Addition of β-Cyclodextrin or Gelatin Ιmproves Organoleptic and Physicochemical Attributes of Aronia Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. β-Cyclodextrin and Gelatin Treatments
2.4. Organoleptic Evaluation and Consumer Preference Test
2.5. Physicochemical Analyses
2.5.1. Determination of Total Phenolic Compounds and Antioxidant Activity
2.5.2. Determination of Total Flavonoids
2.5.3. Determination of Total Anthocyanin Monomers
2.5.4. Determination of Color Due to Polymeric Pigments
2.5.5. Color, pH, and Total Soluble Solids
2.6. Statistical Analysis
3. Results and Discussion
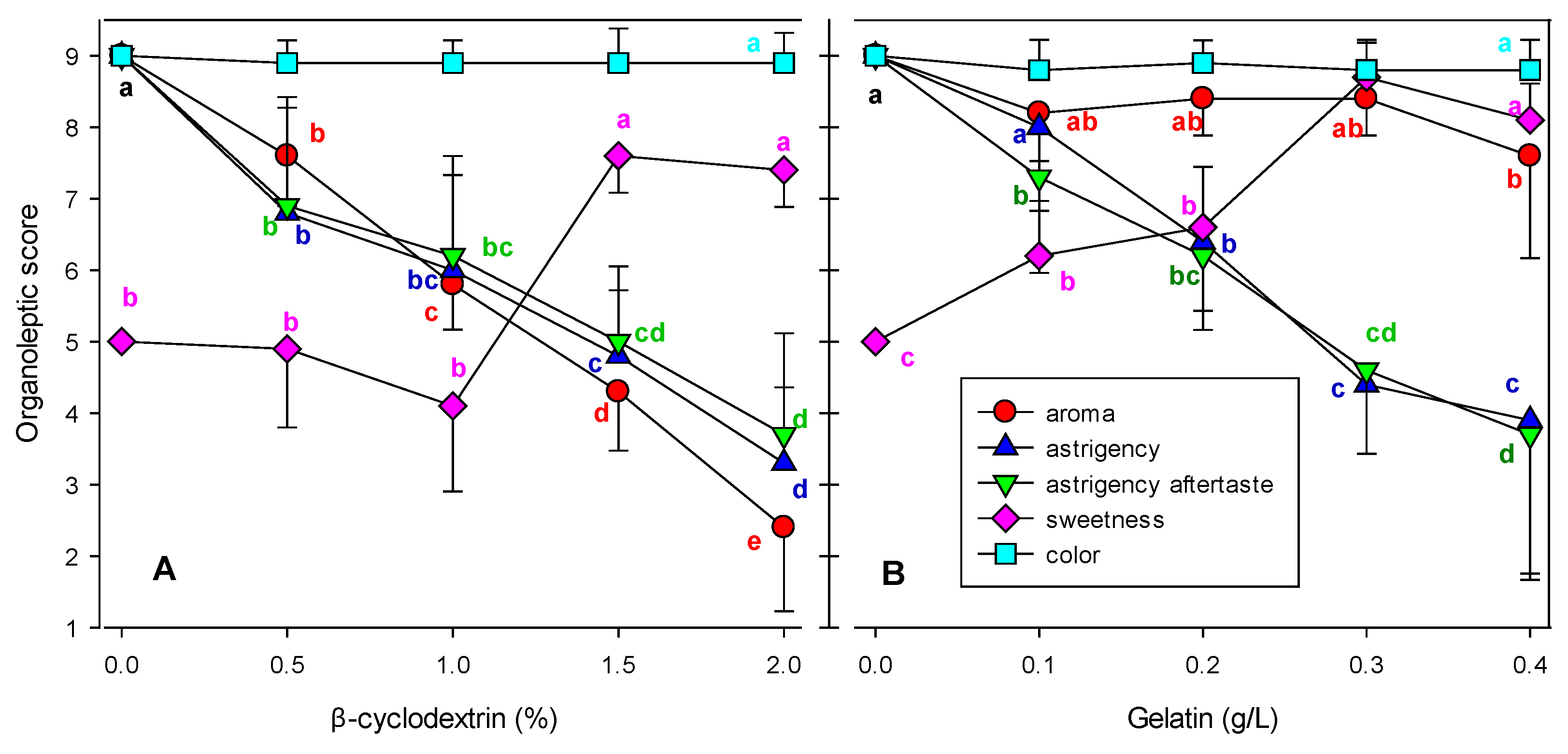
3.1. Organoleptic Evaluation
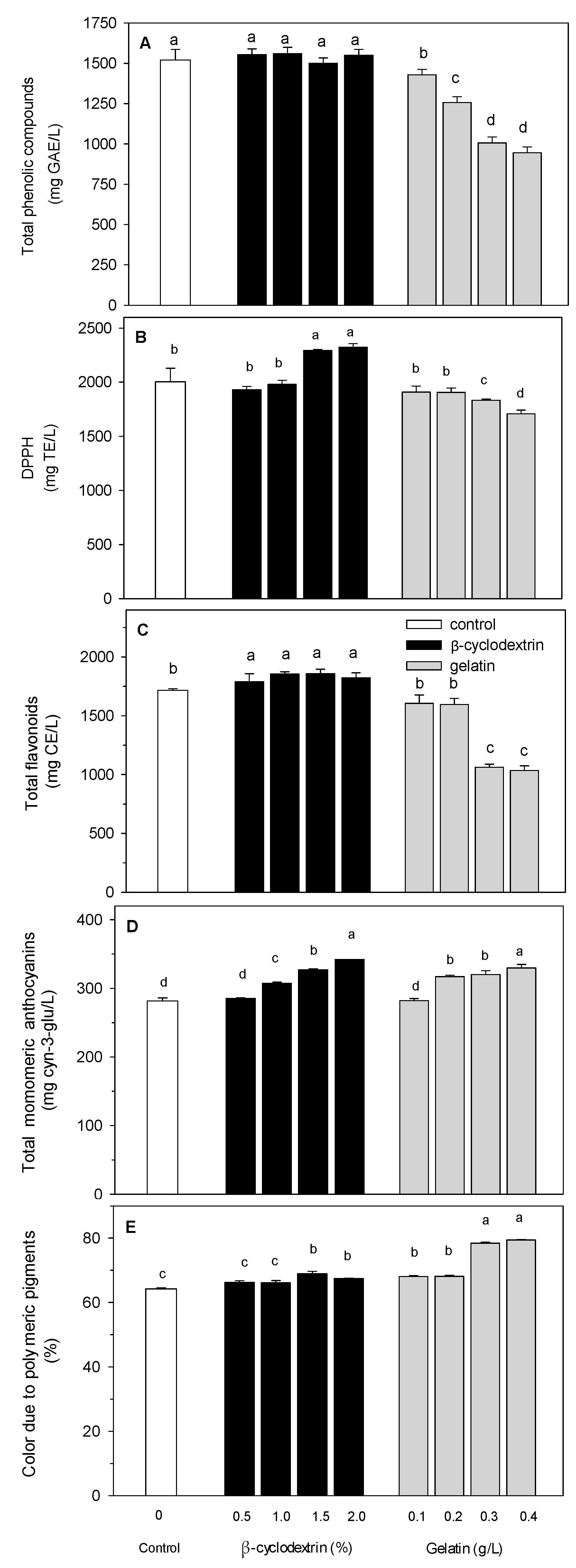
3.2. Effect of Pasteurization
3.3. Effect of β-CD and Gelatin Treatment
3.4. Second Organoleptic (Preference) Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidor, A.; Drozdzynska, A.; Gramza-Michałowska, A. Black Chokeberry (Aronia melanocarpa) and its Products as Potential Health-Promoting Factors—An Overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- King, E.S.; Bolling, B.W. Composition, Polyphenol Bioavailability, and Health Benefits of Aronia Berry: A Review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, X.; Chen, X.; Ding, C.; Dong, L.; Zhang, J.; Sun, S.; Ding, Q.; Khatoom, S.; et al. Chokeberry (Aronia melanocarpa) as a New Functional Food Relationship with Health: An Overview. J. Future Foods 2021, 1, 168–178. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.S. A Review of the Nutritional Profile, Chemical Composition and Potential Health Benefits of Aronia melanocarpa (Chokeberry) Berries and Products. Turk. J. Agric. Food Sci. Technol. 2023, 11, 2027–2043. [Google Scholar] [CrossRef]
- Sarıkaya, B.; Kolay, E.; Guney-Coskun, M.; Ziolkowski, A.Y.; Aktaç, Ş. The Effect of Black Chokeberry (Aronia melanocarpa) on Human Inflammation Biomarkers and Antioxidant Enzymes: A Systematic Review of Randomized Controlled Trials. Nutr. Rev. 2024, 83, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-M.; Zhou, X.; Zhang, J.-L.; Li, T. Research progress of anthocyanin antioxidant function in Aronia melanocarpa. Food Res. Dev. 2017, 38, 220–224. [Google Scholar]
- Xu, J.; Li, F.; Zheng, M.; Sheng, L.; Shi, D.; Song, K. A Comprehensive Review of the Functional Potential and Sustainable Applications of Aronia melanocarpa in the Food Industry. Plants 2024, 13, 3557. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Rohn, S.; Mocan, A. Fermented Black Chokeberry (Aronia melanocarpa (Michx.) Elliott) Products–A Systematic Review on the Composition and Current Scientific Evidence of Possible Health Benefits. Food Res. Int. 2024, 196, 115094. [Google Scholar] [CrossRef]
