Abstract
Probiotic microorganisms from sugary kefir were incorporated into Brazilian non-alcoholic beers to enhance their functional and nutritional properties through aerobic static fermentation over 24 h. Non-alcoholic beers inoculated with sugary kefir showed appropriate acidity (pH reduction from ~3.74 to ~3.52), color, and microbial balance, along with excellent sensory acceptance (scores of 6.9–8.4 on a 9-point hedonic scale). The kefir microbiota included Lacticaseibacillus paracasei, Lacticaseibacillus casei, Lacticaseibacillus paracasei subsp. paracasei, Lacticaseibacillus paracasei subsp. tolerans, Lactobacillus delbrueckii subsp. lactis, Lentilactobacillus parabuchneri, Lentilactobacillus kefiri, Lactococcus lactis, Leuconostoc citreum, Acetobacter lovaniensis, and yeasts such as Saccharomyces cerevisiae, Kluyveromyces lactis, Lachancea meyersii, and Kazachstania aerobia. Genetic analysis confirmed the absence of undesirable or pathogenic microorganisms. Fermentation led to reductions in sucrose (~0.35 to ~0.22 g/L) and °Brix (~5.55 to ~3.80), with increases in lactic acid (~0.55 to ~1.25 g/L) and acetic acid (~0.08 to ~0.14 g/L), confirming active microbial metabolism. Ethanol levels remained within legal limits for non-alcoholic beverages. The process preserved sensory attributes while enriching the beverage with well-documented kefir microorganisms. These findings highlight sugary kefir as a promising biotechnological tool to enhance the functional profile of non-alcoholic beers without compromising their sensory quality.
1. Introduction
Currently, consumer awareness of natural/healthy food consumption has increased significantly. Therefore, there has been great interest in the development of new types of functional foods/beverages with functional, probiotic potential and lower-alcohol beverages [1,2,3,4]. As an example of a non-alcoholic beverage, non-alcoholic beer has been growing in the world market [4,5].
The evolution of craft beer has led consumers to seek new styles, including non-alcoholic options [4,5]. This shift is influenced by lifestyle, health, and safety concerns [4,5,6,7]. Additionally, there is growing interest in functional and probiotic products that combine pleasure with health benefits [1,2,4]. As a rich source of probiotic microorganisms, kefir represents a promising ingredient for enhancing the health value of foods and beverages [1,2,7,8,9,10,11,12,13,14,15,16,17].
Sugary kefir beverage can be considered a dairy-free probiotic beverage because it contains lactic acid bacteria (LAB), acetic acid bacteria (AAB), and yeasts that help to maintain a healthy gut microbiota and the kefir grain grown in a solution containing sugar and water [4,7,10,14]. Sugary kefir grains consist of kefiran, a dextran matrix, and probiotic microorganisms [4,7,10,14]. The sugary kefir grains are jelly-like, translucent, and yellowish [4,7,10,14] (Figure 1). The sugary kefir beverage is appropriate for consumption at room temperature or refrigerated at 4 °C [2].
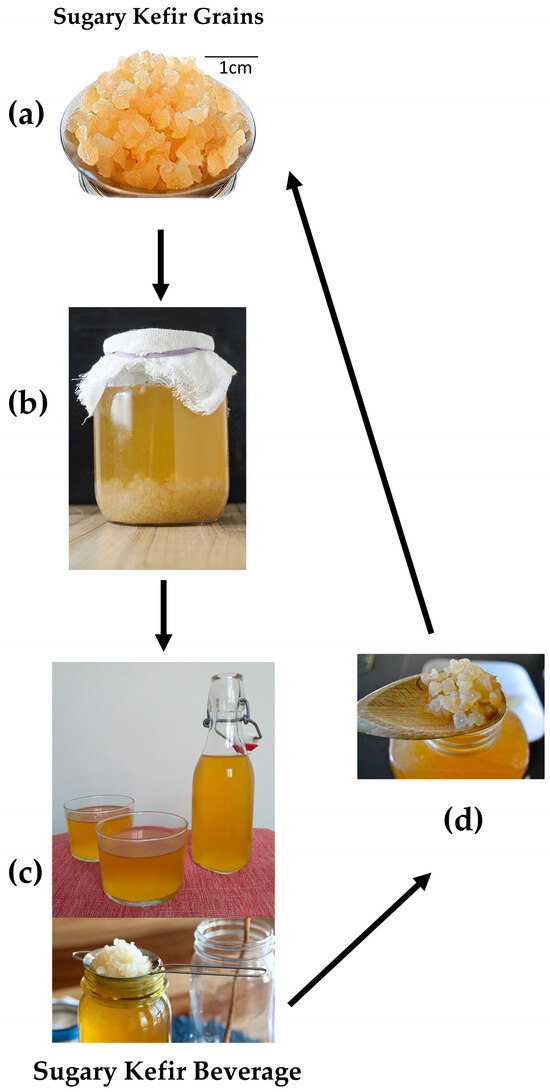
Figure 1.
