Comparative Analysis of Chemical Composition and Antioxidant Activity in Conventional, Civet, and Elephant Coffees: Is There a Definitive Authentication Marker of Elephant Coffee?
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. Sample Preparation
2.3. Chemicals, Reagents, and Standards
2.4. In Vitro Antioxidant Activity
2.4.1. Inhibition of DPPH Radical
2.4.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.4.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4.4. Total Phenolic Content
2.4.5. Total Flavonoid Content
2.5. Chemical Composition
2.5.1. HPLC-UV Analysis of Caffeine and Polyphenols
2.5.2. GC-SMPE-MS Analysis of Coffee Volatiles
2.6. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.2. Content of Caffeine and Phenolic Compounds (HPLC-UV Analysis)
3.3. Profile of Volatile Compounds (GC-SPME-MS)
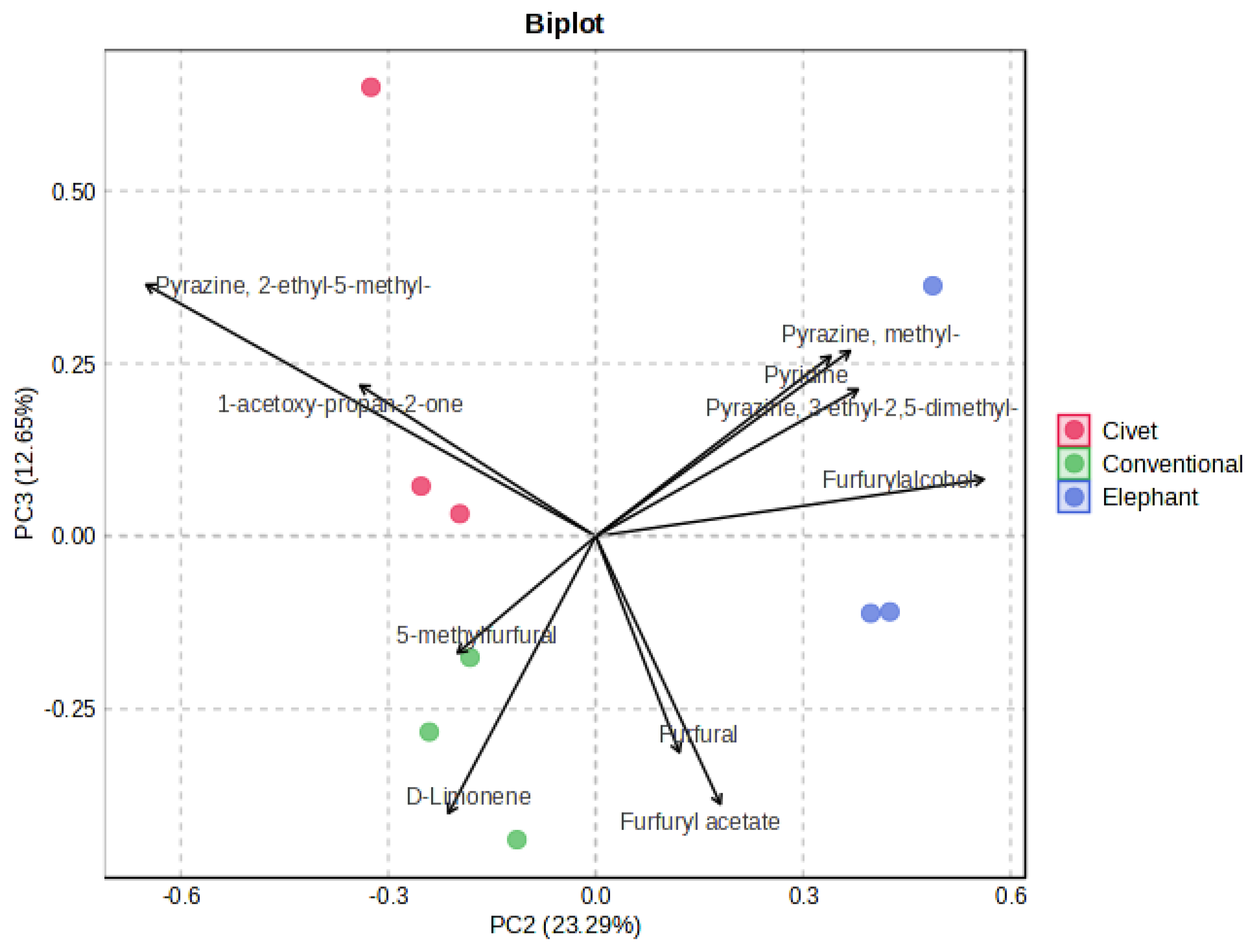
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ridder, M. Coffee Consumption Worldwide from 2012/13 to 2021/22 with a Forecast to 2022/23. Available online: https://www.statista.com/statistics/292595/global-coffee-consumption/ (accessed on 30 October 2023).