- Shi, D.; Xu, J.; Sheng, L.; Song, K. Comprehensive Utilization Technology of Aronia melanocarpa. Molecules 2024, 29, 1388. [Google Scholar] [CrossRef]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of Chokeberry Bioactive Polyphenols during Juice Processing and Stabilization of a Polyphenol-Rich Material from the By-Product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in Chokeberry (Aronia melanocarpa L.) Polyphenols during Juice Processing and Storage. J. Agric. Food Chem. 2013, 62, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Catană, L.; Catană, M.; Iorga, E.; Asănică, A.C.; Lazăr, A.G.; Lazăr, M.A.; Belc, N. Vitamin C and Total Polyphenol Content and Antioxidant Capacity of Fresh and Processed Fruits of Aronia melanocarpa. Sci. Pap. Ser. B Hortic. 2017, 61, 433–440. Available online: http://horticulturejournal.usamv.ro/pdf/2017/Art64.pdf (accessed on 9 March 2025).
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K.; Cyuńczyk, M.; Zujko, M.E. Antioxidant Properties of Chokeberry Products—Assessment of the Composition of Juices and Fibers. Foods 2023, 12, 4029. [Google Scholar] [CrossRef]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative Phytochemical Analysis of Aronia melanocarpa L. Fruit Juices on Bulgarian Market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Park, J. Characterizing and Improving the Oral Sensations and Preference of Polyphenol-Rich Aronia Berry Juice. Digital Commons @ UConn. Available online: https://opencommons.uconn.edu/srhonors_theses/348?utm_source=opencommons.uconn.edu%2Fsrhonors_theses%2F348&utm_medium=PDF&utm_campaign=PDFCoverPages (accessed on 9 March 2025).
- Duffy, V.B.; Rawal, S.; Park, J.; Brand, M.H.; Sharafi, M.; Bolling, B.W. Characterizing and Improving the Sensory and Hedonic Responses to Polyphenol-Rich Aronia Berry Iuice. Appetite 2016, 107, 116–125. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. Sensory property and phenolic profile of aronia juice. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2023; pp. 1–37. [Google Scholar] [CrossRef]
- Kelanne, N.; Laaksonen, O.; Seppälä, T.; Yang, W.; Tuukkanen, K.; Loponen, J.; Yang, B. Impact of Cyclodextrin Treatment on Composition and Sensory Properties of Lingonberry (Vaccinium vitis-idaea) juice. LWT 2019, 113, 108295. [Google Scholar] [CrossRef]
- Santos Buelga, C.; Scalbert, A. Proanthocyanidins and tannin like compounds-nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. An Overview of the Perception and Mitigation of Astringency Associated with Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Gu, L.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fang, W.; Xie, X.; Liu, Y.; Xu, C. Identification of Key Astringent Compounds in Aronia Berry Juice. Food Chem. 2022, 393, 133431. [Google Scholar] [CrossRef]
