Effect of Non-Thermal Treatments of Clear Apple Juice on Exogenous Pectinases
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Sourcing and Sample Preparation
2.2. Processing Parameters
2.2.1. Thermal Pasteurization
2.2.2. Pulsed Electric Field (PEF)
2.2.3. High-Pressure Processing (HPP)
2.3. Analysis of Residual Enzyme Activity
2.3.1. Polygalacturonase (PGX) Assay
2.3.2. Pectin Transeliminase (PTF) Assay
2.3.3. Pectin Esterase Assay (PE)
2.4. Data Analysis, Plotting, and ANOVA Statistics
3. Results and Discussion
3.1. Inactivation of Pectinases
3.1.1. PEF Inactivation
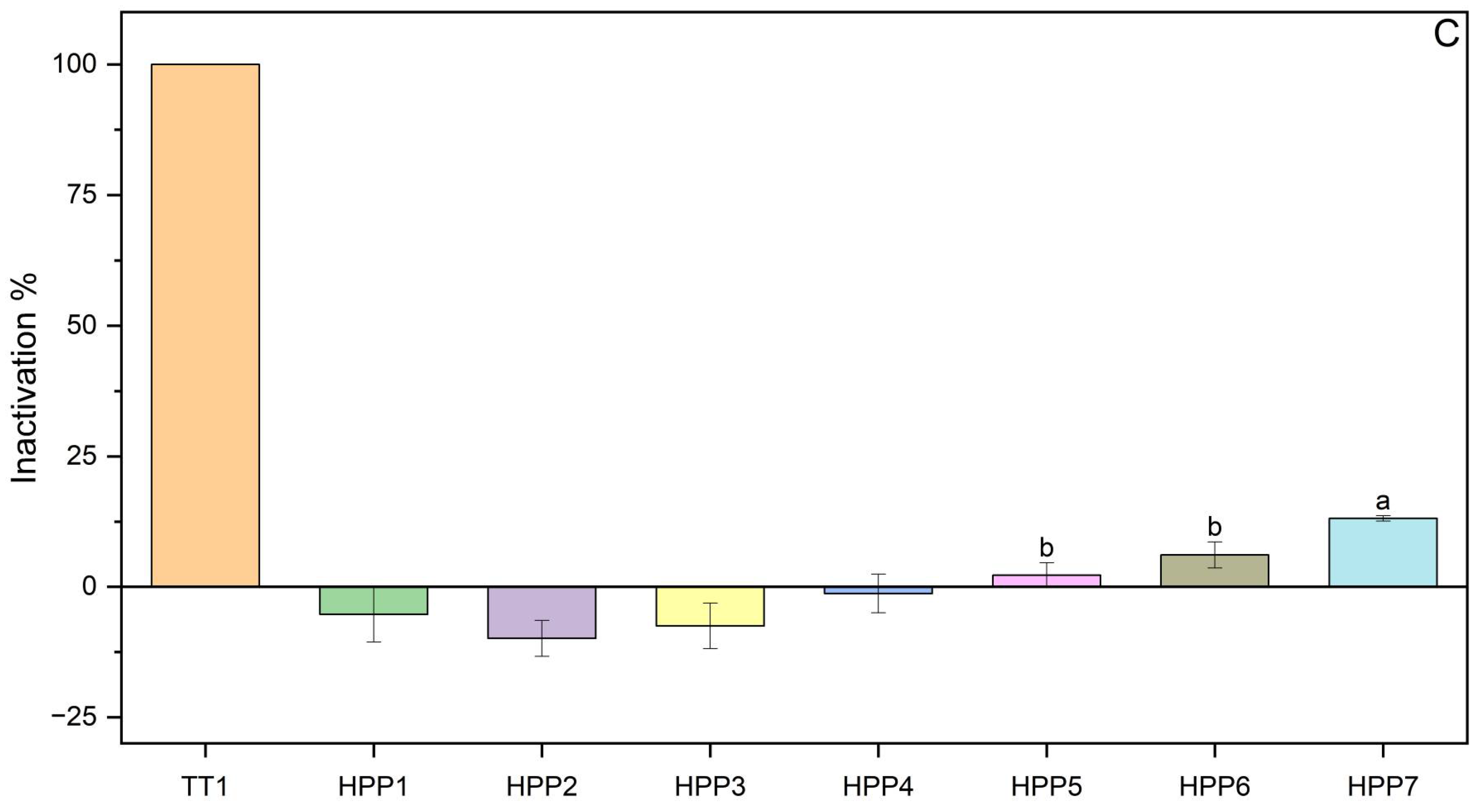
3.1.2. HPP Inactivation
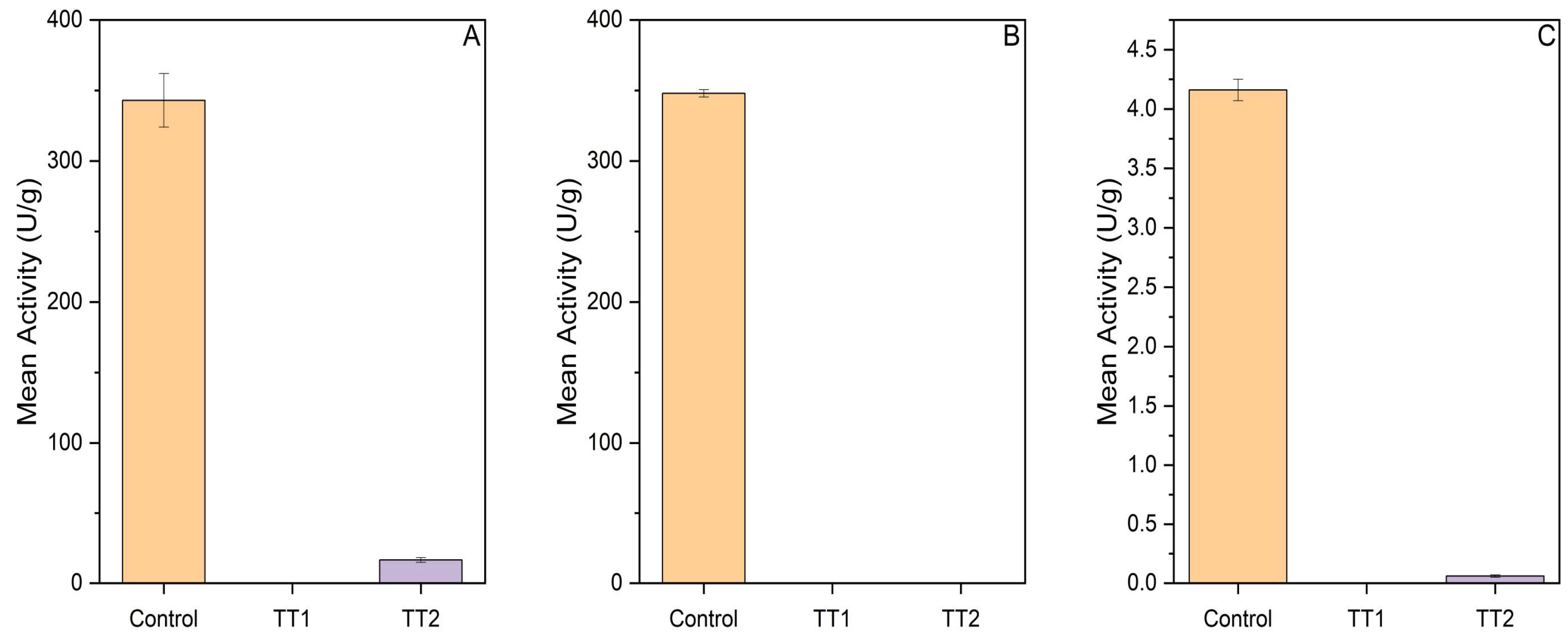
3.1.3. Thermal Pasteurization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallée Marcotte, B.; Verheyde, M.; Pomerleau, S.; Doyen, A.; Couillard, C. Health Benefits of Apple Juice Consumption: A Review of Interventional Trials on Humans. Nutrients 2022, 14, 821. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Tchuenchieu, A.; Essia Ngang, J.; Servais, M.; Dermience, M.; Sado Kamdem, S.; Etoa, F.; Sindic, M. Effect of Low Thermal Pasteurization in Combination with Carvacrol on Color, Antioxidant Capacity, Phenolic and Vitamin C Contents of Fruit Juices. Food Sci. Nutr. 2018, 6, 736–746. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. Effect of Processing Techniques at Industrial Scale on Orange Juice Antioxidant and Beneficial Health Compounds. J. Agric. Food Chem. 2002, 50, 5107–5114. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of Thermal Treatment and Storage on the Stability of Organic Acids and the Functional Value of Grapefruit Juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Kilara, A.; Van Buren, J.P. Clarification of Apple Juice. In Processed Apple Products; Downing, D.L., Ed.; Springer: New York, NY, USA, 1989; pp. 83–96. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shaik, L. Influence of Matrix PH on Batch Thermal Pasteurization of Sweet Lime Juice: Global Kinetic Models for Saccharomyces cerevisiae and Polyphenol Oxidase Inactivation and Degradation of Vitamin C. J. Food Process Eng. 2023, 46, e14437. [Google Scholar] [CrossRef]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Wu, T.; Yuan, Q.; Riaz, A.; Hu, B.; Zhou, L.; Zeng, X. Nutritional, Microbial and Physicochemical Changes in Pear Juice under Ultrasound and Commercial Pasteurization during Storage. J. Food Process Preserv. 