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Febrianto, N.A.; Zhu, F. Coffee Bean Processing: Emerging Methods and Their Effects on Chemical, Biological and Sensory Properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef]
- Farag, M.A.; Mohamed, T.A.; El-Hawary, E.A.; Abdelwareth, A. Metabolite Profiling of Premium Civet Luwak Bio-Transformed Coffee Compared with Conventional Coffee Types, as Analyzed Using Chemometric Tools. Metabolites 2023, 13, 173. [Google Scholar] [CrossRef]
- Adadi, P.; Mensah, E.O.; Blay, B.; Ahmmed, M.K.; Sumaiya, K.; Agyei, D.; Kebede, B. Advancements in Civet Coffee Production and Analytical Techniques: From Aroma Profiling to Market Dynamics and Ethical Considerations. Trends Food Sci. Technol. 2024, 154, 104772. [Google Scholar] [CrossRef]
- Jumhawan, U.; Putri, S.P.; Bamba, T.; Fukusaki, E. Quantification of Coffee Blends for Authentication of Asian Palm Civet Coffee (Kopi Luwak) via Metabolomics: A Proof of Concept. J. Biosci. Bioeng. 2016, 122, 79–84. [Google Scholar] [CrossRef]
- Wang, X.; Lim, L.-T.; Fu, Y. Review of Analytical Methods to Detect Adulteration in Coffee. J. AOAC Int. 2020, 103, 295–305. [Google Scholar] [CrossRef]
- Toci, A.T.; Farah, A.; Pezza, H.R.; Pezza, L. Coffee Adulteration: More than Two Decades of Research. Crit. Rev. Anal. Chem. 2016, 46, 83–92. [Google Scholar] [CrossRef]
- Downey, G. Advances in Food Authenticity Testing, 1st ed.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Held, P. Performing Oxygen Radical Absorbance Capacity Assays with synergyTMHT: ORAC Antioxidant Tests. Application Note; Biotek Instruments: Winooski, VT, USA, 2005. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar]
- Christ, B.; Müller, K.H. On the Serial Determination of the Content of Flavonol Derivatives in Drugs. Arch. Pharm. 1960, 293, 1033–1042. [Google Scholar] [CrossRef]
- Tauchen, J.; Marsik, P.; Kvasnicova, M.; Maghradze, D.; Kokoska, L.; Vanek, T.; Landa, P. In Vitro Antioxidant Activity and Phenolic Composition of Georgian, Central and West European Wines. J. Food Compos. Anal. 2015, 41, 113–121. [Google Scholar] [CrossRef]
- Frankova, A.; Manourova, A.; Kotikova, Z.; Vejvodova, K.; Drabek, O.; Riljakova, B.; Famera, O.; Ngula, M.; Ndiyoi, M.; Polesny, Z.; et al. The Chemical Composition of Oils and Cakes of Ochna Serrulata (Ochnaceae) and Other Underutilized Traditional Oil Trees from Western Zambia. Molecules 2021, 26, 5210. [Google Scholar] [CrossRef]
- Várady, M.; Tauchen, J.; Fraňková, A.; Klouček, P.; Popelka, P. Effect of Method of Processing Specialty Coffee Beans (Natural, Washed, Honey, Fermentation, Maceration) on Bioactive and Volatile Compounds. LWT 2022, 172, 114245. [Google Scholar] [CrossRef]