- Ağçam, E.; Akyıldız, A.; Dündar, B. Thermal Pasteurization and Microbial Inactivation of Fruit Juices. In Fruit Juices; Academic Press: Cambridge, MA, USA, 2018; pp. 309–339. [Google Scholar] [CrossRef]
- Deshaware, S.; Gupta, S.; Singhal, R.; Variyar, P. Influence of Different Pasteurization Techniques on Antidiabetic, Antioxidant and Sensory Quality of Debittered Bitter Gourd Juice during Storage. Food Chem. 2019, 285, 156–162. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.J.; Rupasinghe, H.V. Effect of Thermal and Non-Thermal Pasteurisation on the Microbial Inactivation and Phenolic Degradation in Fruit Juice: A Mini-Review. J. Sci. Food Agric. 2012, 93, 981–986. [Google Scholar] [CrossRef]
- Yi, T.; Fang, W.; Xie, X.; Yuan, B.; Lu, M.; Xu, C. High Pressure Processing (HPP) Improved Safety and Quality of Emerging Aronia Berry Juice: A Pilot Scale Shelf-Life Study. J. Food Sci. Technol. 2021, 59, 755–767. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Trenka, M.; Czaplicka, M.; Maksimowski, D.; Nawirska-Olszańska, A. Selected Properties of Juices from Black Chokeberry (Aronia melanocarpa L.) Fruits Preserved Using the PEF Method. Appl. Sci. 2022, 12, 7008. [Google Scholar] [CrossRef]
- Skąpska, S.; Marszałek, K.; Woźniak, Ł.; Zawada, K.; Wawer, I. Aronia Dietary Drinks Fortified with Selected Herbal Extracts Preserved by Thermal Pasteurization and High Pressure Carbon Dioxide. LWT 2016, 85, 423–426. [Google Scholar] [CrossRef]
- Bray, G.A.; Popkin, B.M. Dietary Sugar and Body Weight: Have we Reached a Crisis in the Epidemic of Obesity and Diabetes? Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef]
- Pohjanheimo, T.; Sandell, M. Explaining the Liking for Drinking Yoghurt: The Role of Sensory Quality, Food Choice Motives, Health Concern and Product Information. Int. Dairy. J. 2009, 19, 459–466. [Google Scholar] [CrossRef]
- Shirvani, A.; Mirzaaghaei, M.; Goli, S.A.H. Application of Natural Fining Agents to Clarify Fruit Juices. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4190–4216. [Google Scholar] [CrossRef] [PubMed]
- Pino-Ramos, L.L.; Gomez-Plaza, E.; Olate-Olave, V.R.; Laurie, V.F.; Bautista-Ortín, A.B. Protein Extracts from Amaranth and Quinoa as Novel Fining Agents for Red Wines. Food Chem. 2024, 448, 139055. [Google Scholar] [CrossRef] [PubMed]
- Mirzaaghaei, M.; Goli, S.A.H.; Fathi, M. Application of Sepiolite in Clarification of Pomegranate Juice: Changes on Quality Characteristics during Process. Int. J. Food Sci. Technol. 2016, 51, 1666–1673. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- Vardin, H.; Fenercioǧlu, H. Study on the Development of Pomegranate Juice Processing Technology: Clarification of Pomegranate Juice. Nahr. Food 2003, 47, 300–303. [Google Scholar] [CrossRef]