2017, 41, e13237. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Hać-Szymańczuk, E.; Kruszewski, B. Impact of Conventional Pasteurization, High Temperature Short Time, Ultra-High Temperature, and Storage Time on Physicochemical Characteristics, Bioactive Compounds, Antioxidant Activity, and Microbiological Quality of Fruit Nectars. Foods 2024, 13, 3963. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E. The Impact of Nonthermal Technologies on the Microbiological Quality of Juices: A Review. Comp. Rev. Food Sci. Food Safe 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Afzal, R.; Madni, G.M.; Zeng, X.; Rahaman, A.; Aadil, R.M. Impact of High-pressure Treatments on Enzyme Activity of Fruit-based Beverages: An Overview. Int. J. Food Sci Technol. 2022, 57, 801–815. [Google Scholar] [CrossRef]
- Bi, X.; Liu, F.; Rao, L.; Li, J.; Liu, B.; Liao, X.; Wu, J. Effects of Electric Field Strength and Pulse Rise Time on Physicochemical and Sensory Properties of Apple Juice by Pulsed Electric Field. Innov. Food Sci. Emerg. Technol. 2013, 17, 85–92. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of High Pressure Processing on the Quality of Acidified Granny Smith Apple Purée Product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Turk, M.F.; Vorobiev, E.; Baron, A. Improving Apple Juice Expression and Quality by Pulsed Electric Field on an Industrial Scale. LWT-Food Sci. Technol. 2012, 49, 245–250. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Zhang, Q.H. (Eds.) Pulsed Electric Fields in Food Processing: Fundamental Aspects and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Oey, I. Effects of High Pressure on Enzymes. In High Pressure Processing of Food; Balasubramaniam, V.M., Barbosa-Cánovas, G.V., Lelieveld, H.L.M., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2016; pp. 391–431. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.-A.; Aadil, R.M.; Trif, M. Applications of Innovative Non-Thermal Pulsed Electric Field Technology in Developing Safer and Healthier Fruit Juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhao, L.; Ma, Y.; Wang, Y.; Sun, Z.; Liao, X. Microorganisms and Some Quality of Red Grapefruit Juice Affected by High Pressure Processing and High Temperature Short Time. Food Bioprocess Technol. 2015, 8, 2096–2108. [Google Scholar] [CrossRef]