- Kováts, E. Gas-Chromatographische Charakterisierung Organischer Verbindungen. Teil 1: Retentionsindices Aliphatischer Halogenide, Alkohole, Aldehyde Und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. [Google Scholar]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Thammarat, P.; Kulsing, C.; Wongravee, K.; Leepipatpiboon, N.; Nhujak, T. Identification of Volatile Compounds and Selection of Discriminant Markers for Elephant Dung Coffee Using Static Headspace Gas Chromatography—Mass Spectrometry and Chemometrics. Molecules 2018, 23, 1910. [Google Scholar] [CrossRef]
- Haile, M.; Bae, H.M.; Kang, W.H. Comparison of the Antioxidant Activities and Volatile Compounds of Coffee Beans Obtained Using Digestive Bio-Processing (Elephant Dung Coffee) and Commonly Known Processing Methods. Antioxidants 2020, 9, 408. [Google Scholar] [CrossRef]
- Wei, F.; Tanokura, M. Chemical Changes in the Components of Coffee Beans during Roasting. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 83–91. [Google Scholar]
- Costa Freitas, A.M.; Mosca, A.I. Coffee Geographic Origin-An Aid to Coffee Differentiation. Food Res. Int. 1999, 32, 565–573. [Google Scholar] [CrossRef]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace Solid-Phase Microextraction-Gas Chromatographic-Time-of-Flight Mass Spectrometric Methodology for Geographical Origin Verification of Coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef]
- Iswanto, T.; Shovitri, M.; Altway, A.; Widjaja, T.; Lisdiyanti, P.; Putra, A.P. Assessment of Caffeine-Degrading Ability on Bacterial Strains Klebsiella Sp. Isolated from Feces of Asian Palm Civet (Luwak). Int. J. Technol. 2023, 14, 100–108. [Google Scholar] [CrossRef]
- Nishiguchi, Y.; Goromaru-shinkai, M.; Kuroda, J.; Kiuchi, S.; Ihara, H. Estimation of Protein, Total Polyphenol, Chlorogenic Acid, Caffeine, and Caffeic Acid Contents in Indonesian Palm Civet Coffee. Int. J. Anal. Bio-Sci. 2017, 5, 53–56. [Google Scholar]
- Febrina, L.; Happyana, N.; Syah, Y.M. Metabolite Profiles and Antidiabetic Activity of the Green Beans of Luwak (Civet) Coffees. Food Chem. 2021, 355, 129496. [Google Scholar] [CrossRef]
- Leino, M.; Kaitaranta, J.; Kallio, H. Comparison of Changes in Headspace Volatiles of Some Coffee Blends during Storage. Food Chem. 1992, 43, 35–40. [Google Scholar] [CrossRef]
- Sakpetch, P.; Benchama, O.; Masniyom, P.; Salaipeth, L.; Kanjan, P. Physicochemical Characteristics and Flavor Profiles of Fermented Fish Sauce (Budu) during Fermentation in Commercial Manufacturing Plant. J. Food Sci. Technol. 2022, 59, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Pennington, T.; Eshima, J.; Smith, B.S. Identification of Volatile Metabolites Produced from Levodopa Metabolism by Different Bacteria Strains of the Gut Microbiome. BMC Microbiol. 2024, 24, 260. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhou, Q.; Cheng, C.; Xing, Z.; Zhou, Y.; Liu, X.; Hua, S.; Wei, W.; Tan, J.; et al. Characterization of Key Aroma Compounds in Cold Brew Coffee Prepared by Negative-Pressure Extraction Technology and Its Changes during Storage. LWT 2024, 197, 115919. [Google Scholar] [CrossRef]