- Alper, N.; Bahceci, K.S.; Acar, J. Influence of Processing and Pomegranate Juice. J. Food Process. Preserv. 2005, 29, 357–368. [Google Scholar] [CrossRef]
- Turfan, Ö.; Türkyilmaz, M.; Yemİş, O.; Özkan, M. Effects of Clarification and Storage on Anthocyanins and Color of Pomegranate Juice Concentrates. J. Food Qual. 2012, 35, 272–282. [Google Scholar] [CrossRef]
- Bagci, P.O. Effective Clarification of Pomegranate Juice: A comparative Study of Pretreatment Methods and their Influence on Ultrafiltration Flux. J. Food Eng. 2014, 141, 58–64. [Google Scholar] [CrossRef]
- Erkan-Koç, B.; Türkyılmaz, M.; Yemiş, O.; Özkan, M. Effects of Various Protein- and Polysaccharide-Based Clarification Agents on Antioxidative Compounds and Colour of Pomegranate Juice. Food Chem. 2015, 184, 37–45. [Google Scholar] [CrossRef]
- Hu, Q.; Tang, G.; Chu, P.K. Cyclodextrin-Based Host–Guest Supramolecular Nanoparticles for Delivery: From Design to Applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Makris, D.P.; Yannakopoulou, K.; Kalogeropoulos, N.; Michali, I.; Karathanos, V.T. Thermal Stability of Anthocyanin Extract of Hibiscus sabdariffa L. in the Presence of β-Cyclodextrin. J. Agric. Food Chem. 2008, 56, 10303–10310. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Brownmiller, C.; Prior, R.L.; Mauromoustakos, A. Improved Stability of Chokeberry Juice Anthocyanins by Β-Cyclodextrin Addition and Refrigeration. J. Agric. Food Chem. 2013, 61, 693–699. [Google Scholar] [CrossRef]
- López-Nicolá, S.J.M.; Andreu-Sevilla, A.J.; Carbonell-Barrachina, A.A.; García-Carmona, F. Effects of addition of A-Cyclodextrin on the sensory quality, volatile compounds, and color parameters of fresh pear juice. J. Agric. Food Chem. 2009, 57, 9668–9675. [Google Scholar] [CrossRef]
- Navarro, P.; Nicolas, T.S.; Gabaldon, J.A.; Mercader-Ros, M.T.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Pérez-López, A.J. Effects of Cyclodextrin Type on Vitamin C, Antioxidant Activity, and Sensory Attributes of a Mandarin Juice Enriched with Pomegranate and Goji Berries. J. Food Sci. 2011, 76, S319–S324. [Google Scholar] [CrossRef]
- Coupland, J.N.; Hayes, J.E. Physical Approaches to Masking Bitter Taste: Lessons from Food and Pharmaceuticals. Pharm. Res. 2014, 31, 2921–2939. [Google Scholar] [CrossRef]
- Huang, R.; Xie, X.; Xu, C. Utilization of Egg White Powders to Mitigate the Astringency of Aronia Berry Juice and Produce Protein-Proanthocyanidin Aggregates with Enhanced Stability during Digestion. Food Chem. 2024, 464, 141748. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent Antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Handb. Food Anal. Chem. 2005, 2, 19–31. [Google Scholar] [CrossRef]
