Non-Conventional Yeasts for Beer Production—Primary Screening of Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Wort Preparation
2.3. Fermentation
2.4. Analytical Procedures
2.4.1. Basic Wort and Beer Parameters
2.4.2. Secondary Metabolites Determination
2.5. Mathematical and Statistical Analysis
2.5.1. Fermentation Dynamic Calculations
2.5.2. Determination of Kinetic Parameters of the Fermentation Processes
- Specific Growth Rate (μ)
- Biomass Yield on Substrate (YX/S)
- Product Yield on Substrate (YP/S) and Biomass (YP/X)
- Specific Substrate Consumption Rate (qs) and Specific Product Formation Rate (qp)
- Volumetric Productivity (Qp)
2.5.3. Principal Component Analysis (PCA)
2.5.4. Correlation Analysis
3. Results and Discussion
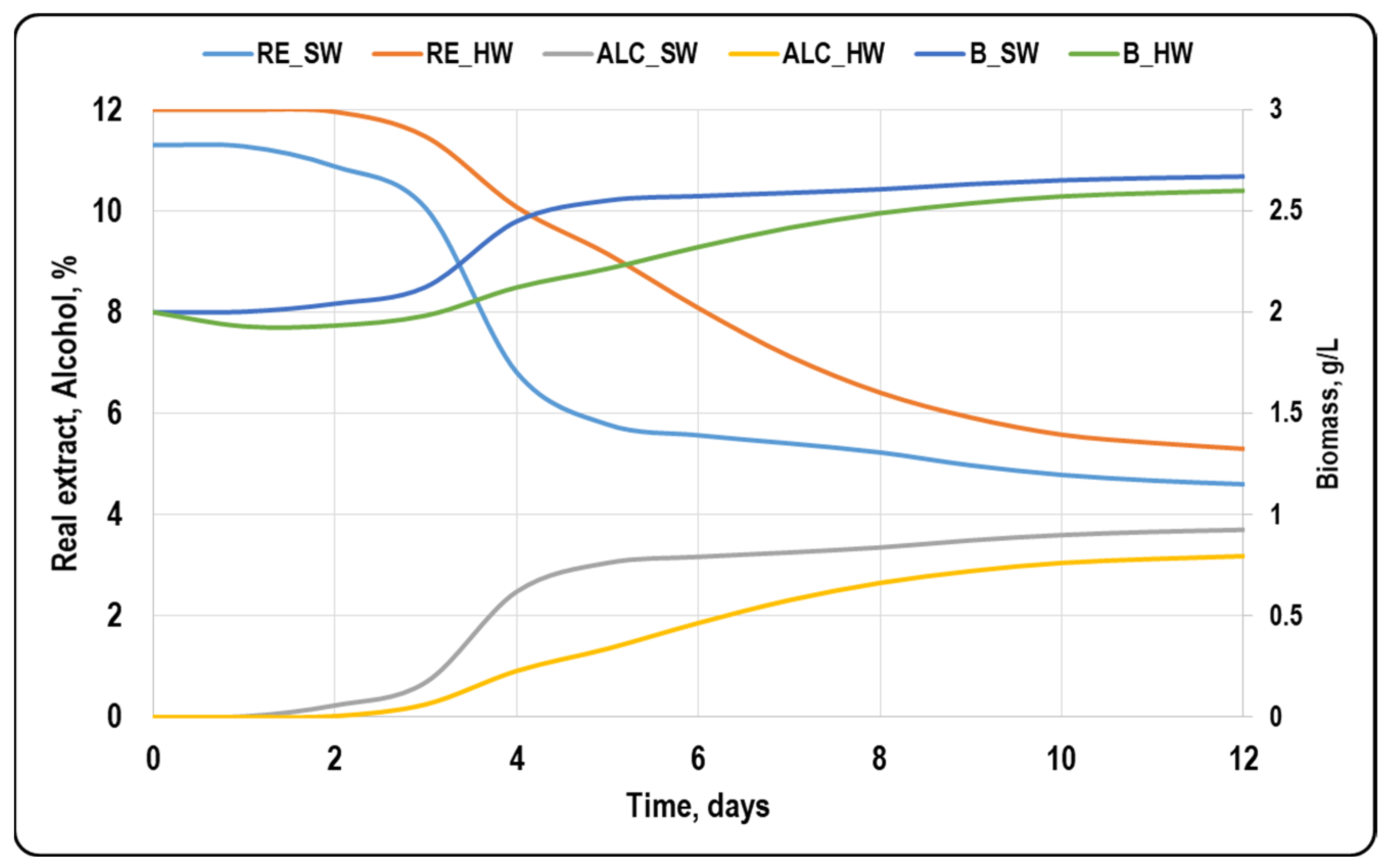
3.1. Basic Beer Parameters
3.2. Secondary Metabolites
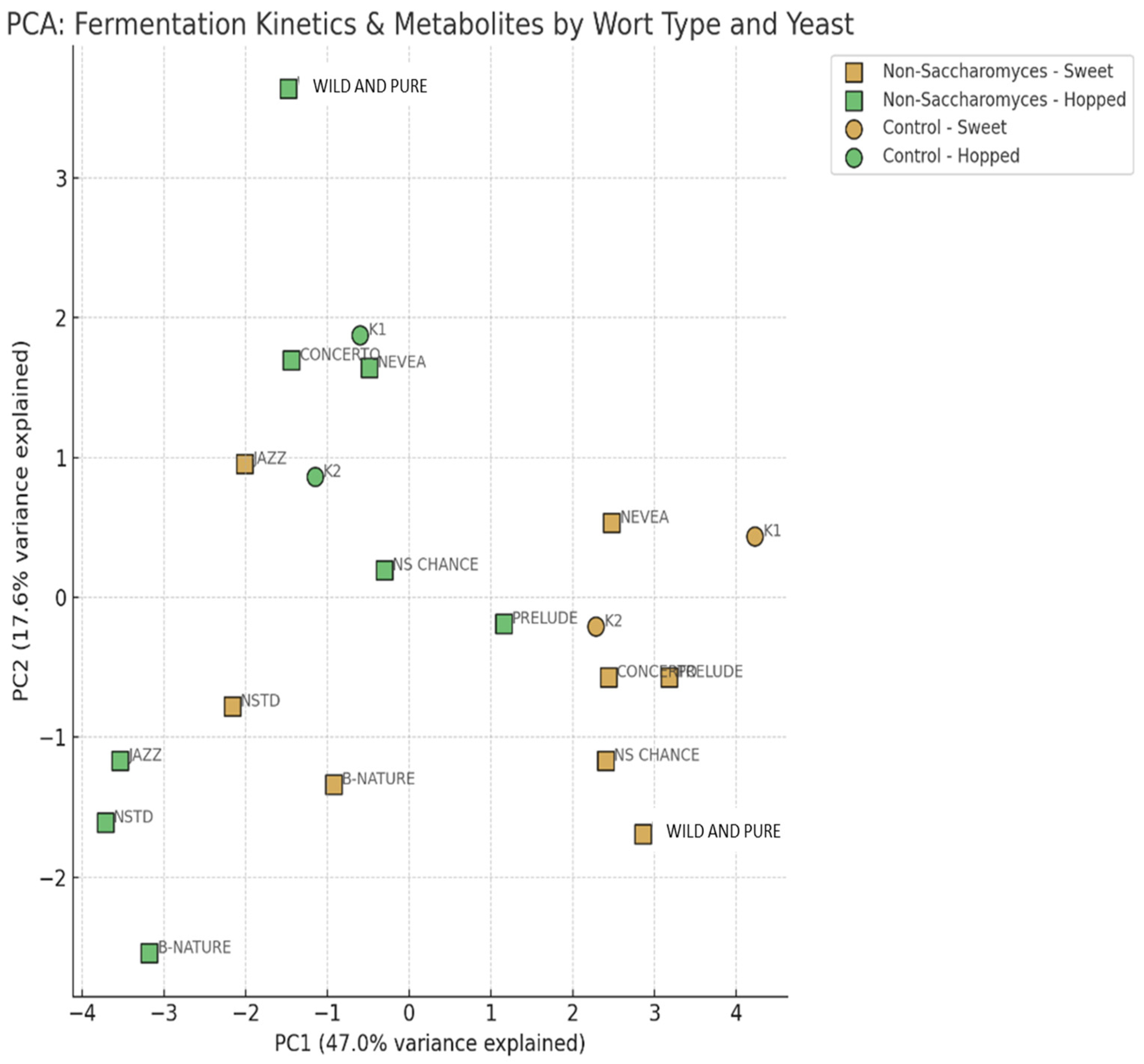
3.3. Comparative Assessment of the Fermentation Kinetics: Statistical Processing and PCA
- Some of the non-Sacharomyces yeasts have profiles similar to classic brewing strains.
- Others could be used as alternatives with distinctive aromatic capacity.
- The type of wort (sweet or hopped) had a significant impact on the positioning in the component space, which should be taken into account in technological selection.