- Wu, H.; Viejo, C.G.; Fuentes, S.; Dunshea, F.R.; Suleria, H.A.R. The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies. Fermentation 2023, 9, 68. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and Chemical Characteristics of Wet Coffee Fermentation Inoculated With Hansinaspora Uvarum and Pichia Kudriavzevii and Their Impact on Coffee Sensory Quality. Front. Microbiol. 2021, 12, 713969. [Google Scholar] [CrossRef]
- Rappert, S.; Botsch, K.C.; Nagorny, S.; Francke, W.; Müller, R. Degradation of 2,3-Diethyl-5-Methylpyrazine by a Newly Discovered Bacterium, Mycobacterium Sp. Strain DM-11. Appl. Environ. Microbiol. 2006, 72, 1437–1444. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Wang, H.; Fang, G.; Li, Y.; Zhang, J.; Liu, K. Microbial Characteristics and Functions in Coffee Fermentation: A Review. Fermentation 2025, 11, 5. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on 3-Ethyl-2-Hydroxy-2-Cyclopenten-1-One. Food Chem. Toxicol. 2012, 50, S646–S649. [Google Scholar] [CrossRef]
- Feng, X.; Hua, R.; Zhang, W.; Liu, Y.; Luo, C.; Li, T.; Chen, X.; Zhu, H.; Wang, Y.; Lu, Y. Comparison of the Gut Microbiome and Resistome in Captive African and Asian Elephants on the Same Diet. Front. Vet. Sci. 2023, 10, 986382. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. NMR-Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar] [CrossRef]
| Sample | DPPH | ORAC | ABTS | TPC | TFC |
|---|---|---|---|---|---|
| μg TE/mg | μg GAE/mg | μg QE/mg | |||
| Conventional coffee | 44.14 ± 13.28 a | 289.99 ± 75.93 a | 125.97 ± 14.22 a | 34.91 ± 4.88 a | 2.15 ± 0.46 a |
| Civet coffee | 32.26 ± 7.97 ab | 314.19 ± 79.60 a | 104.78 ± 15.76 b | 32.69 ± 10.64 b | 1.73 ± 0.40 ab |
| Elephant coffee | 19.67 ± 2.86 b | 250.85 ± 60.35 a | 81.84 ± 7.78 c | 26.35 ± 3.08 b | 1.38 ± 0.28 b |
| Sample | Caffeine | Chlorogenic Acid | Gallic Acid | Cinnamic Acid | Caffeic Acid |
|---|---|---|---|---|---|
| % of dry weight | |||||
| Conventional coffee | 1.33 ± 0.13 a | 0.36 ± 0.04 ab | 0.017 ± 0.0006 a | 0.011 ± 0.0015 a | 0.020 ± 0.002 a |
| RSD (%) | 10.10 | 11.06 | 3.89 | 13.25 | 11.03 |
| Civet coffee | 0.93 ± 0.07 b | 0.44 ± 0.03 a | 0.016 ± 0.0001 a | 0.010 ± 0.0004 a | 0.019 ± 0.001 a |
| RSD (%) | 7.40 | 6.81 | 0.93 | 3.57 | 6.07 |
| Elephant coffee | 0.93 ± 0.03 b | 0.33 ± 0.01 b | 0.018 ± 0.0003 a | 0.009 ± 0.0001 a | 0.017 ± 0.001 a |
| RSD (%) | 2.87 | 4.02 | 1.67 | 1.04 | 4.97 |
| Compound | RI (Measured) 1 | RI (Literature) 2 | Content (% RPA) | ||
|---|---|---|---|---|---|
| Conventional Coffee | Civet Coffee | Elephant Coffee | |||
| 2-methylfuran | 611 | 603 | n.d. | 0.64 ± 0.06 a | 0.94 ± 0.03 a |
| acetic acid | 618 | 602 | 1.33 ± 0.18 a | 1.14 ± 0.19 a | 1.55 ± 0.23 a |
| 3-methylbutanal | 647 | 649 | n.d. | 0.11 ± 0.01 a | 0.185 ± 0.004 a |
| 2-methylbutanal | 654 | 656 | n.d. | 0.64 ± 0.28 a | 1.18 ± 0.32 a |