- Navarro, P.; Melendez-Martinez, A.J.; Heredia, F.; Gabaldon, J.A.; Carbonell-Barrachina, Á.A.; Soler, A.; Perez-Lopez, A.J. Effects of β-Cyclodextrin Addition and Farming Type on Vitamin C, Antioxidant Activity, Carotenoids Profile, and Sensory Analysis in Pasteurised Orange Juices. Int. J. Food Sci. Technol. 2011, 46, 2182–2190. [Google Scholar] [CrossRef]
- Hayoglu, I.; Kola, O.; Kaya, C.; Özer, S.; Turkoglu, H. Chemical and Sensory Properties of Verjuice, a Traditional Turkish Non-Fermented Beverage from Kabarcik and Yediveren Grapes. J. Food Process. Preserv. 2009, 33 (Suppl. S1), 252–263. [Google Scholar] [CrossRef]
- Saint-Cricq de Gaulejac, N.; Glories, Y.; Vivas, N. Recherche des Composes Responsables de L’efft Antiradicalaire dans les Vins. J. Sci. Tech. Tonnellerie 1998, 4, 147–161. [Google Scholar]
- Lehmann, H. Die Aroniabeere und ihre Verarbeitung. Flüss. Obst 1990, 57, 746–752. [Google Scholar]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)-A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Langourieux, S.; Crouzet, J. Interactions between Polysaccharides and Aroma Compounds. Dev. Food Sci. 1995, 37, 1173–1186. [Google Scholar] [CrossRef]
- Rinaldi, A.; Errichiello, F.; Moio, L. Alternative Fining of Sangiovese Wine: Effect on Phenolic Substances and Sensory Characteristics. Aust. J. Grape Wine Res. 2021, 27, 128–137. [Google Scholar] [CrossRef]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour. Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Konno, A.; Misaki, M.; Toda, J.; Wada, T.; Yasumatsu, K. Bitterness Reduction of Naringin and Limonin by β-Cyclodextrin. Agric. Biol. Chem. 1982, 46, 2203–2208. [Google Scholar] [CrossRef]
- Shaw, P.E.; Tatum, J.H.; Wilson, C.W. Improved Flavor of Navel Orange and Grapefruit Juices by Removal of Bitter Components with β-Cyclodextrin Polymer. J. Agric. Food Chem. 1984, 32, 832–836. [Google Scholar] [CrossRef]
- Ćurko, N.; Ganić, K.K.; Tomašević, M.; Gracin, L.; Jourdes, M.; Teissedre, P.L. Effect of Enological Treatments on Phenolic and Sensory Characteristics of Red Wine during Aging: Micro-oxygenation, Sulfur Dioxide, Iron with Copper and Gelatin Fining. Food Chem. 2021, 339, 127848. [Google Scholar] [CrossRef] [PubMed]
- Picouet, P.A.; Hurtado, A.; Jofré, A.; Bañon, S.; Ros, J.M.; Guàrdia, M.D. Effects of Thermal and High-pressure Treatments on the Microbiological, Nutritional and Sensory Quality of a Multi-fruit Smoothie. Food Bioprocess. Technol. 2016, 9, 1219–1232. [Google Scholar] [CrossRef]