3.4. Influence of Hopping on the Growth and Fermentation Activity of Non-Conventional Yeasts
3.5. Sensory Evaluation and Application of the Studied Strains for the Production of Different Beer Styles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roselli, G.; Kerruish, D.; Crow, M.; Smart, K.; Powell, C. The two faces of microorganisms in traditional brewing and the implications for no-and low-alcohol beers. Front. Microbiol. 2024, 15, 1346724. [Google Scholar] [CrossRef]
- Lodolo, E.; Kock, J.; Axcell, B.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef]
- Satora, P.; Pater, A. The Influence of Different Non-Conventional Yeasts on the Odour-Active Compounds of Produced Beers. Appl. Sci. 2023, 13, 2872. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Unravelling the potential of non-conventional yeasts and recycled brewers spent grains (BSG) for non-alcoholic and low alcohol beer (NABLAB). Lwt 2023, 190, 115528. [Google Scholar] [CrossRef]
- Gschaedler, A. Contribution of non-conventional yeasts in alcoholic beverages. Curr. Opin. Food Sci. 2017, 13, 73–77. [Google Scholar] [CrossRef]
- Capece, A.; De Fusco, D.; Pietrafesa, R.; Siesto, G.; Romano, P. Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers. Appl. Sci. 2021, 11, 801. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and non-conventional yeasts in beer production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- Budroni, M.; Zara, G.; Ciani, M.; Comitini, F. Saccharomyces and non-Saccharomyces starter yeasts. BREW Technol. 2017, 1, 81–100. [Google Scholar]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.; Wagner, R.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Callejo, M.J.; Tesfaye, W.; González, M.C.; Morata, A. Craft beers: Current situation and future trends. In New Advances on Fermentation Processes; Martínez-Espinosa, R.M., Ed.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef][Green Version]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae strains isolated from non-brewing environments in beer production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of yeasts in the brewing process: Tradition and innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Methner, Y.; Hutzler, M.; Matoulková, D.; Jacob, F.; Michel, M. Screening for the brewing ability of different non-Saccharomyces yeasts. Fermentation 2019, 5, 101. [Google Scholar] [CrossRef]
- Estela-Escalante, W.; Rosales-Mendoza, S.; Moscosa-Santillán, M.; González-Ramírez, J. Evaluation of the fermentative potential of Candida zemplinina yeasts for craft beer fermentation. J. Inst. Brew. 2016, 122, 530–535. [Google Scholar] [CrossRef]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovšek, U.; Walther, A.; Wendland, J. Adding flavor to beverages with non-conventional yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- Callejo, M.; González, C.; Morata, A. Use of non-Saccharomyces yeasts in bottle fermentation of aged beers. In Brewing Technology; Kanauchi, M., Ed.; IntechOpen: London, UK, 2017; pp. 101–119. [Google Scholar] [CrossRef]
- Estela-Escalante, W. Perspectives and uses of non-Saccharomyces yeasts in fermented beverages. In Frontiers and New Trends in the Science of Fermented Food and Beverages; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Nasuti, C.; Ruffini, J.; Sola, L.; Di Bacco, M.; Raimondi, S.; Candeliere, F.; Solieri, L. Sour Beer as Bioreservoir of Novel Craft Ale Yeast Cultures. Microorganisms 2023, 11, 2138. [Google Scholar] [CrossRef] [PubMed]
- Tijerino, M.; Martín-González, M.; Domingo, J.; Huang, J. Life cycle assessment of craft beer brewing at different scales on a unit operation basis. Sustainability 2023, 15, 11416. [Google Scholar] [CrossRef]
- Lentz, M.; Putzke, T.; Hessler, R.; Luman, E. Genetic and physiological characterization of yeast isolated from ripe fruit and analysis of fermentation and brewing potential. J. Inst. Brew. 2014, 120, 559–564. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Guerrini, S.; Mangani, S.; Barbato, D.; Vallesi, G.; Granchi, L. Exploitation of selected sourdough Saccharomyces cerevisiae strains for the production of a craft raspberry fruit beer. Foods 2023, 12, 3354. [Google Scholar] [CrossRef]
- Einfalt, D. Barley-sorghum craft beer production with Saccharomyces cerevisiae, Torulaspora delbrueckii and Metschnikowia pulcherrima yeast strains. EurFood Res. Technol. 2021, 247, 385–393. [Google Scholar] [CrossRef]
- Toh, D.; Chua, J.; Lu, Y.; Liu, S. Evaluation of the potential of commercial non-Saccharomyces yeast strains of Torulaspora delbrueckii and Lachancea thermotolerans in beer fermentation. Int. J. Food Sci. Technol. 2020, 55, 2049–2059. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Testa, B.; Letizia, F.; Kycyk, O.; Ruci, M.; Kongoli, R.; Lamce, F.; Lioha, I.; Sulaj, K.; et al. Application of Saccharomyces cerevisiae 31 and Metschnikowia pulcherrima 62, isolated from Albanian vineyards, as new starters in the production of ale style beers. In Proceedings of the II-International Biological and Life Sciences Congress Biolic, Antalya, Turkey, 30 October–1 November 2024; p. 410. [Google Scholar]
- Ellis, D.; Kerr, E.; Schenk, G.; Schulz, B. Metabolomics of Non-Saccharomyces Yeasts in Fermented Beverages. Beverages 2022, 8, 41. [Google Scholar] [CrossRef]
- Postigo, V.; Esteban, S.; Arroyo, T. Lachancea thermotolerans, an innovative alternative for sour beer production. Beverages 2023, 9, 20. [Google Scholar] [CrossRef]
- Fu, X.; Guo, L.; Li, Y.; Chen, X.; Song, Y.; Li, S. Transcriptional Analysis of Mixed-Culture Fermentation of Lachancea Thermotolerans and Saccharomyces Cerevisiae for Natural Fruity Sour Beer. Fermentation 2024, 10, 180. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamce, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zapryanova, P.; Gaytanska, Y.; Shopska, V.; Kostov, G. Non-conventional yeasts in beer production—A review. BIO Web Conf. 2025, 170, 01015. [Google Scholar] [CrossRef]
- Gerhards, S.; Talaverano, M.; Andrés, A.; Sánchez, C.; Lozano, J.; García-Latorre, C.; Rodrigo, S. Different Dry Hopping and Fermentation Methods: Influence on Beer Nutritional Quality. J. Sci. Food Agric. 2020, 101, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Sanekata, A.; Tanigawa, A.; Takoi, K.; Nakayama, Y.; Tsuchiya, Y. Identification and Characterization of Geranic Acid as a Unique Flavor Compound of Hops (Humulus lupulus L.) Variety Sorachi Ace. J. Agric. Food Chem. 2018, 66, 12285–12295. [Google Scholar] [CrossRef]
- EBC Analytica. Available online: https://brewup.eu/ebc-analytica/beer (accessed on 21 April 2025).