| pentan-2,3-dione | 684 | 696 | 0.13 ± 0.02 a | 0.28 ± 0.12 a | 0.30 ± 0.11 a |
| propanoic acid | 701 | 702 | 0.34 ± 0.15 a | 0.21 ± 0.06 a | 0.36 ± 0.09 a |
| pyrazine | 724 | 672 | 0.20 ± 0.05 a | 0.28 ± 0.03 ab | 0.43 ± 0.03 b |
| pyridine | 737 | 740 | 2.68 ± 0.44 a | 5.65 ± 0.78 b | 6.28 ± 0.06 b |
| methylpyrazine | 824 | 824 | 6.97 ± 0.21 a | 7.26 ± 0.56 a | 8.84 ± 1.01 a |
| furfural | 839 | 829 | 8.63 ± 0.28 a | 4.65 ± 0.36 b | 7.00 ± 0.62 a |
| furfuryl alcohol | 862 | 866 | 30.79 ± 1.95 | 23.72 ± 4.14 | 32.73 ± 3.14 |
| 1-acetoxy-propan-2-one | 872 | 876 | 4.86 ± 0.35 | 5.12 ± 1.16 | 3.57 ± 0.38 |
| 2-methylbutanoic acid | 878 | 886.2 | 0.32 ± 0.05 | 0.36 ± 0.12 | 0.20 ± 0.03 |
| furfuryl formate, | 909 | 902 | 1.63 ± 0.39 | 1.52 ± 0.45 | 1.09 ± 0.17 |
| 5-methylfurfural | 968 | 969 | 21.21 ± 2.37 | 14.43 ± 2.23 | 15.77 ± 2.25 |
| furfuryl acetate | 996 | 998 | 14.46 ± 0.00 | 20.96 ± 0.56 | 18.00 ± 0.03 |
| 2-ethyl-5-methylpyrazine | 1003 | 1004 | 2.69 ± 0.39 a | 7.01 ± 1.06 b | n.d. |
| N-acetyl-4H-pyridine | 1024 | 1038 | 1.28 ± 0.13 | 0.86 ± 0.13 | 1.13 ± 0.25 |
| (+)-limonene | 1033 | 1035 | 4.53 ± 0.63 a | 0.41 ± 0.06 b | 0.82 ± 0.21 b |
| 2,3-dimethyl-2-cyclopenten-1-one | 1044 | 1052 | 0.95 ± 0.16 | 0.81 ± 0.14 | 0.67 ± 0.21 |
| 1-(1H-pyrrol-2-yl)-ethanone | 1067 | 1063 | 0.37 ± 0.02 | 0.44 ± 0.08 | 0.46 ± 0.06 |
| 3-ethyl-2,5-dimethylpyrazine | 1081 | 1083 | 1.94 ± 0.21 | 2.16 ± 0.38 | 3.73 ± 0.63 |
| 3-ethyl-2-hydroxy-2-cyclopenten-1-one | 1098 | 1082 | 0.48 ± 0.03 a | 0.73 ± 0.02 b | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájíček, J.; Hoca, G.; Várady, M.; Maršík, P.; Fraňková, A.; Tauchen, J. Comparative Analysis of Chemical Composition and Antioxidant Activity in Conventional, Civet, and Elephant Coffees: Is There a Definitive Authentication Marker of Elephant Coffee? Beverages 2025, 11, 79. https://doi.org/10.3390/beverages11030079
Hájíček J, Hoca G, Várady M, Maršík P, Fraňková A, Tauchen J. Comparative Analysis of Chemical Composition and Antioxidant Activity in Conventional, Civet, and Elephant Coffees: Is There a Definitive Authentication Marker of Elephant Coffee? Beverages. 2025; 11(3):79. https://doi.org/10.3390/beverages11030079
Chicago/Turabian StyleHájíček, Jan, Gökçe Hoca, Matúš Várady, Petr Maršík, Adéla Fraňková, and Jan Tauchen. 2025. "Comparative Analysis of Chemical Composition and Antioxidant Activity in Conventional, Civet, and Elephant Coffees: Is There a Definitive Authentication Marker of Elephant Coffee?" Beverages 11, no. 3: 79. https://doi.org/10.3390/beverages11030079
APA StyleHájíček, J., Hoca, G., Várady, M., Maršík, P., Fraňková, A., & Tauchen, J. (2025). Comparative Analysis of Chemical Composition and Antioxidant Activity in Conventional, Civet, and Elephant Coffees: Is There a Definitive Authentication Marker of Elephant Coffee? Beverages, 11(3), 79. https://doi.org/10.3390/beverages11030079








