Formulation of Black Soybean Yogurt and Evaluation of Changes in the Bioactive Profile and Other Compositional Aspects During Fermentation and Storage
Abstract
1. Introduction
2. Materials and Methods
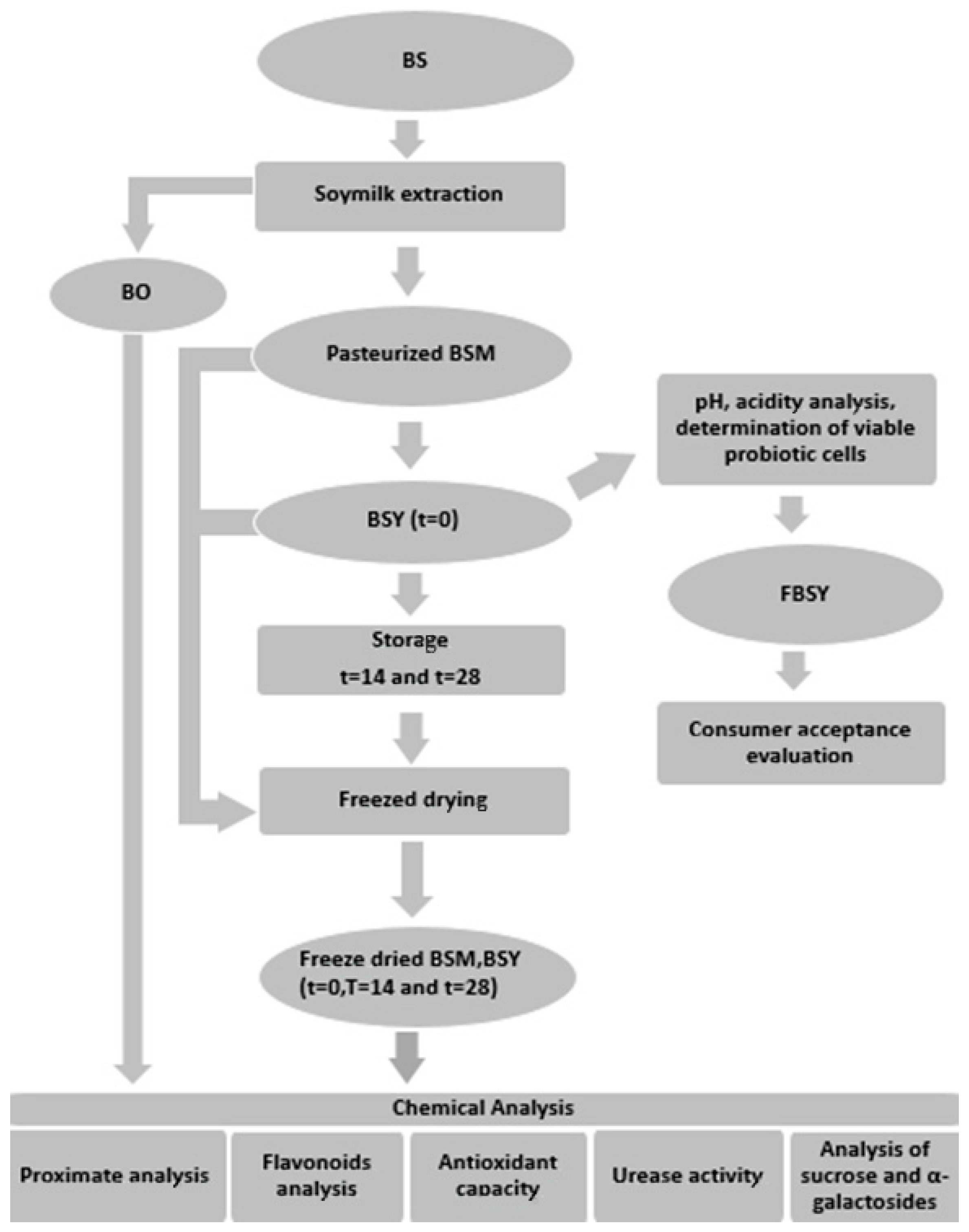
2.1. Experimental Design
2.2. Raw Materials
2.3. Product Development and Storage Conditions
2.3.1. BSM Processing
2.3.2. BSY and Flavored BSY Production
2.3.3. Storage Conditions and Freeze-Drying
2.4. Chemical and Physicochemical Analyses
2.4.1. Determination and Viable Probiotic Cells of L. acidophilus, pH, and Titratable Acidity
2.4.2. Proximate Analyses
2.4.3. Urease Activity
2.4.4. Analysis of Sucrose and α-Galactosides
2.4.5. Analysis of Flavonoids
2.4.6. Antioxidant Capacity (AC) Assays
2.5. Consumer’s Acceptance Test with Formulated BSY
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of BS, BSM, BSY, and BO
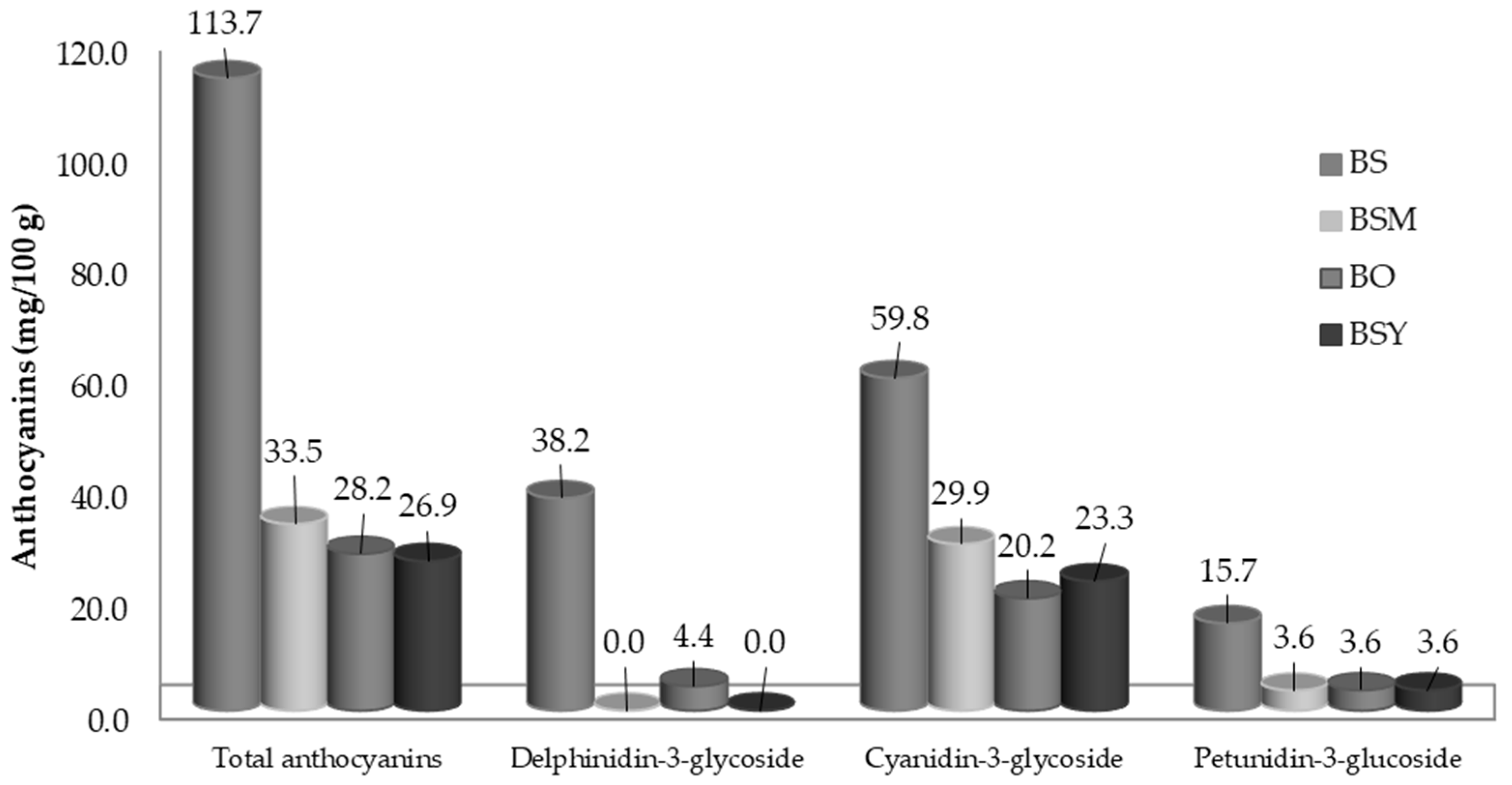
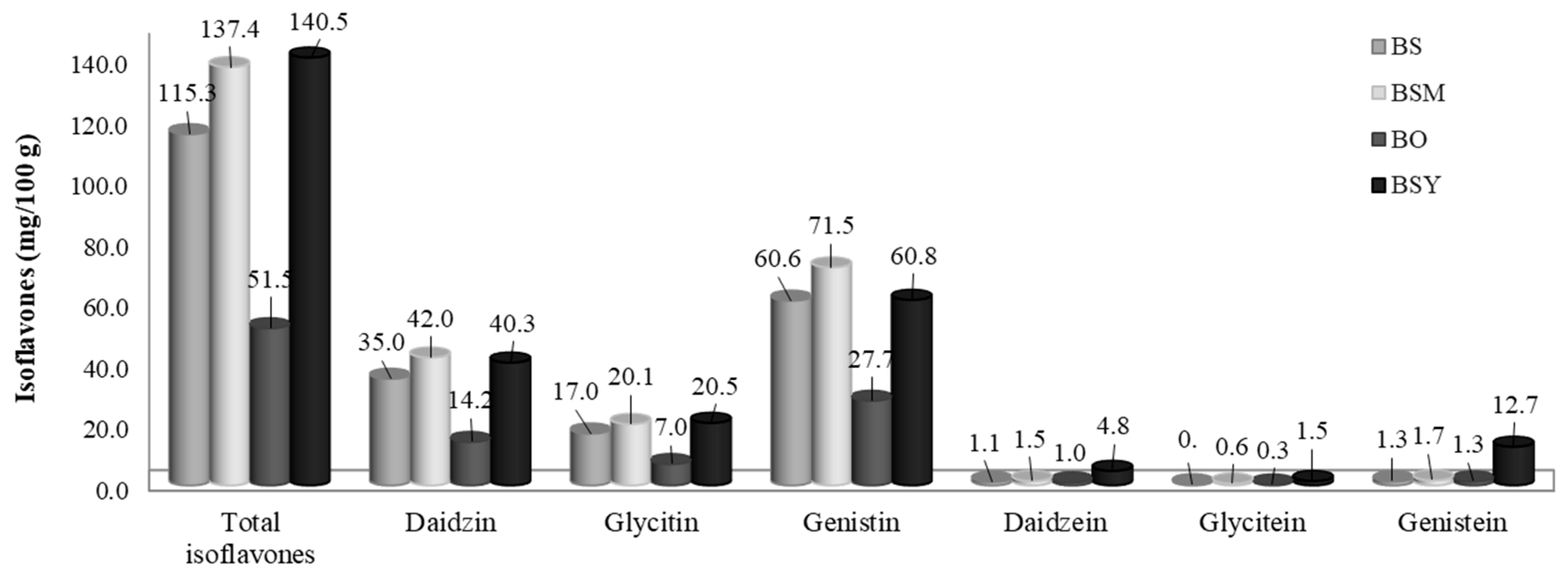
3.2. Analysis of Flavonoids of BS, BSM, BSY, and BO
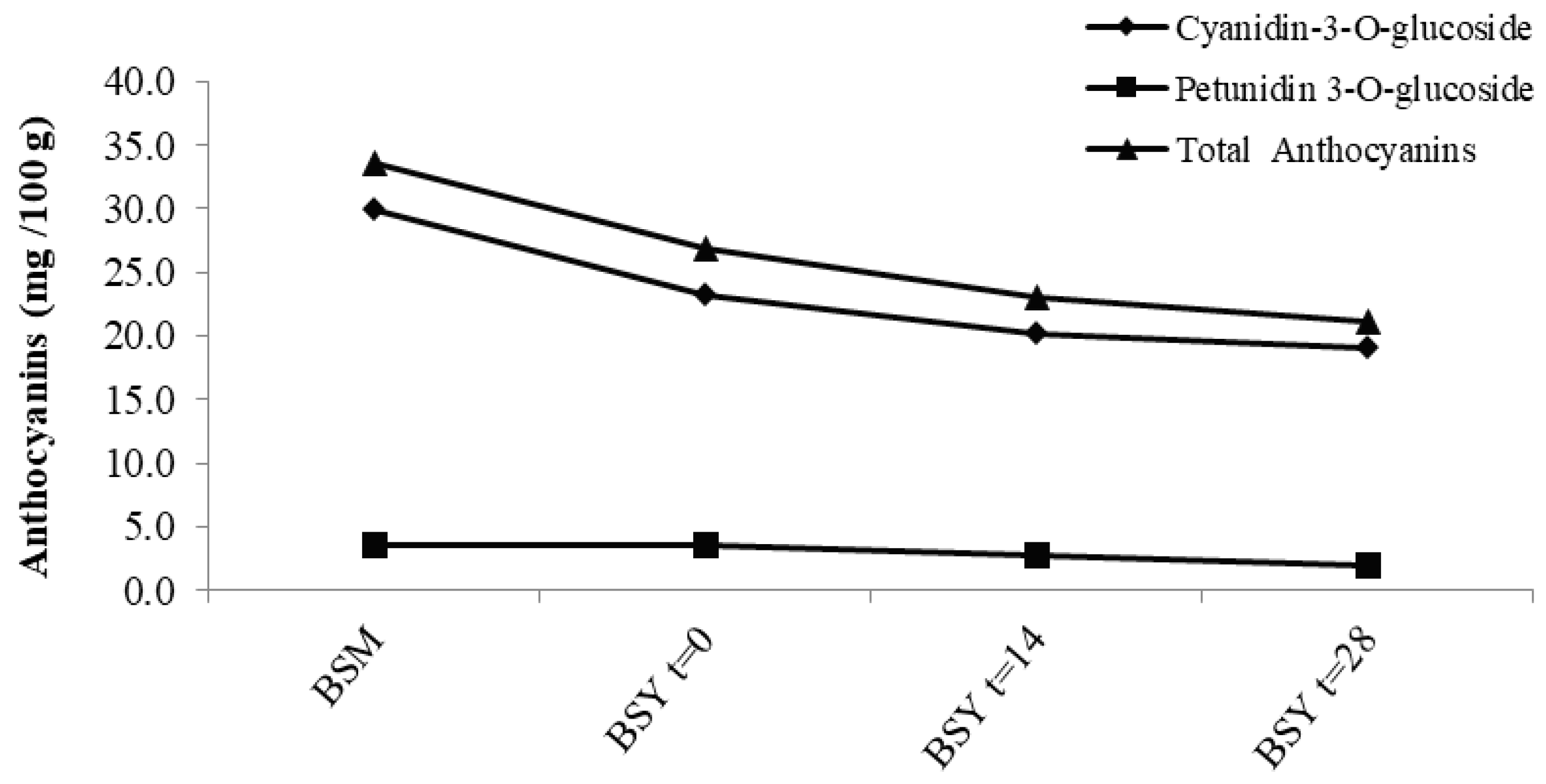
3.2.1. Anthocyanins
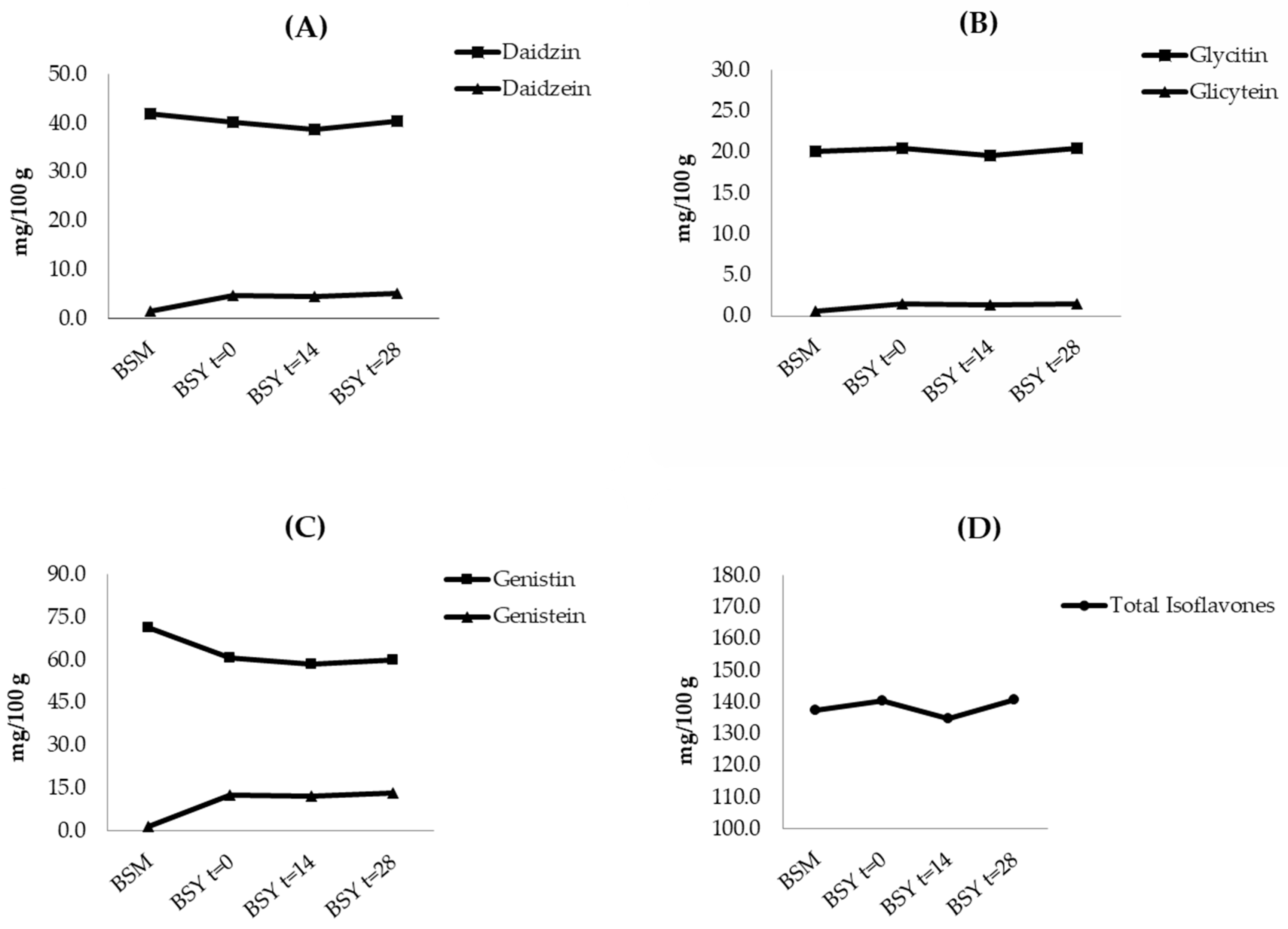
3.2.2. Isoflavones
3.3. Antioxidant Capacity Assays of BS, BSM, and BSY
3.4. Analysisof Sucrose and α-Galactosides of BSM and BSY
3.5. Urease Activity (UA) of BS, BSM, BSY, and BO
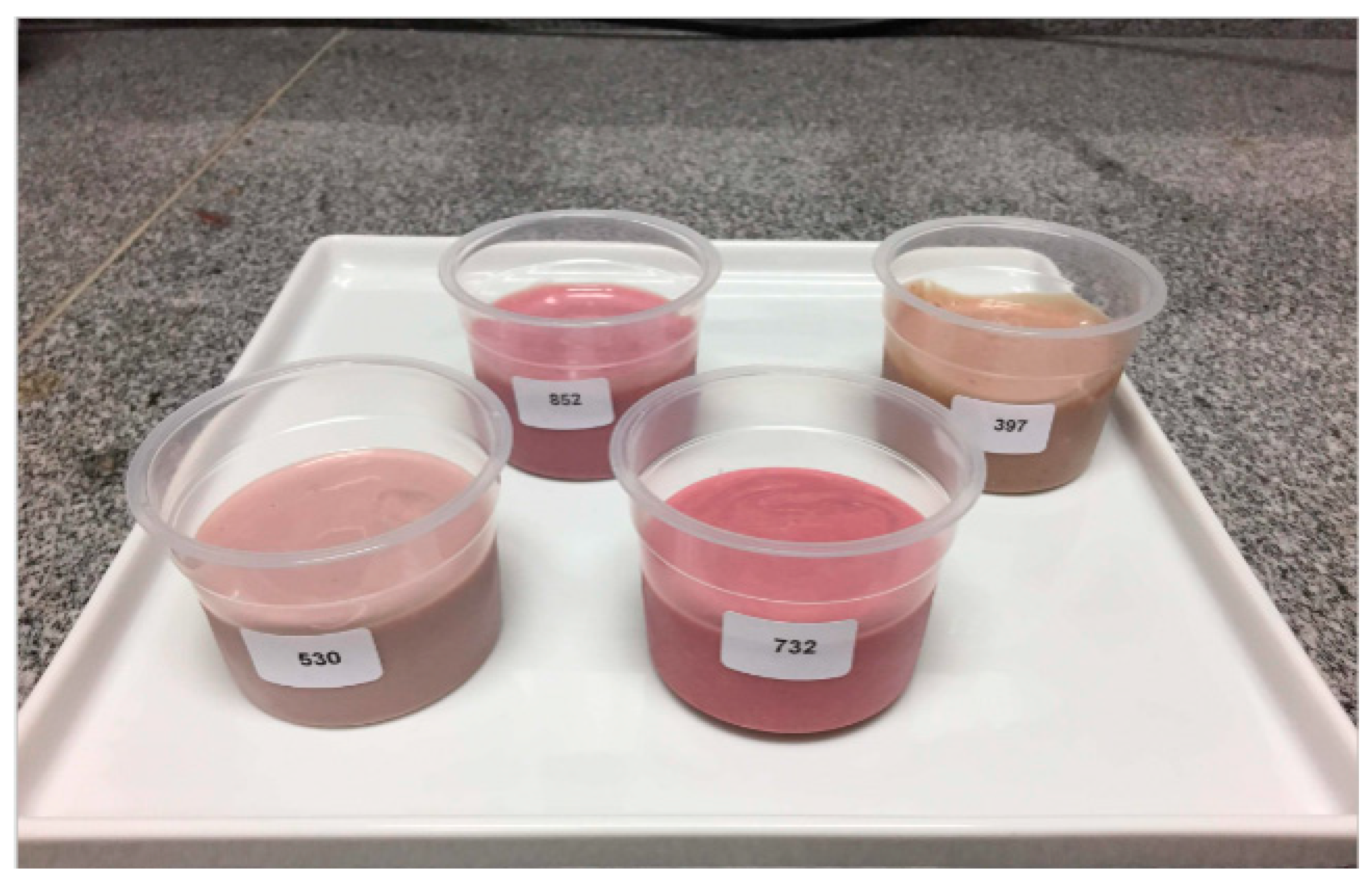
3.6. Consumers’ Acceptance Test of BSY and Flavored BSY
3.7. Analyses of Chemical Components After Storage
3.7.1. Flavonoids Analyses
3.7.2. Antioxidant Capacity Assays
3.7.3. Determination of Viable Probiotic Cells of L. acidophilus
3.7.4. Analysis of Sucrose and α-Galactosides
3.7.5. Determination of pH and Titratable Acidity
4. Conclusions and Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, C.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Anjum, S.; Rana, S.; Dasila, K.; Agnihotri, V.; Pandey, A.; Pande, V. Comparative nutritional and antimicrobial analysis of Himalayan black and yellow soybean and their okara. J. Sci. Food Agric. 