- Fernandez, M.; Denoya, G.; Jagus, R.; Vaudagna, S.; Agüero, M. Microbiological, Antioxidant and Physicochemical Stability of a Fruit and Vegetable Smoothie Treated by High Pressure Processing and Stored at Room Temperature. LWT 2019, 105, 206–210. [Google Scholar] [CrossRef]
- Kulcan, A.A.; Öziyci, H.R.; Karhan, M. Quality Stability of Clear Pomegranate Juice Treated with Cyclodextrin. J. Food Sci. Technol. 2019, 56, 4139–4146. [Google Scholar] [CrossRef]
- Błaszczak, W.; Amarowicz, R.; Górecki, A.R. Antioxidant Capacity, Phenolic Composition and Microbial Stability of Aronia Juice Subjected to High Hydrostatic Pressure Processing. Innov. Food Sci. Emerg. Technol. 2016, 39, 141–147. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Végvári, G.; Ladányi, M.; Ficzek, G.; Stéger-Máté, M. Degradation of Anthocyanin Content in Sour Cherry Juice during Heat Treatment. Food Technol. Biotechnol. 2015, 53, 354–360. [Google Scholar] [CrossRef]
- Dubrovi, I.; Herceg, Z.; Režek Jambrak, A.; Badanjak Sabolovic, M.; Verica, D.U. Effect of High Intensity Ultrasound and Pasteurization on Anthocyanin Content in Strawberry Juice. Food Technol. Biotechnol. 2013, 49, 196–204. [Google Scholar]
- Patras, A.; Brunton, N.P.; Tiwari, B.K.; Butler, F. Stability and Degradation Kinetics of Bioactive Compounds and Colour in Strawberry Jam during Storage. Food Bioprocess Technol. 2011, 4, 1245–1252. [Google Scholar] [CrossRef]
- Martynenko, A.; Chen, Y. Degradation Kinetics of Total Anthocyanins and Formation of Polymeric Color in Blueberry Hydrothermodynamic (HTD) Processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Kunsági-Máté, S.; May, B.; Tschiersch, C.; Fetzer, D.; Horváth, I.; Kollár, L.; Nikfardjam, M.P. Transformation of Stacked π-π-Stabilized Malvidin-3-O-glucoside-Catechin Complexes Towards Polymeric Structures Followed by Anisotropy Decay Study. Food Res. Int. 2011, 44, 23–27. [Google Scholar] [CrossRef]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest Date Affects Aronia Juice Polyphenols, Sugars, and Antioxidant Activity, but not Anthocyanin Stability. Food Chem. 2015, 187, 189–196. [Google Scholar] [CrossRef]
- Aree, T.; Jongrungruangchok, S. Crystallographic Evidence for β-Cyclodextrin Inclusion Complexation Facilitating the Improvement of Antioxidant Activity of Tea (+)-Catechin and (−)-Epicatechin. Carbohydr. Polym. 2015, 140, 362–373. [Google Scholar] [CrossRef]
- Dorris, M.R.; Voss, D.M.; Bollom, M.A.; Krawiec-Thayer, M.P.; Bolling, B.W. Browning Index of Anthocyanin-Rich Fruit Juice Depends on pH and Anthocyanin Loss More Than the Gain of Soluble Polymeric Pigments. J. Food Sci. 2018, 83, 911–921. [Google Scholar] [CrossRef]