- Dars, A.G.; Hu, K.; Liu, Q.; Abbas, A.; Xie, B.; Sun, Z. Effect of Thermo-Sonication and Ultra-High Pressure on the Quality and Phenolic Profile of Mango Juice. Foods 2019, 8, 298. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Salmeron-Ochoa, I.; Gutierrez-Mendez, N.; Ramaswamy, H.S.; Ortega-Rivas, E. Shelf Life Studies on Apple Juice Pasteurised by Ultrahigh Hydrostatic Pressure. LWT-Food Sci. Technol. 2015, 62, 915–919. [Google Scholar] [CrossRef]
- Szczepańska, J.; Pinto, C.A.; Skąpska, S.; Saraiva, J.A.; Marszałek, K. Effect of Static and Multi-Pulsed High Pressure Processing on the Rheological Properties, Microbial and Physicochemical Quality, and Antioxidant Potential of Apple Juice during Refrigerated Storage. LWT 2021, 150, 112038. [Google Scholar] [CrossRef]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the Impact of High Pressure, Pulsed Electric Field and Thermal Pasteurization on Quality Attributes of Cloudy Apple Juice Using Targeted and Untargeted Analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Lacey, K.L.; Moreno-Barreto, A.; Pavón-Vargas, D.; Cattani, L.; Rinaldi, M.; Rainieri, S.; Dhenge, R. A Quality Assessment of Strawberry Nectar Stabilized by Thermal and High-Pressure Processing Conditions. J. Food Process. Preserv. 2023, 2023, 5481142. [Google Scholar] [CrossRef]
- Garg, G.; Singh, A.; Kaur, A.; Singh, R.; Kaur, J.; Mahajan, R. Microbial Pectinases: An Ecofriendly Tool of Nature for Industries. 3 Biotech 2016, 6, 47. [Google Scholar] [CrossRef]
- Osorio, O.; Martínez-Navarrete, N.; Moraga, G.; Carbonell, J.V. Effect of Thermal Treatment on Enzymatic Activity and Rheological and Sensory Properties of Strawberry Purees. Food Sci. Technol. Int. 2008, 14, 103–108. [Google Scholar] [CrossRef]