2022, 102, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Kuo, L.C.; Cheng, W.Y.; Wu, R.Y. Hydrolysis of black soybean isoflavone glycosides by Bacillus subtilis natto. Appl. Microbiol. Biotechnol. 2006, 73, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, K.M. Changes occurring in compositional components of black soybeans maintained at room temperature for different storage periods. Food Chem. 2012, 131, 161–169. [Google Scholar] [CrossRef]
- Kumar, M.; Suhag, R.; Hasan, M.; Dhumal, S.; Radha; Pandiselvam, R.; Senapathy, M.; Sampathrajan, V.; Punia, S.; Sayed, A.A.S.; et al. Black soybean (Glycine max (L.) Merr.): Paving the way toward new nutraceutical. Crit. Rev. Food Sci. Nutr. 2023, 63, 6208–6234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Hao, X.; Ji, X.; Zhu, Y.; Chen, X.; Yao, Y. A systematic review of black soybean (Glycine max (L.) Merr.): Nutritional composition, bioactive compounds, health benefits, and processing to application. Food Front. 2024, 5, 1188–1211. [Google Scholar] [CrossRef]
- Li, Y.; Qi, B. Phytochemicals in Soybeans: Bioactivity and Health Benefits, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–400. [Google Scholar]
- Leksono, B.Y.; Cahyanto, M.N.; Rahayu, E.S.; Yanti, R.; Utami, T. Enhancement of antioxidant activities in black soy milk through isoflavone aglycone production during indigenous lactic acid bacteria fermentation. Fermentation 2022, 8, 326. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Hsieh, J.F. The conversion and deglycosylation of isoflavones and anthocyanins in black soymilk process. Food Chem. 2018, 261, 8–14. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of action of prebiotics and their effects on gastrointestinal disorders in adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yu, R.-C.; Yang, H.-Y.; Chou, C.-C. Sugar and acid contents in soymilk fermented with lactic acid bacteria alone or simultaneously with bifidobacteria. Food Microbiol. 2003, 20, 333–338. [Google Scholar] [CrossRef]
