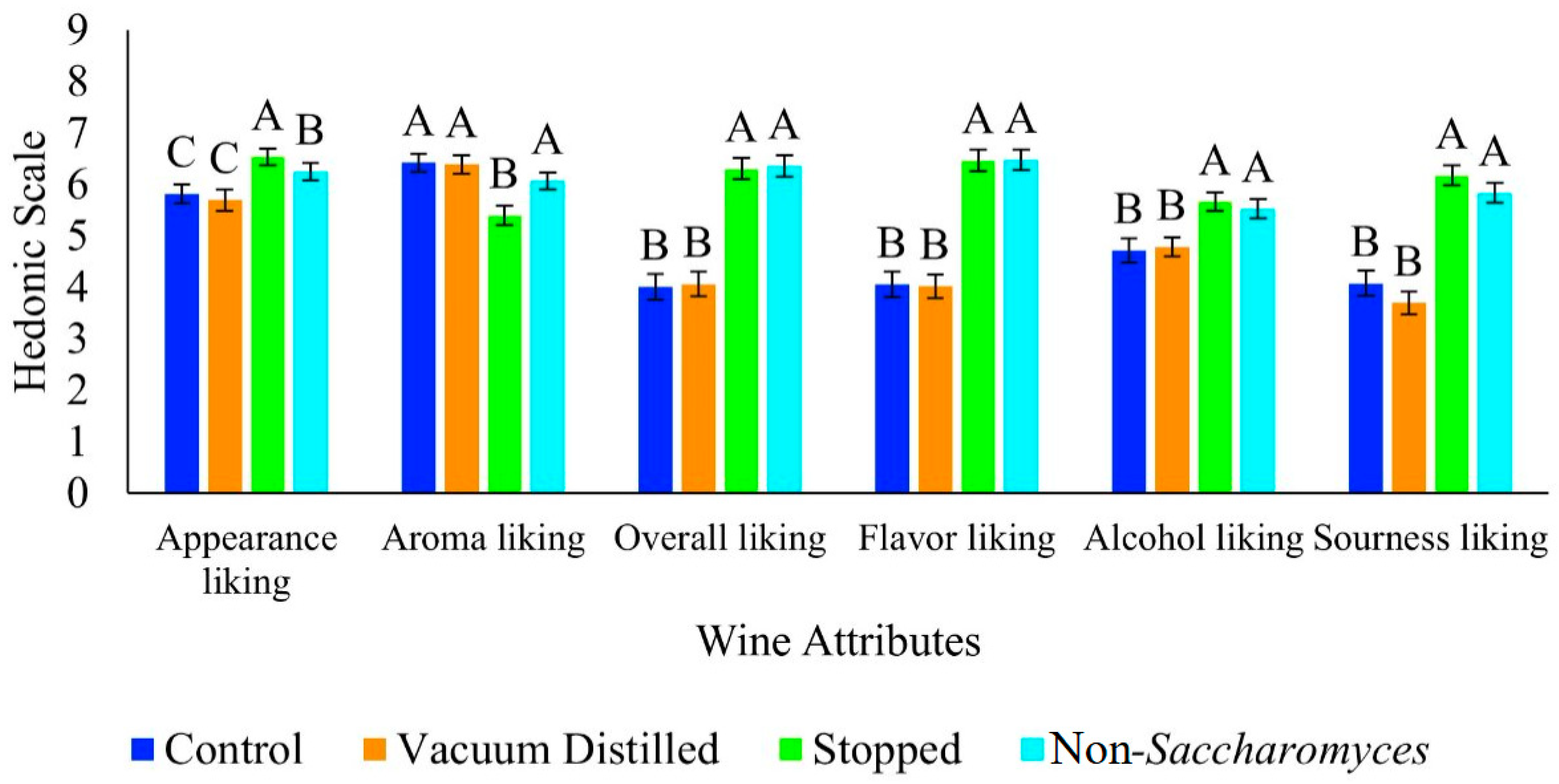
| Wine Type | Time (h) | pH | TA (g/L Tartaric Acid) | Color Hue | Color Intensity |
|---|---|---|---|---|---|
| Juice ^ | 0 | 3.22 ± 0.01 A | 8.1 ± 0.15 AB | 0.661 ± 0.01 D | 3.313 ± 0.03 A |
| Control | 121 | 3.17 ± 0.05 A | 9.3 ± 0.26 A | 1.102 ± 0.03 A | 1.207 ± 0.04 C |
| Vacuum-Distilled | 121 | 3.19 ± 0.04 A | 9.1 ± 0.23 A | 1.103 ± 0.01 A | 1.184 ± 0.06 C |
| Stopped | 3 | 3.22 ± 0.01 A | 8.6 ± 0.92 AB | 0.797 ± 0.03 C | 2.296 ± 0.46 B |
| Non-Saccharomyces | 45 | 3.24 ± 0.01 A | 7.7 ± 0.38 B | 0.943 ± 0.01 B | 3.398 ± 0.17 A |
| Compound | LRI | Odor Descriptors | Approximate Concentration (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| Juice | Control | Distilled | Stopped | Non-Sacc. | |||
| Acids | |||||||
| Hexanoic acid | 982 | rancid | ----- | ----- | ----- | ----- | 0.02 ± 0.02 |
| Octanoic acid | 1169 | sweat, cheese | 0.06 ± 0.03 | 0.57 ± 0.33 | 0.79 ± 0.91 | 0.17 ± 0.16 | 0.03 ± 0.01 |
| Nonanoic acid | 1260 | green, fat | 0.05 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.04 | 0.02 ± 0.03 | 0.01 ± 0.01 |
| Decanoic acid | 1373 | rancid fat | ----- | 0.29 ± 0.05 | 0.32 ± 0.05 | ----- | 0.07 ± 0.02 |
| SUBTOTAL | 0.07 ± 0.03 | 0.97 ± 0.39 e | 1.22 ± 1.00 e | 0.27 ± 0.20 e | 0.13 ± 0.02 e | ||
| Alcohols | |||||||
| Isoamyl alcohol | 757 | floral, fruity | ----- | 24.26 ± 5.04 | 22.31 ± 2.13 | 6.73 ± 1.37 | 0.52 ± 0.02 |
| 3-Hexenol | 865 | grass | 0.08 ± 0.05 | ----- | ----- | 0.03 ± 0.01 | 0.02 ± 0.01 |
| 2-Hexen-1-ol | 874 | leaf, green, wine, fruit | 0.06 ± 0.01 | ----- | ----- | ----- | ----- |
| Hexanol | 876 | floral, fruity | 0.58 ± 0.22 | 0.15 ± 0.03 | 0.14 ± 0.01 | ----- | 0.22 ± 0.01 |
| 1-Octanol | 1068 | floral, fruity, citrus | 0.03 ± 0.04 | 0.07 ± 0.01 | 0.07 ± 0.01 | ----- | ----- |
| Phenethyl alcohol | 1108 | alcohol, honey, roses, sweet | 0.13 ± 0.19 | 1.61 ± 0.05 | 1.82 ± 0.67 | 1.02 ± 0.31 | 0.04 ± 0.01 |
| Decanol | 1266 | fat | ----- | ----- | 0.06 ± 0.04 | ----- | ----- |
| Dodecanol | 1469 | fatty acids, coconut, banana | 0.02 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 | ----- | ----- |
| SUBTOTAL | 0.88 ± 0.21 | 26.25 ± 5.10 A | 24.43 ± 2.77 A | 7.78 ± 1.66 B | 0.28 ± 0.02 B | ||
| Aldehydes | |||||||