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2007. [Google Scholar]
- Marinov, M. Practice for Analysis and Control of Alcohol Beverages and Ethanol; Academic Publisher of UFT: Plovdiv, Bulgaria, 2010; pp. 72–79. [Google Scholar]
- Parcunev, I.; Naydenova, V.; Kostov, G.; Yanakiev, Y.; Popova, Z.; Kaneva, M.; Ignatov, I. Modeling Of Alcohol Fermentation In Brewing—Some Practical Approaches. In Proceedings of the 26th European Conference on Modelling and Simulation, Shaping Reality through Simulation, Koblenz, Germany, 29 May–1 June 2012; pp. 434–440. [Google Scholar]
- Pyrovolou, K.; Tataridis, P.; Revelou, P.-K.; Strati, I.F.; Konteles, S.J.; Tarantilis, P.A.; Houhoula, D.; Batrinou, A. Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile. Appl. Sci. 2024, 14, 1199. [Google Scholar] [CrossRef]
- Shopska, V.; Dzhivoderova-Zarcheva, M.; Kostov, G. Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core. Beverages 2024, 10, 87. [Google Scholar] [CrossRef]
- Nasuti, C.; Solieri, L. Yeast Bioflavoring in Beer: Complexity Decoded and Built up Again. Fermentation 2024, 10, 183. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Mikulski, D.; Kłosowski, G.; Głowacki, A. Application of white grape pomace in the brewing technology and its impact on the concentration of esters and alcohols, physicochemical parameteres and antioxidative properties of the beer. Food Chem. 2022, 367, 130646. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Keukeleire, D. Fundamentals of Beer and Hop Chemistry. Quím. Nova 2000, 23, 108–112. [Google Scholar] [CrossRef]
- Gomes, F.D.O.; Guimarães, B.P.; Ceola, D.; Ghesti, G.F. Advances in dry hopping for industrial brewing: A review. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Sanekata, A.; Tanigawa, A.; Takoi, K.; Nakayama, Y.; Tsuchiya, Y. Interesting Behavior of Geranic Acid during the Beer Brewing Process: Why Could Geranic Acid Remain at a Higher Level Only in the Beer Using Sorachi Ace Hops? J. Agric. Food Chem. 2023, 71, 18489–18498. [Google Scholar] [CrossRef]
- McCabe, A.; Keyes, J.; Hemetsberger, H.; Kurr, C.; Albright, B.; Ward, M.; Cole, C. Aroma Profile Development in Beer Fermented with Azacca, Idaho-7, and Sultana Hops. Molecules 2023, 28, 5802. [Google Scholar] [CrossRef]
- Klimczak, K.; Cioch-Skoneczny, M.; Duda-Chodak, A. Effects of Dry-Hopping on Beer Chemistry and Sensory Properties—A Review. Molecules 2023, 28, 6648. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, K.; Shellhammer, T. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66, 9121–9126. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; O’Sullivan, T.; van Sinderen, D. Enhancing the Microbiological Stability of Malt and Beer—A Review. J. Inst. Brew. 2005, 111, 355–371. [Google Scholar] [CrossRef]