- Chun, J.; Kim, J.S.; Kim, J.H. Enrichment of isoflavones aglycone in soymilk by fermentation with single and mixed cultures of Streptococcus infantarius 12 and Weisellia sp. 4. Food Chem. 2008, 109, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sasi, M.; Kumar, S.; Hasan, M.; S.R., A.; Garcia-Gutierrez, E.; Kumari, S.; Prakash, O.; Nain, L.; Sachdev, A.; Dahuja, A. Current trends in the development of soy-based foods containing probiotics and paving the path for soy-synbiotics. Crit. Rev. Food Sci. Nutr. 2022, 3, 9995–10013. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [CrossRef]
- Desmawati, D.; Sulastri, D. Phytoestrogens and their health effect. Open Access Maced. J. Med. Sci. 2019, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.-H.; Jeong, E.-J. A Study on Manufacturing Black Soybean Yogurt. Korean J. Food Nutr. 2007, 20, 289–294. [Google Scholar]
- Ye, M.; Ren, L.; Wu, Y.; Wang, Y.; Liu, Y. Quality characteristics and antioxidant activity of hickory-black soybean yogurt. LWT-Food Sci. Technol. 2013, 51, 314–318. [Google Scholar] [CrossRef]
- Winarti, S.; Sarofa, U.; Islami, B.Y. Physicochemical Properties of Black Soygurt Made from Black Soybeans (BS) and Black Sticky Rice (BR). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1125, 012103. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Cao, Y.; Liu, B.; Ruan, C.Q. Study on the production technology of solidifying black soybean yogurt. J. Food Saf. Qual. 2022, 13, 774–781. [Google Scholar]
- Hasan, M.; Meena, N.L.; Krishnan, V.; Rudra, S.G.; Dahuja, A. Impact of storage on probiotic viability, nutritional and sensory quality of fermented soymilk produced from different soybean varieties. Legume Res. Int. J. 2023, 46, 721–727. [Google Scholar] [CrossRef]
- Rahmawati, A.A.; Setiani, B.E.; Pramono, Y.B.; Al-Baarri, A.N. The Fermentation Time Effect against the Isoflavones Profiles of Genistein and Daidzein of Blacksoyghurt as a Potential Functional-Probiotic Drink. Int. J. Adv. Sci. Eng. Inf. Technol. 2023, 13, 522–529. [Google Scholar] [CrossRef]
- Suhaimi, Q.; Kamarudin, W.S.S.W.; Nizori, A.; Sikin, A.M. Effect of different extraction methods on antioxidant and sensorial properties of pasteurized black soymilk. Indones. Food Sci. Technol. J. 2023, 7, 23–29. [Google Scholar] [CrossRef]