Sugary kefir beverage production. Sugary kefir grains (a). Sugary kefir grains fermentation (b). Sugary kefir beverage (c). Sugary kefir grains recovered for re-fermentation (d).
Non-alcoholic beers have emerged as a promising matrix for functional enrichment due to the growing consumer demand for healthier, low-calorie beverages that still offer complexity in flavor and social appeal [1,2,3,4,5]. Recent trends show a significant increase in the consumption of non-alcoholic beers, driven by lifestyle shifts toward wellness, moderation in alcohol intake, and interest in gut health [1,2,3,4,5]. Within this context, incorporating probiotics into non-alcoholic beers aligns with consumer interest in multifunctional products that combine pleasure with health benefits.
Previous studies have explored the addition of probiotics in various non-dairy matrices, including fruit juices, teas, and kefir [1,2,7,8,9,10,11,12,13,14,15,16,17]. However, attempts to integrate probiotics—particularly kefir grains—into non-alcoholic beers remain limited. Most existing research focuses on traditional dairy-based kefir or fermented beverages outside the scope of beer matrices [1,2,7,8,9,10,11,12,13,14,15,16,17]. To date, there is a notable gap in the literature regarding the use of kefir cultures in non-alcoholic beers, especially within the Brazilian market, where both craft beer and probiotic consumption are gaining popularity. This study aims to fill this gap by evaluating the viability and sensory impact of kefir inoculation in non-alcoholic beer, offering a novel approach to functional beverage development.
Therefore, this study aimed to evaluate the feasibility of using Brazilian commercial non-alcoholic beers as substrates for the inoculation of sugary kefir grains, with the goal of developing beverages with potential functional properties. Microbial viability and taxonomic identification were assessed, alongside physicochemical analyses conducted before and after the fermentation process. It is important to highlight that the primary objective was to characterize the resulting kefir-enriched non-alcoholic beers, rather than to scientifically validate their health benefits in humans. Further functional evaluations, including in vitro and in vivo studies, are planned as part of the continuation of this research.
2. Materials and Methods
2.1. Materials
Three commercial brands of “Non-Alcoholic Beers—NAB”, commonly consumed in Brazil, were purchased at the local market in Salvador, Bahia, Brazil. The beers were named: NAB1, NAB2, and NAB3. The sugary kefir grains come from the Federal University of the Recôncavo Baiano (UFRB), Cruz das Almas, BA, Brazil. Brown sugar was sourced from the local market of Salvador, BA, Brazil.
2.2. Substrate for Sugary Kefir Grains Adaptation
Over a seven-day period, sugary kefir grains were adapted in a brown sugar solution (12 °Brix) prepared with water in triplicate. Each Erlenmeyer flask was filled with 125 mL of the sugar solution, to which 12.5 g of sugary kefir grains were added, resulting in a final concentration of 10% (w/w), assuming a density of approximately 1 g/mL. The fermentation was conducted at 25 °C in a CIENLAB BOD incubator (model CE-300F, Cinelab, São Paulo, SP, Brazil), under static conditions with ambient aeration. The substrate (12 °Brix brown sugar solution) was renewed every 24 h. All materials and solutions used in the experiment were sterilized by autoclaving at 121 °C for 20 min to ensure aseptic conditions.
2.3. Inoculation Process of Sugary Kefir Grains in Commercial Non-Alcoholic Beers
After the adaptation period, sugary kefir grains were inoculated at a rate of 10% of the volume of the non-alcoholic beers in triplicate (corresponding to the volume of each commercial beer brand analyzed). The fermentation process took place in a BOD CIENLAB incubator (model CE-300F, Cinelab, São Paulo, SP, Brazil) at a temperature of 25 °C, under static conditions with ambient aeration and for a period of 24 h. After the fermentation process, the kefir grains were recovered through a sieve, and the non-alcoholic beers were properly closed with plastic film and stored at 4 °C in a BOD CIENLAB (model CE-300F, Cinelab, São Paulo, SP, Brazil) for subsequent microbiological and physical-chemical analyses. The experiments were performed in triplicate for each non-alcoholic beer brand. The controls used were commercial beers without sugary kefir grains inoculation. Physicochemical and microbiological analyses were performed at T0 and T24 (for control and experimental samples).