- Haakensen, M.; Schubert, A.; Ziola, B. Broth and Agar Hop-Gradient Plates Used to Evaluate the Beer-Spoilage Potential of Lactobacillus and Pediococcus Isolates. Int. J. Food Microbiol. 2009, 130, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, K.; Cioch-Skoneczny, M.; Poreda, A. Evaluation of Non-Saccharomyces Yeast for Low-Alcohol Beer Production. Appl. Sci. 2024, 14, 6755. [Google Scholar] [CrossRef]
- Gamero, A.; Dijkstra, A.; Smit, B.; de Jong, C. Aromatic Potential of Diverse Non-Conventional Yeast Species for Winemaking and Brewing. Fermentation 2020, 6, 50. [Google Scholar] [CrossRef]
| Yeast Strains | Manufacturer |
|---|---|
| Saccharomyces pastorianus Saflager W34/70 | Fermentis by Lesaffre, Lambersart, France |
| Saccharomyces cerevisiae Safale US-05 | Fermentis by Lesaffre, Lambersart, France |
| Torulaspora delbrueckii Viniferm NS-TD | Agrovin, Alcázar de San Juan, Spain |
| Torulaspora delbrueckii Viniflora PRELUDE | Novonesis, Bagsværd, Denmark |
| Metschnikowia pulcherrima EXELLENCE B-Nature BIOPROTECTION | Lamothe-Abiet, Bordeaux, France |
| Lachancea thermotolerans Viniferm Ns-CHANCE | Agrovin, Alcázar de San Juan, Spain |
| Lachancea thermotolerans JAZZ | Lamothe-Abiet, Bordeaux, France |
| Lachancea thermotolerans NEVEA | SAS Sofralab, Magenta, France |
| Lachancea thermotolerans/formerly Klyuveromyces thermotolerans/ Viniflora CONCERTO | Novonesis, Bagsværd, Denmark |
| Saccharomyces cerevisiae/Torulaspora delbrueckii Oenoferm Wild and Pure | ERBSLÖH Gaisenheim GmbH, Gaisenheim, Germany |
| Wort Type | Initial Extract, °P | pH |
|---|---|---|
| Sweet wort | 11.3 ± 0.1 | 6.22 ± 0.02 |
| Hopped wort | 12.0 ± 0.1 | 6.06 ± 0.01 |
| Yeast Strain | Wort Type | Fermentation Time, Days | Real Extract, °P | Alcohol, % w/w | RDF, % | pH |
|---|---|---|---|---|---|---|
| S. pastorianus Saflager W34/70 | Sweet wort | 5 | 4.7 ± 0.10 | 3.70 ± 0.15 | 59.80 | 4.75 ± 0.15 |
| Hopped wort | 9 | 4.8 ± 0.15 | 3.88 ± 0.14 | 61.72 | 4.93 ± 0.07 | |
| S. cerevisiae Safale US-05 | Sweet wort | 7 | 4.9 ± 0.16 | 3.47 ± 0.11 | 57.37 | 3.34 ± 0.03 |
| Hopped wort | 12 | 3.8 ± 0.14 | 4.42 ± 0.13 | 68.83 | 4.46 ± 0.08 | |
| T. delbrueckii NS-TD | Sweet wort | 8 | 5.3 ± 0.12 | 3.12 ± 0.13 | 54.06 | 3.50 ± 0.09 |
| Hopped wort | 9 | 4.6 ± 0.16 | 3.88 ± 0.14 | 63.18 | 4.61 ± 0.11 | |