- Anwar, S.; Perdana, T.; Rachmadi, M.; Noor, T.I. Traceability information model for sustainability of black soybean supply chain: A systematic literature review. Sustainability 2022, 14, 9498. [Google Scholar] [CrossRef]
- Asghar, A.; Afzaal, M.; Saeed, F.; Ahmed, A.; Ateeq, H.; Shah, Y.A.; Islam, F.; Hussain, M.; Akram, N.; Shah, M.A. Valorization and food applications of okara (soybean residue): A concurrent review. Food Sci. Nutr. 2023, 11, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Esteves, T.C.F.; Calado, V.; Farah, A.; de Faria-Machado, A.F.; de Oliveira Godoy, R.L.; de Araujo Santiago, M.C.P.; Felberg, I. Impact of thermal processing parameters during black soymilk extraction and pasteurization on key bioactive compounds and antioxidant capacity. J. Food Nutr. Res. 2022, 10, 503–510. [Google Scholar] [CrossRef]
- Walter, E.H.M.; Carneiro, M.S.; Felberg, I.; De Oliveira, D.R.; Costa, S.D.O.; Conte, C. Obtenção de bebidas fermentadas por probióticos a partir de diferentes matérias-primas da soja. Embrapa Comun. Téc. 2016, 219, 4p. [Google Scholar]
- Tharmaraj, N.; Shah, N.P. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J. Dairy Sci. 2003, 86, 2288–2296. [Google Scholar] [CrossRef]
- International Dairy Federation (IDF). Fermented and non-fermented milk products: Detection and enumeration of Lactobacillus acidophilus. Bull. IDF 1995, 306, 23–33. [Google Scholar]
- AOAC. Official Methods of Analysis: AOAC, 18th ed.; 3rd revision; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- ISO/TS 22113:2012/IDF/RM204:2012. Milk and Milk Products—Determination of the Titratable Acidity of Milk Fat (Publication Last Reviewed and Confirmed in 2022. Available online: https://www.iso.org/standard/56167.html (accessed on 10 December 2022).
- AOAC. Official Methods of Analysis: AOAC, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the AOCS, 6th ed.; AOCS International: Gauthrsburg, MD, USA, 2009. [Google Scholar]