- Rai, P.; Majumdar, G.C.; DasGupta, S.; De, S. Optimizing Pectinase Usage in Pretreatment of Mosambi Juice for Clarification by Response Surface Methodology. J. Food Eng. 2004, 64, 397–403. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1332/2008 of 16 December 2008 on Food Enzymes and Amending Council Directive 83/417/EEC, Council Regulation (EC) No 1493/1999, Directive 2000/13/EC, Council Directive 2001/112/EC and Regulation (EC) No 258/97. Official Journal of the European Union 2008, L 354, 7–15. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0007:0015:en:PDF (accessed on 15 March 2025).
- European Parliament and Council. Regulation (EU) 2019/1381 of 20 June 2019 on the Transparency and Sustainability of the EU Risk Assessment in the Food Chain and Amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC. Official Journal of the European Union 2019, L 231, 1–28. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019R1381 (accessed on 15 March 2025).
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Food Manufacturing Processes and Technical Data Used in the Exposure Assessment of Food Enzymes. EFSA J. 2023, 21, e08094. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1333/2008 of 16 December 2008 on Food Additives (Text with EEA relevance). Official Journal of the European Union 2008, L 354, 16–33. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj (accessed on 15 March 2025).
- Maller, A. Use of Pectinases in Juice Clarification Processes: A Scoping Review. NTNF 2025, 8, 816–826. [Google Scholar] [CrossRef]
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Ho, S.Y.; Mittal, G.S.; Cross, J.D. Effects of High Field Electric Pulses on the Activity of Selected Enzymes. J. Food Eng. 1997, 31, 69–84. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; De Ancos, B. High-Pressure Processing Effect on Nutrients and Their Stability; CRC Press: Boca Raton, FL, USA, 2017; pp. 85–104. ISBN 978-1-4987-3902-3. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W.; Hogan, E.; Kelly, A. High-Pressure Processing of Foods: An Overview. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–24. [Google Scholar] [CrossRef]
- Abera, G. Review on High-Pressure Processing of Foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Neogen Megazymes. Available online: https://prod-docs.megazyme.com/documents/Data_Sheet/P-PGACIT-10G_DATA.pdf (accessed on 26 March 2025).