| T. delbrueckii PRELUDE | Sweet wort | 5 | 5.2 ± 0.16 | 3.06 ± 0.16 | 54.93 | 4.70 ± 0.06 |
| Hopped wort | 6 | 5.7 ± 0.11 | 3.18 ± 0.11 | 53.21 | 4.86 ± 0.07 | |
| M. pulcherrima B-NATURE | Sweet wort | 12 | 4.6 ± 0.14 | 3.70 ± 0.12 | 60.69 | 3.70 ± 0.09 |
| Hopped wort | 12 | 5.3 ± 0.15 | 3.18 ± 0.13 | 56.73 | 4.50 ± 0.11 | |
| L. thermotolerans Ns-CHANCE | Sweet wort | 5 | 6.0 ± 0.18 | 2.84 ± 0.15 | 48.12 | 3.53 ± 0.12 |
| Hopped wort | 16 | 3.3 ± 0.10 | 3.30 ± 0.16 | 73.45 | 3.63 ± 0.08 | |
| L. thermotolerans JAZZ | Sweet wort | 8 | 6.4 ± 0.15 | 3.06 ± 0.16 | 43.95 | 4.04 ± 0.06 |
| Hopped wort | 11 | 5.8 ± 0.11 | 3.30 ± 0.11 | 51.77 | 4.25 ± 0.06 | |
| L. thermotolerans NEVEA | Sweet wort | 5 | 6.0 ± 0.15 | 3.00 ± 0.13 | 48.12 | 3.64 ± 0.06 |
| Hopped wort | 9 | 5.5 ± 0.18 | 3.30 ± 0.10 | 47.02 | 3.93 ± 0.04 | |
| L. thermotolerans CONCERTO | Sweet wort | 5 | 5.6 ± 0.17 | 3.06 ± 0.10 | 51.41 | 4.02 ± 0.06 |
| Hopped wort | 9 | 5.9 ± 0.16 | 3.30 ± 0.16 | 52.59 | 4.50 ± 0.05 | |
| S. cerevisiae/T. delbrueckii WILD and PURE | Sweet wort | 5 | 5.5 ± 0.14 | 2.95 ± 0.08 | 53.17 | 3.88 ± 0.10 |
| Hopped wort | 8 | 6.4 ± 0.12 | 3.06 ± 0.10 | 47.84 | 4.67 ± 0.11 |
| Esters | Aldehydes | Higher Alcohols | |||||
|---|---|---|---|---|---|---|---|
| SW | HW | SW | HW | SW | HW | ||
| S. pastorianus | Saflager W34/70 | 88.15 ± 5.56 | 136.5 ± 5.47 | 8.07 ± 0.33 | 6.05 ± 0.85 | 10.26 ± 1.02 | 14.62 ± 1.43 |
| S. cerevisiae | Safale US-05 | 88.15 ± 4.87 | 72.03 ± 4.43 | 12.11 ± 0.40 | 8.07 ± 1.10 | 9.7 ± 1.10 | 9.95 ± 1.05 |
| M. pulcherrima | B-NATURE | 72.03 ± 3.88 | 72.03 ± 4.56 | 32.29 ± 1.22 | 8.07 ± 1.10 | 3.53 ± 0.42 | 2.39 ± 0.32 |
| T. delbrueckii | NSTD | 184.85 ± 8.63 | 168.73 ± 3.26 | 10.09 ± 1.10 | 76.69 ± 4.43 | 11.09 ± 1.23 | 8.19 ± 1.10 |
| T. delbrueckii | PRELUDE | 168.73 ± 5.21 | 72.03 ± 4.56 | 10.09 ± 1.60 | 10.09 ± 1.11 | 4.91 ± 0.56 | 4.03 ± 0.33 |
| L. thermotolerans | CONCERTO | 104.27 ± 5.44 | 120.38 ± 4.76 | 26.24 ± 2.26 | 12.11 ± 1.83 | 11.59 ± 1.59 | 12.98 ± 0.88 |
| L. thermotolerans | NEVEA | 152.61 ± 5.86 | 200.96 ± 7.86 | 26.24 ± 2.72 | 12.11 ± 1.85 | 11.34 ± 1.34 | 14.49 ± 1.05 |
| L. thermotolerans | JAZZ | 152.62 ± 6.33 | 72.03 ± 4.56 | 10.09 ± 1.10 | 16.15 ± 1.66 | 4.92 ± 0.66 | 1.76 ± 0.23 |
| L. thermotolerans | NS CHANCE | 120.38 ± 7.75 | 72.03 ± 3.45 | 10.09 ± 1.10 | 22.2 ± 2.26 | 3.28 ± 0.43 | 8.06 ± 1.10 |
| S. cerevisiae/T. delbrueckii | WILD and PURE | 72.03 ± 4.59 | 184.84 ± 6.34 | 26.23 ± 2.10 | 8.07 ± 0.99 | 8.82 ± 1.00 | 24.32 ± 1.33 |
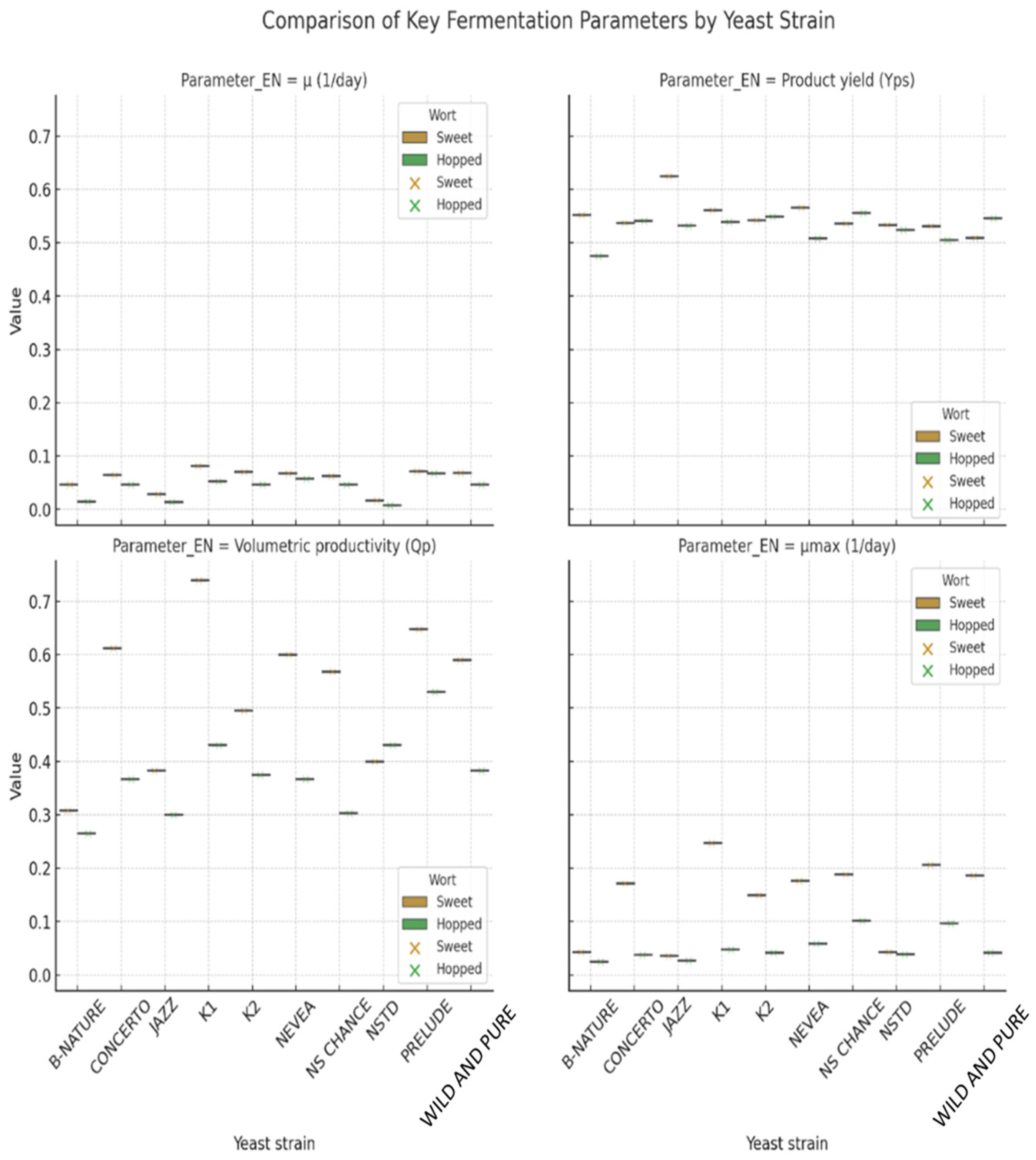