- Macrae, R. HPLC in Food Analysis, 2nd ed.; Academic Press: New York, NY, USA, 1998; p. 77. [Google Scholar]
- Pereira, J.N.; Godoy, R.L.O.; Felberg, I.; Esteves, T.C.F.; Santiago, M.C.P.A.; Carrão-Panizzi, M.C. Avaliação de metodologias de extração e caracterização do perfil de antocianinas em soja preta por cromatografia líquida de alta eficiência (CLAE) e espectrometria de massas (MS). In Proceedings of the XXIV Congresso Brasileiro de Ciência e Tecnologia de Alimentos, Aracaju, Brazil, 25–29 September, 2014. [Google Scholar]
- Pereira, J.N. Avaliação de diferentes linhagens de soja preta quanto as suas características físico-químicas, nutricionais e compostos bioativos. Master’s Thesis, Universidade Federal Rural do Rio de Janeiro, Seropédica, Brazil, 2015. [Google Scholar]
- Gouvêa, A.C.M.S.; Melo, A.; Santiago, M.C.P.A.; Peixoto, F.M.; Freitas, V.; Godoy, R.L.O.; Ferreira, I.M.P.L.V.O. Identification and quantification of anthocyanins in fruits from Neomitranthes obscura (DC.) N. Silveira, an endemic species from Brazil, by comparison of chromatographic methodologies. Food Chem. 2015, 185, 277–283. [Google Scholar] [CrossRef]
- Gouvêa, A.C.M.S.; Santiago, M.C.P.A.; Pacheco, S.; Godoy, R.L.O.; Cabral, L.M.C. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutenosideo) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci. Technol. 2012, 32, 43–46. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- MacFie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Cho, K.M.; Ha, T.J.; Lee, Y.B.; Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Jeong, S.H.; Kang, Y.M.; Lee, J.H. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J. Funct. Foods 2013, 5, 1065–1076. [Google Scholar] [CrossRef]
- Felberg, I.; Carrão-Panizzi, M.C.; Pereira, J.d.N.; Godoy, R.L.D.O.; Freitas, S.C.d.; Santiago, M.C.P.d.A.; Pacheco, S.; Calado, V.M.d.A. Características químicas, nutricionais e compostos bioativos em genótipos de soja preta. Bol. Pesqui. Desenvolv. 2019, 33. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1112506/1/BPD332019sojapreta.pdf (accessed on 10 December 2022).