- Albersheim, P.; Killias, U. Studies Relating to the Purification and Properties of Pectin Transeliminase. Arch. Biochem. Biophys. 1962, 97, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, Z.I. [18] Pectic Enzymes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1955; Volume 1, pp. 158–166. [Google Scholar] [CrossRef]
- Espachs-Barroso, A.; Barbosa-Cánovas, G.V.; Martín-Belloso, O. Microbial and Enzymatic Changes in Fruit Juice Induced by High-Intensity Pulsed Electric Fields. Food Rev. Int. 2003, 19, 253–273. [Google Scholar] [CrossRef]
- Raso, J.; Álvarez, I. Pulsed Electric Field Processing: Cold Pasteurization. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Álvarez, I. The Influence of Process Parameters for the Inactivation of Listeria monocytogenes by Pulsed Electric Fields. Int. J. Food Microbiol. 2003, 87, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yu, L.; Wang, W.; Gai, L.; Wang, J. Comparing the Pulsed Electric Field Resistance of the Microorganisms in Grape Juice: Application of the Weibull Model. Food Control 2014, 35, 241–251. [Google Scholar] [CrossRef]
- Rastogi, N.K. Application of High-Intensity Pulsed Electrical Fields in Food Processing. Food Rev. Int. 2003, 19, 229–251. [Google Scholar] [CrossRef]
- Meneses, N.; Jaeger, H.; Knorr, D. pH-Changes during Pulsed Electric Field Treatments—Numerical Simulation and in Situ Impact on Polyphenoloxidase Inactivation. Innov. Food Sci. Emerg. Technol. 2011, 12, 499–504. [Google Scholar] [CrossRef]
- Cho, S.W.; Lee, S.; Shin, W. The X-Ray Structure of Aspergillus aculeatus Polygalacturonase and a Modeled Structure of the Polygalacturonase-Octagalacturonate Complex. J. Mol. Biol. 2001, 311, 863–878. [Google Scholar] [CrossRef]
- Kent, L.M.; Loo, T.S.; Melton, L.D.; Mercadante, D.; Williams, M.A.K.; Jameson, G.B. Structure and Properties of a Non-Processive, Salt-Requiring, and Acidophilic Pectin Methylesterase from Aspergillus niger Provide Insights into the Key Determinants of Processivity Control. J. Biol. Chem. 2016, 291, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of Pulsed Electric Fields on the Activities of Microorganisms and Pectin Methyl Esterase in Orange Juice. J. Food Sci. 2000, 65, 1359–1363. [Google Scholar] [CrossRef]