| μmax (Day−1) | Yx/s | Yp/s | Yp/x | qs | qp | Qp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SW | HW | SW | HW | SW | HW | SW | HW | SW | HW | SW | HW | SW | HW | ||
| S. pastorianus | Saflager W34/70 | 0.081 | 0.053 | 0.100 | 0.090 | 0.561 | 0.539 | 5.606 | 5.969 | 0.809 | 0.583 | 0.453 | 0.314 | 0.740 | 0.431 |
| S. cerevisiae | Safale US-05 | 0.070 | 0.047 | 0.100 | 0.092 | 0.542 | 0.549 | 5.420 | 6.000 | 0.702 | 0.508 | 0.381 | 0.279 | 0.496 | 0.375 |
| M. pulcherrima | B-NATURE | 0.046 | 0.014 | 0.100 | 0.090 | 0.552 | 0.475 | 5.520 | 5.300 | 0.464 | 0.159 | 0.256 | 0.075 | 0.308 | 0.265 |
| T. delbrueckii | NSTD | 0.017 | 0.007 | 0.100 | 0.091 | 0.533 | 0.524 | 5.330 | 5.791 | 0.168 | 0.082 | 0.090 | 0.043 | 0.400 | 0.431 |
| T. delbrueckii | PRELUDE | 0.071 | 0.067 | 0.100 | 0.089 | 0.531 | 0.505 | 5.310 | 5.680 | 0.713 | 0.755 | 0.379 | 0.381 | 0.648 | 0.530 |
| L. thermotolerans | CONCERTO | 0.064 | 0.047 | 0.100 | 0.089 | 0.537 | 0.541 | 5.370 | 6.110 | 0.639 | 0.528 | 0.343 | 0.285 | 0.612 | 0.367 |
| L. thermotolerans | NEVEA | 0.067 | 0.057 | 0.100 | 0.089 | 0.566 | 0.508 | 5.660 | 5.690 | 0.668 | 0.641 | 0.378 | 0.325 | 0.600 | 0.367 |
| L. thermotolerans | JAZZ | 0.028 | 0.014 | 0.100 | 0.089 | 0.625 | 0.532 | 6.245 | 6.000 | 0.281 | 0.153 | 0.175 | 0.082 | 0.383 | 0.300 |
| L. thermotolerans | NS-CHANCE | 0.062 | 0.046 | 0.100 | 0.092 | 0.536 | 0.556 | 5.359 | 6.050 | 0.620 | 0.500 | 0.332 | 0.278 | 0.568 | 0.303 |
| S. cerevisiae/T. delbrueckii | WILD and PURE | 0.069 | 0.046 | 0.100 | 0.088 | 0.509 | 0.546 | 5.086 | 6.245 | 0.685 | 0.526 | 0.349 | 0.287 | 0.590 | 0.383 |
| μmax (day−1) | Yp/s | qp | Qp | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SW | HW | SW | HW | SW | HW | SW | HW | ||
| M. pulcherrima | B-NATURE | 0.574 | 0.271 | 0.985 | 0.881 | 0.565 | 0.240 | 0.416 | 0.615 |
| T. delbrueckii | NSTD | 0.208 | 0.141 | 0.950 | 0.973 | 0.198 | 0.137 | 0.541 | 1.000 |
| T. delbrueckii | PRELUDE | 0.882 | 1.274 | 0.947 | 0.937 | 0.836 | 1.212 | 0.876 | 1.229 |
| L. thermotolerans | CONCERTO | 0.790 | 0.887 | 0.958 | 1.004 | 0.757 | 0.907 | 0.827 | 0.851 |
| L.thermotolerans | NEVEA | 0.826 | 1.086 | 1.010 | 0.942 | 0.834 | 1.035 | 0.811 | 0.851 |
| L.thermotolerans | JAZZ | 0.347 | 0.258 | 1.114 | 0.988 | 0.387 | 0.259 | 0.517 | 0.696 |
| L. thermotolerans | NS CHANCE | 0.767 | 0.873 | 0.956 | 1.032 | 0.733 | 0.885 | 0.768 | 0.702 |
| S. cerevisiae/T. delbrueckii | WILD and PURE | 0.848 | 0.873 | 0.907 | 1.014 | 0.769 | 0.914 | 0.797 | 0.887 |
| μmax (day−1) | Yp/s | qp | Qp | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SW | HW | SW | HW | SW | HW | SW | HW | ||