- Lee, M.; Hong, G.-E.; Zhang, H.; Yang, C.-Y.; Han, K.-H.; Mandal, P.K.; Lee, C.-H. Production of the isoflavone aglycone and antioxidant activities in black soymilk using fermentation with Streptococcus thermophilus S10. Food Sci. Biotechnol. 2015, 24, 537–544. [Google Scholar] [CrossRef]
- Hong, G.E.; Mandal, P.K.; Lim, K.W.; Lee, C.H. Fermentation increases isoflavone aglycone contents in black soybean pulp. Asian J. Anim. Vet. Adv. 2012, 7, 502–511. [Google Scholar] [CrossRef]
- Liu, K. Food use of whole soybeans. In Soybeans: Chemistry, Production, Processing, and Utilization, 3rd ed.; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Urbana, IL, USA, 2008; pp. 441–481. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Zuidam, N.J. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Lu, F.; Liu, Y.; Li, B. Okara dietary fiber and hypoglycemic effect of okara foods. Bioact. Carbohydr. Diet. Fibre 2013, 2, 126–132. [Google Scholar] [CrossRef]
- Wu, C.; Teng, F.; McClements, D.J.; Zhang, S.; Li, Y.; Wang, Z. Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res. Int. 2020, 134, 109251. [Google Scholar] [CrossRef]
- Yang, H.-F.; Tso, T.; Huang, C.-C.; Huang, W.-N. Okara supplementation relieves fatigue and muscle damage that occur during exercise training. J. Food Nutr. Res. 2015, 3, 352–356. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiologia, 8th ed.; Artmed: Porto Alegre, Brazil, 2005; 894p. [Google Scholar]
- Koh, K.; Youn, J.E.; Kim, H.S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr.) varieties. J. Food Sci. Technol. 2014, 51, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, J.H.; Shin, S.-O.; Shin, S.-H.; Han, S.-I.; Kim, H.-T.; Ko, J.-M.; Lee, M.-H.; Park, K.-Y. Changes in anthocyanin and isoflavone concentrations in black seed-coated soybean at different planting locations. J. Crop Sci. Biotechnol. 2009, 12, 79–86. [Google Scholar] [CrossRef]
- Wu, H.-J.; Deng, J.-C.; Yang, C.-Q.; Zhang, J.; Zhang, Q.; Wang, X.-C.; Yang, F.; Yang, W.-Y.; Liu, J. Metabolite profiling of isoflavones and anthocyanins in black soybean [Glycine max (L.) Merr.] seeds by HPLC-MS and geographical differentiation analysis in Southwest China. Anal. Methods 2017, 9, 792–802. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.-R.; Ma, K.-H.; Cho, Y.-H.; Lee, G.-A.; Chung, J.-W. Anthocyanin and isoflavone contents in Korean black soybean landraces and their antioxidant activities. Plant Breed. Biotechnol. 2016, 4, 441–452. [Google Scholar] [CrossRef]
- Esteves, T.; Felberg, I.; Calado, V.; Carrão-Panizzi, M. Effect of Black Soymilk Processing Conditions on Anthocyanins Content. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 56–60. [Google Scholar] [CrossRef]
- Leithardt, D.R.F.; Yamashita, A.N.; Beléia, A.D.P. Determinação de isoflavonas e antocianinas em soja preta. Braz. J. Food Res. 2018, 9, 112–124. [Google Scholar] [CrossRef]
- De Moraes, F.M.L.; Hirozawa, S.S.; Prudencio, S.H.; Ida, E.I.; Garcia, S. Petit Suisse from black soybean: Bioactive compounds and antioxidant properties during development process. Int. J. Food Sci. Nutr. 2014, 65, 470–475. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Isoflavones, flavan-3-ols, phenolic acids, total phenolic profiles, and antioxidant capacities of soymilk as affected by ultra-high temperature and traditional processing methods. J. Agric. Food Chem. 2009, 57, 4706–4717. [Google Scholar] [CrossRef]
- Cheng, K.C.; Lin, J.T.; Liu, W.H. Extracts from fermented black soybean milk exhibit antioxidant and cytotoxic activities. Food Technol. Biotechnol. 2011, 49, 111–117. [Google Scholar]
- Leksono, B.Y.; Cahyanto, M.N.; Utami, T. Antioxidant activity of isoflavone aglycone from fermented black soymilk supplemented with sucrose and skim milk using Indonesian indigenous lactic acid bacteria. Appl. Food Biotechnol. 2021, 8, 285–295. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chou, C.-C. Enhancement of aglycone, vitamin K2 and superoxide dismutase activity of black soybean through fermentation with Bacillus subtilis BCRC 14715 at different temperatures. J. Agric. Food Chem. 2009, 57, 10695–10700. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.C.; Dini, J.P.; Lavandier, C.; Rupasinghe, H.P.V.; Faulkner, H.; Poysa, V.; Buzzell, D.; Degrandis, S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002, 37, 1117–1123. [Google Scholar] [CrossRef]
- Wang, H.-J.; Murphy, P.A. Mass balance study of isoflavones during soybean processing. J. Agric. Food Chem. 1996, 44, 2377–2383. [Google Scholar] [CrossRef]
- Guimarães, R.M.; Silva, T.E.; Lemes, A.C.; Boldrin, M.C.F.; da Silva, M.A.P.; Silva, F.G.; Egea, M.B. Okara: A soybean by-product as an alternative to enrich vegetable paste. LWT-Food Sci. Technol. 2018, 92, 593–599. [Google Scholar] [CrossRef]
- Slavin, M.; Kenworthy, W.; Yu, L.L. Antioxidant properties, phytochemical composition, and antiproliferative activity of Maryland-grown soybeans with colored seed coats. J. Agric. Food Chem. 2009, 57, 11174–11185. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M.T.; Franceschi, S.; Lerici, C.R. Loss and/or formation of antioxidants during processing and storage. Cancer Lett. 1997, 114, 71–74. [Google Scholar] [CrossRef]