- Giner, J.; Gimeno, V.; Espachs, A.; Elez, P.; Barbosa-Cánovas, G.V.; Martín, O. Inhibition of Tomato (Licopersicon esculentum Mill.) Pectin Methylesterase by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2000, 1, 57–67. [Google Scholar] [CrossRef]
- Giner, J.; Grouberman, P.; Gimeno, V.; Martín, O. Reduction of Pectinesterase Activity in a Commercial Enzyme Preparation by Pulsed Electric Fields: Comparison of Inactivation Kinetic Models. J. Sci. Food Agric. 2005, 85, 1613–1621. [Google Scholar] [CrossRef]
- Di Matteo, A.; Giovane, A.; Raiola, A.; Camardella, L.; Bonivento, D.; De Lorenzo, G.; Cervone, F.; Bellincampi, D.; Tsernoglou, D. Structural Basis for the Interaction between Pectin Methylesterase and a Specific Inhibitor Protein. Plant Cell 2005, 17, 849–858. [Google Scholar] [CrossRef]
- Jolie, R.P.; Christiaens, S.; De Roeck, A.; Fraeye, I.; Houben, K.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Pectin Conversions under High Pressure: Implications for the Structure-Related Quality Characteristics of Plant-Based Foods. Trends Food Sci. Technol. 2012, 24, 103–118. [Google Scholar] [CrossRef]
- Riahi, E.; Ramaswamy, H.S. High-Pressure Processing of Apple Juice: Kinetics of Pectin Methyl Esterase Inactivation. Biotechnol. Prog. 2003, 19, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Sandate-Flores, L.; Rostro-Alanis, M.D.J.; Mancera-Andrade, E.I.; Esquivel-Hernandez, D.A.; Brambila-Paz, C.; Parra-Saldívar, R.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.; Rodríguez-Rodríguez, J. Using High Hydrostatic Pressures to Retain the Antioxidant Compounds and to Reduce the Enzymatic Activity of a Pitaya–Pineapple (Stenocereus sp.–Fragaria ananassa) Beverage. J. Food Sci. Technol. 2017, 54, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Asaka, M.; Aoyama, Y.; Nakanishi, R.; Hayashi, R. Purification of a Latent Form of Polyphenoloxidase from La France Pear Fruit and Its Pressure-Activation. Biosci. Biotechnol. Biochem. 