- Andreu-Sevilla, A.J.; Lopez-Nicolas, J.M.; Carbonell-Barrachina, A.; Garcıa-Carmona, F. Comparative Effect of the Addition of a-, b-, or c-cyclodextrin on Main Sensory and Physico-Chemical Parameters. J. Food Sci. 2011, 76, 347–353. [Google Scholar] [CrossRef]
- Jafari, S.; Shiekh, K.A.; Mishra, D.K.; Kijpatanasilp, I.; Assatarakul, K. Combined Effects of Clarifying Agents Improve Physicochemical, Microbial and Sensorial Qualities of Fresh Indian Gooseberry (Phyllanthus emblica L.) Juice during Refrigerated Storage. Foods 2024, 13, 290. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, e04628. [Google Scholar] [CrossRef]



| First Set of Samples | Second Set of Samples | ||||
|---|---|---|---|---|---|
| UPA/PAS | β-CD (%) | Gelatin (mL/L) | Organoleptic Evaluation (5-Member Trained Panel) | Physico-Chemical Analysis | Consumer Preference (28-Member Untrained Panel) |
| UPA | 0 (control) | 0 control | - | √ | - |
| PAS | 0 (control) | 0 control | √ | √ | √ |
| PAS | 0.5 | 0.1 | √ | √ | - |
| PAS | 1.0 | 0.2 | √ | √ | - |
| PAS | 1.5 | 0.3 | √ | √ | - |
| PAS | 2.0 | 0.4 | √ | √ | √ |
| Juice | Total Phenolic Content (mgGAE/L) | DPPH Scavenging Activity (mgTE/L) | Total Flavonoids (mg CE/L) | Total Monomeric Anthocyanins (mg cyn-3-glu/L) | Total Color Due Topolymeric Pigments (%) |
|---|---|---|---|---|---|
| UPA | 1613.1 ± 37.6 A | 2255.6 ± 64.8 A | 1699.4 ± 23.8 A | 340.7 ± 7.96 A | 61.6 ± 0.6 A |
| PAS | 1591.9 ± 36.2 A | 2003.1 ± 127.1 B | 1716.9 ± 13.3 A | 281.4 ± 4.42 B | 64.0 ± 0.3 B |
| Lightness (L*) | Redness (a*) | Yellowness (b*) | Soluble Solids Content (%) | pH | |
| UPA | 17.08 ± 0.23 A | 2.65 ± 0.11 A | 1.76 ± 0.03 A | 20.2 ± 0.20 A | 4.00 ± 0.01 A |
| PAS | 16.70 ± 0.26 A | 2.69 ± 0.19 A | 1.67 ± 0.10 A | 20.5 ± 0.12 A | 4.00 ± 0.01 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkoutzina, K.; Mourtzinos, I.; Gerasopoulos, D. Addition of β-Cyclodextrin or Gelatin Ιmproves Organoleptic and Physicochemical Attributes of Aronia Juice. Beverages 2025, 11, 115. https://doi.org/10.3390/beverages11040115
Gkoutzina K, Mourtzinos I, Gerasopoulos D. Addition of β-Cyclodextrin or Gelatin Ιmproves Organoleptic and Physicochemical Attributes of Aronia Juice. Beverages. 2025; 11(4):115. https://doi.org/10.3390/beverages11040115
Chicago/Turabian StyleGkoutzina, Kalliopi, Ioannis Mourtzinos, and Dimitrios Gerasopoulos. 2025. "Addition of β-Cyclodextrin or Gelatin Ιmproves Organoleptic and Physicochemical Attributes of Aronia Juice" Beverages 11, no. 4: 115. https://doi.org/10.3390/beverages11040115
APA StyleGkoutzina, K., Mourtzinos, I., & Gerasopoulos, D. (2025). Addition of β-Cyclodextrin or Gelatin Ιmproves Organoleptic and Physicochemical Attributes of Aronia Juice. Beverages, 11(4), 115. https://doi.org/10.3390/beverages11040115