- Feng, S.; Saw, C.L.; Lee, Y.K.; Huang, D. Novel process of fermenting black soybean [Glycine max (L.) Merrill] yogurt with dramatically reduced flatulence-causing oligosaccharides but enriched soy phytoalexins. J. Agric. Food Chem. 2008, 56, 10078–10084. [Google Scholar] [CrossRef]
- Lima, M.R.; Morais, S.A.N.; Costa, F.G.P.; Pinheiro, S.G.; Dantas, L.S.; Cavalcante, L.E. Atividade ureática. Rev. Eletrônica Nutritime. 2011, 8, 1606–1611. [Google Scholar]
- Paulsen, M.R. Measurement and maintenance of soybean quality. In Soybeans: Chemistry, Production, Processing, and Utilization; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Urbana, IL, USA, 2008; pp. 151–192. [Google Scholar] [CrossRef]
- Duarte, G.; Felberg, I.; Calado, V.; DePaula, J.; de Jesus, M.S.C.; Deliza, R.; Miguel, M.A.L.; Farah, A. Fermented Soy-coffee Pudding Dessert Containing Probiotics: Product Formulation and Evaluation of Compositional Changes during Fermentation. J. Food Nutr. Res. 2023, 11, 333–344. [Google Scholar] [CrossRef]
- Chun, J.; Know, D.-Y.; Kim, J.S.; Kim, J.-H. Sensory properties of soy yoghurts prepared from yellow and black soymilk using Streptococcus infantarius 12 and Weisellia sp. 4. J. Sci. Food Agric. 2008, 88, 1845–1849. [Google Scholar] [CrossRef]
- Felberg, I.; Deliza, R.; Farah, A.; Calado, V.; Donangelo, C.M. Formulation of a soy–coffee beverage by response surface methodology and internal preference mapping. J. Sens. Stud. 2010, 25, 226–234. [Google Scholar] [CrossRef]
- Nie, Q.; Feng, L.; Hu, J.; Wang, S.; Chen, H.; Huang, X.; Nie, S.; Xiong, T.; Xie, M. Effect of fermentation and sterilization on anthocyanins in blueberry. J. Sci. Food Agric. 2017, 97, 1459–1466. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, J.; Wang, X.; Sun, X.; Hackman, R.M.; Li, Z.; Feng, X. Evaluation of antioxidant capacity and flavor profile change of pomegranate wine during fermentation and aging process. Food Chem. 2017, 232, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Hayes, K. Beta-glycosidase activity toward different glycosidic forms of isoflavones. J. Agric. Food Chem. 2005, 53, 4918–4924. [Google Scholar] [CrossRef]
- Lim, E.S. Antioxidant capacity of small black soymilk fermented with ROS-resistant probiotics. Food Sci. Biotechnol. 2024, 34, 653–664. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Zahrani, A.J.A.; Shori, A.B. Improve the antioxidant activity and viability of B. longum and B. animalis subsp lactis in fermented soy and almond milk. Food Sci. and Technol. 2023, 43, e118122. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C.; Lee, Y.-C. Effect of lactic acid fermentation on enrichment of antioxidant properties and bioactive isoflavones in soybean. J. Food Sci. 2005, 70, 215–220. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K.; Sahoo, D. Bioactive molecules in fermented soybean products and their potential health benefits. In Fermented Foods, Part II: Technological Interventions; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 97–121. [Google Scholar]
- Pinthong, R.; Macrae, R.; Rothwell, J. The development of soya-based yoghurt. I Acid production by lactic acid bacteria. II Sensory evaluation and analysis of volatiles. J. Food Technol. 1980, 15, 647–659. [Google Scholar] [CrossRef]
- Mital, B.K.; Steinkraus, K.H. Utilization of oligosaccharides by lactic acid bacteria during fermentation of soymilk. J. Food Sci. 1975, 40, 114–118. [Google Scholar] [CrossRef]
- Trindade, C.S.F.; Terzi, S.C.; Trugo, L.C.; Modesta, R.C.D.; Couri, S. Development and sensory evaluation of soy milk based yoghurt. Arch. Latinoam. Nutr. 2001, 51, 100–104. [Google Scholar]
- Esteves, T.C.F. Desenvolvimento de alimento fermentado de soja tipo “iogurte”: Avaliação da estabilidade física. Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Rinaldoni, N.A.; Campderrós, M.E.; Pérez Padilla, A. Physicochemical and sensory properties of yogurt from ultrafiltered soymilk concentrate added with inulin. LWT-Food Sci. Technol. 2012, 45, 142–147. [Google Scholar] [CrossRef]

| Component | BS | BSM | BSY | BO |
|---|---|---|---|---|
| (g/100 g, dwb) | ||||
| Water | 8.25 ± 0.22 | 92.45 ± 0.03 | 92.76 ± 0.16 | 74.17 ± 0.18 |
| Proteins | 43.34 ± 0.07 | 49.42 ± 0.17 | 50.22 ± 0.56 | 24.56 ± 0.06 |
| Lipids | 19.90 ± 0.13 | 17.35 ± 0.01 | 20.11 ± 0.13 | 10.97 ± 0.11 |
| Ashes | 5.43 ± 0.23 | 5.84 ± 0.01 | 5.85 ± 0.05 | 3.53 ± 0.00 |
| Carbohydrates and other constituents, including dietary fiber ** | 31.34 ± 0.43 | 27.40 ± 0.17 | 23.82 ± 0.64 | 60.95 ± 0.05 |
| Sample | ORAC | DPPH (EC50) | |
|---|---|---|---|
| (μmol QE/g, dwb) | (μmol TE/g, dwb) | (mg/L, dwb) | |
| BS | 36.44 ± 2.26 a | 156.15 ± 9.67 a | 1.56 ± 0.24 a |
| BSM | 35.91 ± 1.46 a | 156.40 ± 1.45 a | 0.58 ± 0.02 b |
| BSY | 31.80 ± 0.06 a | 139.07 ± 4.17 b | 0.28 ± 0.07 b |
| Sample | Sucrose | Raffinose | Stachyose | Total |
|---|---|---|---|---|
| (g/100 g, dwb) | ||||
| BSM | 7.14 ± 0.06 a | 1.42 ± 0.01 a | 5.57 ± 0.01 a | 14.13 ± 0.06 a |