1994, 58, 1486–1489. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative Study of Enzymes, Phenolics, Carotenoids and Color of Apricot Nectars Treated by High Hydrostatic Pressure and High Temperature Short Time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Tomlin, B.D.; Jones, S.E.; Reyes-De-Corcuera, J.I. High Hydrostatic Pressure Protection of a Pectinase Cocktail against Thermal Inactivation. J. Food Eng. 2013, 116, 674–680. [Google Scholar] [CrossRef]
- Tomlin, B.D.; Jones, S.E.; Teixeira, A.A.; Correll, M.J.; Reyes-De-Corcuera, J.I. Kinetics of Viscosity Reduction of Pectin Solutions Using a Pectinase Formulation at High Hydrostatic Pressure. J. Food Eng. 2014, 129, 47–52. [Google Scholar] [CrossRef]
- Yong, S.X.M.; Song, C.P.; Choo, W.S. Impact of High-Pressure Homogenization on the Extractability and Stability of Phytochemicals. Front. Sustain. Food Syst. 2021, 4, 593259. [Google Scholar] [CrossRef]
- Rodrigo, D.; Cortés, C.; Clynen, E.; Schoofs, L.; Loey, A.V.; Hendrickx, M. Thermal and High-Pressure Stability of Purified Polygalacturonase and Pectinmethylesterase from Four Different Tomato Processing Varieties. Food Res. Int. 2006, 39, 440–448. [Google Scholar] [CrossRef]
- Bermejo-Prada, A.; Van Buggenhout, S.; Otero, L.; Houben, K.; Van Loey, A.; Hendrickx, M.E. Kinetics of Thermal and High-Pressure Inactivation of Avocado Polygalacturonase. Innov. Food Sci. Emerg. Technol. 2014, 26, 51–58. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Lorenzo, J.M.; Marszałek, K. The Influence of Static and Multi-Pulsed Pressure Processing on the Enzymatic and Physico-Chemical Quality, and Antioxidant Potential of Carrot Juice During Refrigerated Storage. Food Bioprocess Technol. 2021, 14, 52–64. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Safety Evaluation of the Food Enzyme Pectin Lyase from the Genetically Modified Trichoderma reesei Strain RF6199. EFSA J. 2022, 20, e07675. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; De Diego, S.; Perez-Mateos, M.; Busto, M.D. Kinetic Properties and Thermal Behaviour of Polygalacturonase Used in Fruit Juice Clarification. Food Chem. 2004, 88, 209–217. [Google Scholar] [CrossRef]