| Hexanal | 813 | grass, tallow, fat | 0.21 ± 0.07 | ----- | ----- | ----- | ----- |
| p-Tolualdehyde | 1079 | floral | ----- | 0.08 ± 0.07 | 0.10 ± 0.01 | ----- | ----- |
| 2,4-Dimethylbenzaldehyde | 1199 | mild, sweet, bitter almond | ----- | 0.03 ± 0.01 | 0.06 ± 0.03 | ----- | ----- |
| SUBTOTAL | 0.21 ± 0.07 | 0.08 ± 0.07 AB | 0.15 ± 0.03 A | 0 ± 0 B | 0 ± 0 B | ||
| Benzene | |||||||
| 1,2,3,5-Tetramethylbenzene | 1124 | camphor | ----- | 0.06 ± 0.01 | 0.07 ± 0.01 | ----- | ----- |
| Naphthalene | 1154 | tar | ----- | 0.39 ± 0.34 | 0.59 ± 0.10 | ----- | ----- |
| SUBTOTAL | ----- | 0.45 ± 0.34 AB | 0.66 ± 0.10 A | 0 ± 0 B | 0 ± 0 B | ||
| Esters | |||||||
| Ethyl butanaote | 826 | butter, sweet, perfumed, fruity | ----- | ----- | ----- | 0.02 ± 0.01 | ----- |
| Isoamyl acetate | 882 | banana | ----- | 16.54 ± 1.60 | 17.41 ± 0.21 | 0.66 ± 0.08 | ----- |
| Methyl N-hydroxybenzenecarboximidoate | 921 | 0.12 ± 0.08 | 0.42 ± 0.68 | 0.16 ± 0.24 | 0.02 ± 0.02 | ----- | |
| Ethyl hexanoate | 1002 | apple peel, fruit | ----- | 6.77 ± 0.91 | 7.98 ± 0.79 | 0.50 ± 0.16 | 0.09 ± 0.01 |
| Hexyl acetate | 1002 | fruit, herb | ----- | 1.66 ± 0.75 | 2.33 ± 0.12 | 0.03 ± 0.01 | 0.01 ± 0.01 |
| 2-Ethylhexanol | 1037 | rose, green | 0.03 ± 0.03 | 0.02 ± 0.02 | ----- | ----- | ----- |
| Ethyl 2,4-hexadienoate | 1089 | pineapple, celery | 0.07 ± 0.04 | ----- | ----- | ----- | ----- |
| Ethyl succinate | 1164 | wine, fruit | 0.07 ± 0.09 | ----- | ----- | ----- | ----- |
| Ethyl octanoate | 1197 | fruit, fat | 0.40 ± 0.59 | 8.65 ± 0.31 | 9.37 ± 1.31 | 1.31 ± 0.23 | 0.08 ± 0.03 |
| β-Phenethyl acetate | 1260 | rose, honey, tobacco | ----- | 0.51 ± 0.04 | 0.57 ± 0.12 | 0.28 ± 0.01 | 0.01 ± 0.01 |
| Ethyl 9-decanoate | 1369 | caprylic, fruity, apple | ----- | ----- | 0.03 ± 0.01 | 0.07 ± 0.01 | ----- |
| Ethyl decanoate | 1379 | grape | 0.12 ± 0.15 | 2.15 ± 0.12 | 2.69 ± 0.27 | 0.35 ± 0.07 | 0.02 ± 0.01 |
| Isoamyl octanoate | 1594 | fruity, spicy, orange, pear, melon | ----- | 0.08 ± 0.01 | 0.10 ± 0.02 | ----- | ----- |
| Ethyl dodecanoate | 1594 | caprylic, soapy, estery | ----- | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 |
| SUBTOTAL | 0.77 ± 0.94 | 37.03 ± 2.95 A | 40.68 ± 0.52 A | 3.25 ± 0.53 B | 0.21 ± 0.03 B | ||
| Ketones | |||||||