| M. pulcherrima | B-NATURE | 0.661 | 0.306 | 1.018 | 0.866 | 0.672 | 0.270 | 0.621 | 0.707 |
| T. delbrueckii | NSTD | 0.239 | 0.160 | 0.983 | 0.955 | 0.235 | 0.154 | 0.806 | 1.150 |
| T. delbrueckii | PRELUDE | 1.016 | 1.444 | 0.980 | 0.920 | 0.995 | 1.367 | 1.306 | 1.413 |
| L. thermotolerans | CONCERTO | 0.910 | 1.005 | 0.991 | 0.986 | 0.900 | 1.022 | 1.234 | 0.979 |
| L.thermotolerans | NEVEA | 0.951 | 1.231 | 1.044 | 0.925 | 0.992 | 1.167 | 1.210 | 0.978 |
| L.thermotolerans | JAZZ | 0.400 | 0.292 | 1.152 | 0.970 | 0.460 | 0.292 | 0.771 | 0.800 |
| L. thermotolerans | NS CHANCE | 0.884 | 0.989 | 0.989 | 1.014 | 0.872 | 0.997 | 1.145 | 0.807 |
| S. cerevisiae/T. delbrueckii | WILD and PURE | 0.976 | 0.990 | 0.938 | 0.996 | 0.915 | 1.030 | 1.190 | 1.020 |
| Metabolites | μ | Qp | Yp/s |
|---|---|---|---|
| Esters | 0.55 | 0.62 | 0.48 |
| Aldehydes | −0.15 | −0.33 | −0.21 |
| Higher Alcohols | 0.44 | 0.38 | 0.35 |
| Yeast Strain | Key Traits | Suggested Beer Styles |
|---|---|---|
| Torulaspora delbrueckii (Prelude, NS-TD) | Fruity esters Good hop tolerance Moderate higher alcohols | Blonde Ale Specialty Ales Dry-Hopped Pale Ales |
| Lachancea thermotolerans (Nevea, Concerto, NS-Chance) | Low pH Moderate alcohol | Sour Beers Fruited Gose |
| Metschnikowia pulcherrima (B-Nature) | Slow fermentation Low alcohol | Low-Alcohol Beers Specialty Herbal Beers |
| S. cerevisiae/T. delbrueckii (Wild and Pure) | Aromatic complexity Moderate acidity High higher alcohols | New England IPA Mixed-Fermentation Beers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapryanova, P.; Gaytanska, Y.; Shopska, V.; Denkova-Kostova, R.; Kostov, G. Non-Conventional Yeasts for Beer Production—Primary Screening of Strains. Beverages 2025, 11, 114. https://doi.org/10.3390/beverages11040114
Zapryanova P, Gaytanska Y, Shopska V, Denkova-Kostova R, Kostov G. Non-Conventional Yeasts for Beer Production—Primary Screening of Strains. Beverages. 2025; 11(4):114. https://doi.org/10.3390/beverages11040114
Chicago/Turabian StyleZapryanova, Polina, Yordanka Gaytanska, Vesela Shopska, Rositsa Denkova-Kostova, and Georgi Kostov. 2025. "Non-Conventional Yeasts for Beer Production—Primary Screening of Strains" Beverages 11, no. 4: 114. https://doi.org/10.3390/beverages11040114
APA StyleZapryanova, P., Gaytanska, Y., Shopska, V., Denkova-Kostova, R., & Kostov, G. (2025). Non-Conventional Yeasts for Beer Production—Primary Screening of Strains. Beverages, 11(4), 114. https://doi.org/10.3390/beverages11040114