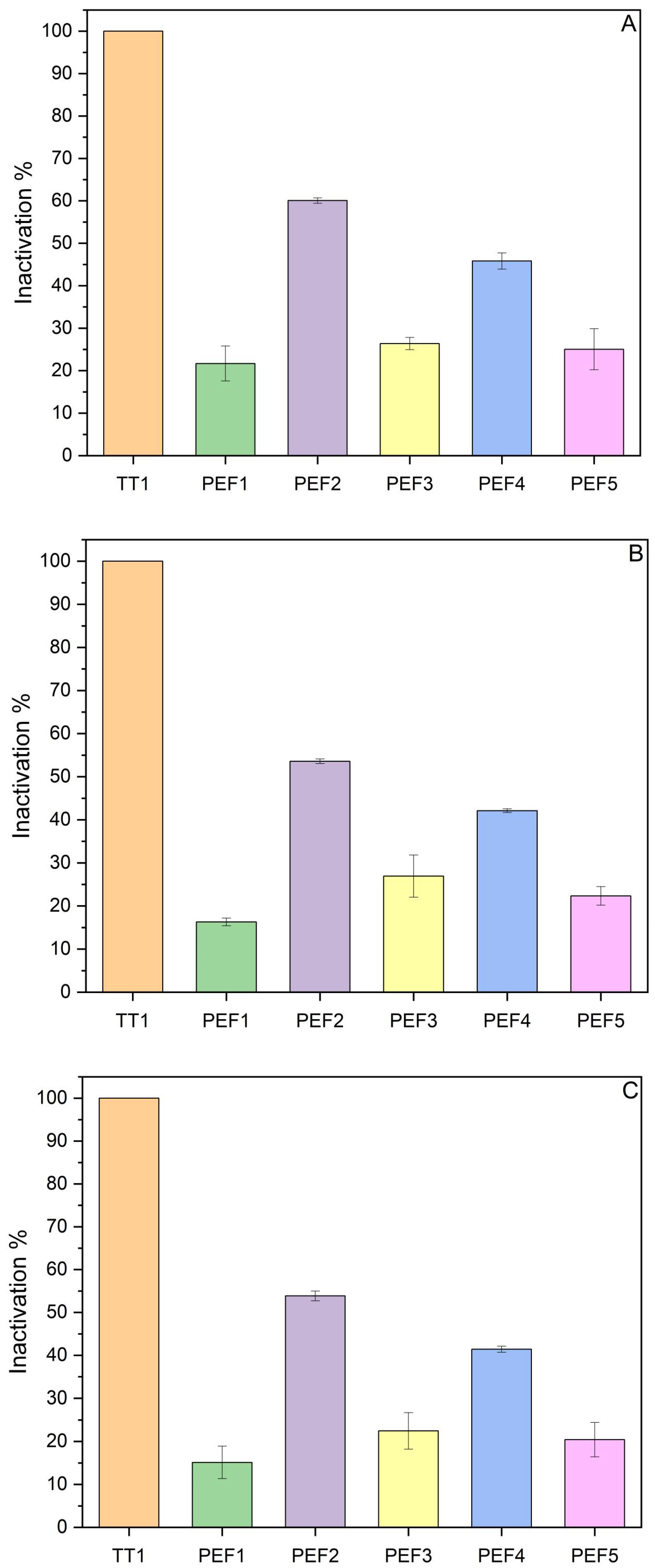
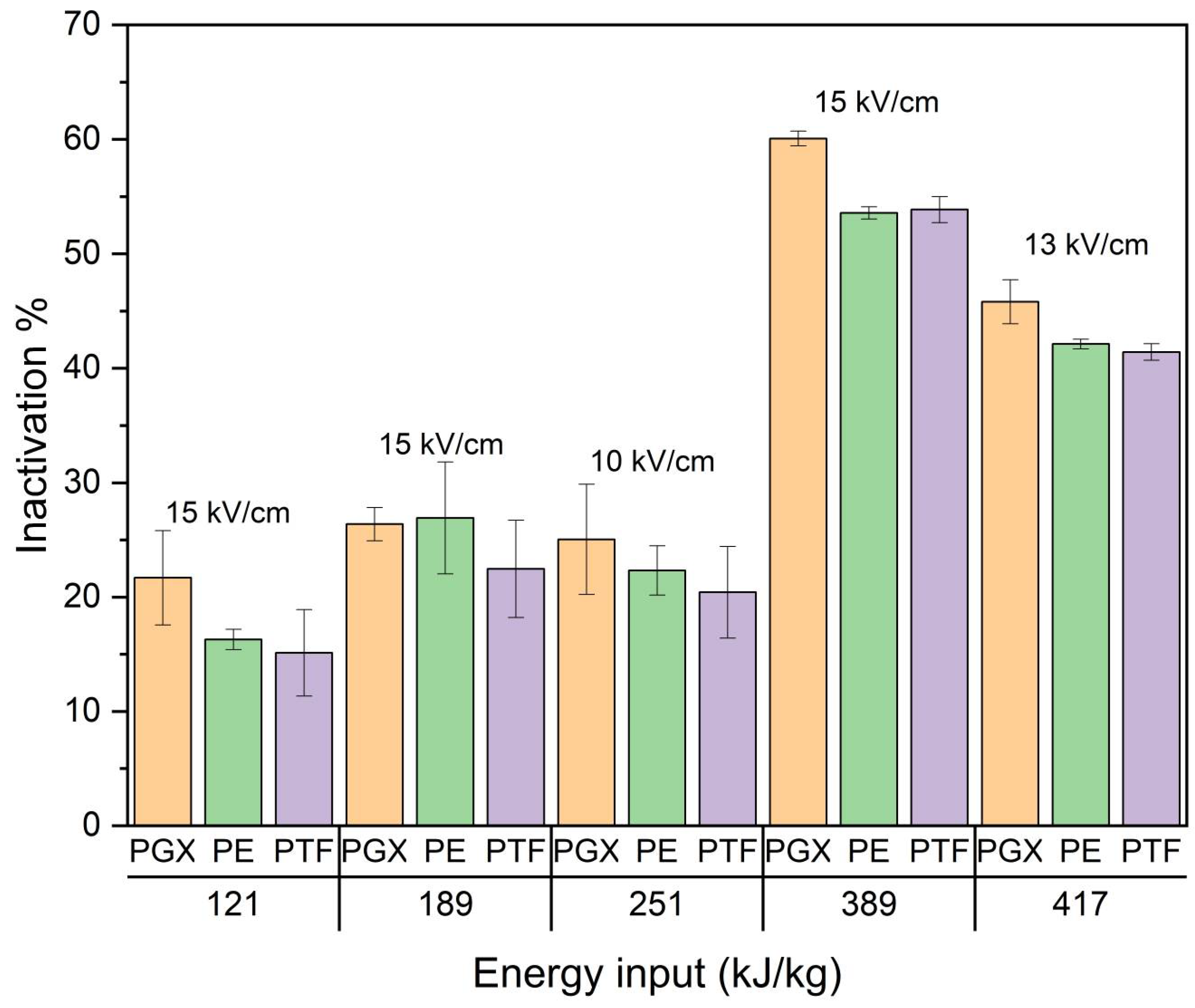

| Test | Electric Field Intensity (kV/cm) | Pulse Witdth (μs) | Frequency (Hz) | Current (A) | Specific Energy (kJ/kg) |
|---|---|---|---|---|---|
| PEF 1 | 15 | 10 | 10 | 210 | 121 |
| PEF 2 | 15 | 10 | 20 | 336 | 389 |
| PEF 3 | 15 | 10 | 15 | 228 | 189 |
| PEF 4 | 13 | 10 | 30 | 277 | 417 |
| PEF 5 | 10 | 10 | 40 | 161 | 251 |
| Test | Pressure (MPa) | Time (min) |
|---|---|---|
| HPP 1 | 250 | 2 |
| HPP 2 | 250 | 4 |
| HPP 3 | 250 | 6 |
| HPP 4 | 400 | 4 |
| HPP 5 | 600 | 4 |
| HPP 6 | 600 | 8 |
| HPP 7 | 600 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavarise, A.; Puzović, A.; Moreno Barreto, A.F.; Pavon Vargas, D.; Goessinger, M.; Mikulič Petkovšek, M.; Rinaldi, M.; Haselmair-Gosch, C.; Cattani, L.; Halbwirth, H. Effect of Non-Thermal Treatments of Clear Apple Juice on Exogenous Pectinases. Beverages 2025, 11, 113. https://doi.org/10.3390/beverages11040113
Zavarise A, Puzović A, Moreno Barreto AF, Pavon Vargas D, Goessinger M, Mikulič Petkovšek M, Rinaldi M, Haselmair-Gosch C, Cattani L, Halbwirth H. Effect of Non-Thermal Treatments of Clear Apple Juice on Exogenous Pectinases. Beverages. 2025; 11(4):113. https://doi.org/10.3390/beverages11040113
Chicago/Turabian StyleZavarise, Alberto, Alema Puzović, Andres Felipe Moreno Barreto, Dario Pavon Vargas, Manfred Goessinger, Maja Mikulič Petkovšek, Massimiliano Rinaldi, Christian Haselmair-Gosch, Luca Cattani, and Heidi Halbwirth. 2025. "Effect of Non-Thermal Treatments of Clear Apple Juice on Exogenous Pectinases" Beverages 11, no. 4: 113. https://doi.org/10.3390/beverages11040113
APA StyleZavarise, A., Puzović, A., Moreno Barreto, A. F., Pavon Vargas, D., Goessinger, M., Mikulič Petkovšek, M., Rinaldi, M., Haselmair-Gosch, C., Cattani, L., & Halbwirth, H. (2025). Effect of Non-Thermal Treatments of Clear Apple Juice on Exogenous Pectinases. Beverages, 11(4), 113. https://doi.org/10.3390/beverages11040113