| BSY t = 0 | 0.97 ± 0.01 b | 0.69 ± 0.01 b | 5.16 ± 0.03 b | 6.83 ± 0.05 b |
| Black Soybean Yogurt | Mean Acceptance ± SD |
|---|---|
| Unflavored | 5.6 ±1.99 c |
| Prune flavor | 5.9 ± 2.37 c |
| Grape flavor | 7.0 ± 1.81 b |
| Strawberry flavor | 7.6 ± 1.51 a |
| Black Soybean Yogurt | Consumer’s Segments | ||
|---|---|---|---|
| Cluster 1 (n = 30) | Cluster 2 (n = 33) | Cluster 3 (n = 40) | |
| Unflavored | 4.1 ef | 4.7 e | 7.4 abc |
| Prune flavor | 3.2 f | 7.0 bcd | 7.0 bcd |
| Strawberry flavor | 6.4 cd | 7.7 ab | 8.3 a |
| Grape flavor | 6.5 cd | 6.0 d | 8.2 a |
| Black Soybean Yogurt | Consumer Assessors’ Frequency of Consumption | |||
|---|---|---|---|---|
| Never (n = 19) | Rarely (n = 58) | Often (n = 24) | Daily (n = 2) | |
| Unflavored | 5.6 cde | 5.3 de | 6.0 bcd | 8.0 a |
| Prune flavor | 4.8 e | 5.9 cd | 6.7 abc | 7.5 a |
| Strawberry flavor | 6.7 ab | 7.6 a | 8.0 a | 9.0 a |
| Grape flavor | 7.0 ab | 6.7 ab | 7.5 a | 8.5 a |
| Anthocyanins | BSM | BSY t = 0 | BSY t = 14 | BSY t = 28 |
|---|---|---|---|---|
| (g/100 g, dwb) | ||||
| Delphinidin-3-glucoside | ND | ND | ND | ND |
| Cyanidin-3-glucoside | 29.94 ± 1.59 a | 23.26 ± 0.19 b | 20.19 ± 0.25 c | 19.10 ± 0.49 c |
| Petunidin-3-glucoside | 3.61 ± 0.14 a | 3.62 ± 0.02 a | 2.78 ± 0.28 b | 1.95 ± 0.19 c |
| Anthocyanins Total | 33.55 ± 1.73 a | 26.88 ± 0.21 b | 22.97 ± 0.53 c | 21.05 ± 0.30 c |
| Isoflavones | BSM | BSY t = 0 | BSY t= 14 | BSY t = 28 | |
|---|---|---|---|---|---|
| (mg/100 g, dwb) | |||||
| Glucosides | Daidzin | 41.95 ± 0.50 a | 40.27± 1.19 a | 38.72 ± 2.42 a | 40.33 ± 0.50 a |
| Glyciti n | 20.14 ± 0.23 a | 20.52± 0.58 a | 19.61 ± 1.28 a | 20.48 ± 0.25 a | |
| Genistin | 71.52 ± 0.56 a | 60.84± 0.87 b | 58.40 ± 2.39 b | 59.91 ± 0.71 b | |
| Aglicones | Daidzein | 1.46± 0.02 a | 4.78± 0.35 b | 4.59 ± 0.44 b | 5.11± 0.02 b |
| Glycitein | 0.63± 0.05 a | 1.45± 0.05 b | 1.40 ± 0.12 b | 1.52± 0.00 b | |
| Genistein | 1.69± 0.00 a | 12.68± 1.09 b | 12.16 ± 1.23 b | 13.45 ± 0.14 b | |
| Total Isoflavones | 137.39 ± 1.35 a | 140.53 ± 4.14 a | 134.86 ± 7.89 a | 140.81 ± 1.61 a | |
| Sample | ORAC (μmol QE/g, dwb) | ORAC (μmol TE/g, dwb) | DPPH (EC50) (mg/L, dwb) |
|---|---|---|---|
| BSM | 35.91 ± 1.46 a | 156.40 ± 1.45 a | 0.58 ± 0.02 a |
| BSY t = 0 | 31.80 ± 0.06 b | 139.07 ± 4.17 b | 0.28 ± 0.07 b |
| BSY t = 14 | 36.78 ± 1.48 a | 156.48 ± 4.73 a | 0.22 ± 0.04 b |
| BSY t = 28 | 34.28 ± 0.45 ab | 150.51 ± 2.49 a | 0.18 ± 0.03 b |
| Sample | L. acidophilus LA-5® (UFC/g) | L. acidophilus LA-5® (UFC/Portion of 200 g) |
|---|---|---|
| BSM | 0.0 | 0.0 |
| BSY t = 0 | 7.30 × 107 | 1.46 × 1010 |
| BSY t = 14 | 4.80 × 106 | 9.6 × 108 |
| BSY t = 28 | 2.20 × 105 | 4.40 × 107 |
| Sample | Sucrose | Raffinose | Stachyose | Total |
|---|---|---|---|---|
| (g/100 g ± SD, dwb) | ||||
| BSM | 7.14 ± 0.06 a | 1.42 ± 0.01 a | 5.57 ± 0.01 a | 14.13 ± 0.06 a |
| BSY t = 0 | 0.97 ± 0.01 b | 0.69 ± 0.01 b | 5.16 ± 0.03 b | 6.83 ± 0.05 b |
| BSYt = 14 | 0.61 ± 0.01 c | 0.71 ± 0.01 b | 4.74 ± 0.01 c | 6.06 ± 0.01 c |
| BSYt = 28 | 0.30 ± 0.01 d | 0.70 ± 0.01 d | 4.27 ± 0.02 d | 5.27 ± 0.03 d |
| Sample | pH | Total Acidity in Lactic Acid (g/100 g) |
|---|---|---|
| BSM | 6.46 ± 0.00 | 0.10 ± 0.00 |
| BSY t = 0 | 4.50 ± 0.00 | 0.44 ± 0.00 |
| BSY t = 14 | 4.56 ± 0.00 | 0.44 ± 0.01 |
| BSY t = 28 | 4.59 ± 0.00 | 0.44 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, T.C.F.; Felberg, I.; Farah, A.; Faria-Machado, A.F.d.; Walter, E.H.M.; Santiago, M.C.P.d.A.; Pacheco, S.; Antoniassi, R.; Deliza, R.; Carrão-Panizzi, M.C.; et al. Formulation of Black Soybean Yogurt and Evaluation of Changes in the Bioactive Profile and Other Compositional Aspects During Fermentation and Storage. Beverages 2025, 11, 103. https://doi.org/10.3390/beverages11040103
Esteves TCF, Felberg I, Farah A, Faria-Machado AFd, Walter EHM, Santiago MCPdA, Pacheco S, Antoniassi R, Deliza R, Carrão-Panizzi MC, et al. Formulation of Black Soybean Yogurt and Evaluation of Changes in the Bioactive Profile and Other Compositional Aspects During Fermentation and Storage. Beverages. 2025; 11(4):103. https://doi.org/10.3390/beverages11040103
Chicago/Turabian StyleEsteves, Thiana Claudia Freire, Ilana Felberg, Adriana Farah, Adelia Ferreira de Faria-Machado, Eduardo Henrique Miranda Walter, Manuela Cristina Pessanha de Araujo Santiago, Sidney Pacheco, Rosemar Antoniassi, Rosires Deliza, Mercedes Concórdia Carrão-Panizzi, and et al. 2025. "Formulation of Black Soybean Yogurt and Evaluation of Changes in the Bioactive Profile and Other Compositional Aspects During Fermentation and Storage" Beverages 11, no. 4: 103. https://doi.org/10.3390/beverages11040103
APA StyleEsteves, T. C. F., Felberg, I., Farah, A., Faria-Machado, A. F. d., Walter, E. H. M., Santiago, M. C. P. d. A., Pacheco, S., Antoniassi, R., Deliza, R., Carrão-Panizzi, M. C., & Calado, V. (2025). Formulation of Black Soybean Yogurt and Evaluation of Changes in the Bioactive Profile and Other Compositional Aspects During Fermentation and Storage. Beverages, 11(4), 103. https://doi.org/10.3390/beverages11040103