| 2-Octanone | 981 | fruity, green, floral, herbaceous | 0.14 ± 0.06 | 0.13 ± 0.11 | ----- | ----- | ----- |
| β-Damascenone | apple, rose, honey | ----- | 0.05 ± 0.05 | ----- | 0.04 ± 0.01 | 0.04 ± 0.03 | |
| SUBTOTAL | 0.14 ± 0.06 | 0.17 ± 0.15 e | 0 ± 0 e | 0.04 ± 0.01 e | 0.04 ± 0.03 e | ||
| Phenols | |||||||
| 2,4-Di-tert-butylphenol | 1481 | citrus, violet, hops, floral, berry | ----- | 0.35 ± 0.04 | 0.45 ± 0.12 | ----- | ----- |
| SUBTOTAL | 0 ± 0 | 0.35 ± 0.04 A | 0.45 ± 0.12 A | 0 ± 0 B | 0 ± 0 B | ||
| Terpenes | |||||||
| Limonene | lemon | ----- | ----- | ----- | 0.05 ± 0.02 | 0.06 ± 0.01 | |
| Linalool oxide | 1084 | flower, wood | 0.05 ± 0.05 | ----- | ----- | ----- | 0.02 ± 0.01 |
| Linalool | 1092 | flower, lavender | 0.26 ± 0.09 | 0.21 ± 0.18 | 0.34 ± 0.09 | 0.09 ± 0.01 | 0.04 ± 0.01 |
| SUBTOTAL | 0.30 ± 0.13 | 0.20 ± 0.18 e | 0.34 ± 0.09 e | 0.14 ± 0.03 e | 0.12 ± 0.0 e | ||
| Terpene alcohol | |||||||
| 3,7-Dimethyl-1,5,7-octatrien-3-ol | 1082 | hyacinth | ----- | 0.12 ± 0.03 | ----- | 0.15 ± 0.02 | 0.07 ± 0.03 |
| SUBTOTAL | 0 ± 0 | 0.12 ± 0.03 A | 0 ± 0 B | 0.15 ± 0.02 A | 0.07 ± 0.03 A | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalera, A.A.; Patricio Morillo, P.C.; Budner, D.; Thompson-Witrick, K.A.; MacIntosh, A.J. Chemical and Sensory Attributes of Different Ethanol Reduction Methods in Muscadine Wine Production. Beverages 2025, 11, 146. https://doi.org/10.3390/beverages11050146
Escalera AA, Patricio Morillo PC, Budner D, Thompson-Witrick KA, MacIntosh AJ. Chemical and Sensory Attributes of Different Ethanol Reduction Methods in Muscadine Wine Production. Beverages. 2025; 11(5):146. https://doi.org/10.3390/beverages11050146
Chicago/Turabian StyleEscalera, Alexandra A., Patricia C. Patricio Morillo, Drew Budner, Katherine A. Thompson-Witrick, and Andrew J. MacIntosh. 2025. "Chemical and Sensory Attributes of Different Ethanol Reduction Methods in Muscadine Wine Production" Beverages 11, no. 5: 146. https://doi.org/10.3390/beverages11050146
APA StyleEscalera, A. A., Patricio Morillo, P. C., Budner, D., Thompson-Witrick, K. A., & MacIntosh, A. J. (2025). Chemical and Sensory Attributes of Different Ethanol Reduction Methods in Muscadine Wine Production. Beverages, 11(5), 146. https://doi.org/10.3390/beverages11050146