2.4. Fermentation Kinetics: Physicochemical Analyses
The quantification of soluble solids was conducted using a digital refractometer (model DR 201-95, Kruss, Matthews, NC, EUA), operating within a range of 0 to 32 °Brix, and the results were expressed in °Brix. The pH values were assessed via direct potentiometric readings using a calibrated digital pH meter (model K39-1014B, Kasvi, São José dos Pinhais, PR, Brazil). To evaluate titratable acidity, 10 mL of each non-alcoholic beer sample (including controls and post-fermentation) was transferred to an Erlenmeyer flask, followed by the addition of 50 mL of distilled water and three drops of phenolphthalein indicator. The solution was titrated with 0.1 N sodium hydroxide until the appearance of a persistent pale pink coloration, indicating the endpoint of the titration [2,17].
The identification and quantification of sucrose, lactic acid, acetic acid, and ethanol were performed using High-Performance Liquid Chromatography (HPLC, Series 200, Perkin Elmer, Waltham, MA, EUA), equipped with both a refractive index detector (RID) and a UV/Vis detector. Chromatographic separation was achieved using a Rezex ROA-Organic Acid H+ column (300 × 7.8 mm, Phenomenex, Torrance, CA, USA), with the thermostat at 65 °C. The mobile phase consisted of 5 mM sulfuric acid (H2SO4) in isocratic elution at a flow rate of 0.6 mL/min. Prior to injection, all samples underwent centrifugation at 10,000 rpm for 10 min and were subsequently filtered through 0.22 μm pore-size membrane filters to remove particulate matter. Each chromatographic run used a 20 μL injection volume, and analyses were conducted in triplicate. RID was employed for the detection of ethanol and sugars, while the UV/Vis detector, set at 210 nm, was used for monitoring organic acids. Quantification of analytes was carried out through external calibration with analytical-grade standards: sucrose (0.1–2.0 g/L), lactic acid (0.1–2.0 g/L), acetic acid (0.05–1.0 g/L), and ethanol (0.1–5.0 g/L). All calibration curves demonstrated excellent linearity, with coefficients of determination (R2) exceeding 0.99.
2.5. Microbial Genetic Profile Analysis and Species-Level Detection
The genetic profile of the microbial community (bacteria and yeasts) of this study was performed according to methodologies of previous studies [18,19] and compared with the results of genetic profiles obtained by Tavares et al. [20]. Samples of sugary kefir grains and non-alcoholic beers (controls, T0 and T24) were collected for microbial genetic profile analysis [20]. GenBank research (http://www.ncbi.nlm.nih.gov/BLAST/) (accessed on 12 December 2024) was performed to determine the closest known relatives of the ribosomal DNA sequences obtained in this study [21].
2.6. Comparative Analysis of Color Scales for Non-Alcoholic Beers According to the EBC (European Brewing Convention) and SRM (Standard Reference Method)
The color evaluation of non-alcoholic beers was carried out according to the EBC (European Brewing Convention) and SRM (Standard Reference Method) color scales, accepted by Brazilian legislation that establishes the identity and quality standards for brewery products, “MAPA—Ministry of Agriculture, Livestock and Supply. Normative Instruction No. 65, of December 10, 2019. Establishes the identity and quality standards for beverages. Official Gazette of the Union (DOU), Brasília, Brazil, 11 December 2019. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-n-65-de-10-de-dezembro-de-2019-232666262 (accessed on 12 December 2024)”. Beers were compared according to T0 and T24.
To evaluate the color, a CR-5—Konica Minolta benchtop colorimeter was used, previously calibrated on a white surface. The color parameter used was L*. The L value provides luminosity, ranging from white (L = 100) to black (L = 0). L = 100 corresponds to the lowest values on the EBC and SRM scales, and L = 0 corresponds to the highest values on the EBC and SRM scales.
2.7. Sensory Analysis
A sensory evaluation of the non-alcoholic beer samples (control, T0, and T24) was conducted with the participation of 100 untrained assessors. The panel consisted of male and female volunteers between 20 and 55 years of age, including students, faculty, and technical staff from the Federal University of Bahia, Brazil [20]. Each participant assessed the samples using a structured 9-point hedonic scale, where 1 corresponded to “dislike extremely” and 9 to “like extremely”. The attributes evaluated included overall acceptability, appearance, color, flavor, and texture. To analyze the relative prominence of each sensory descriptor mentioned, Smith’s Salience Index was applied to calculate the total salience of the attributes cited by all participants.
The project entitled “Kefir Sensory Analysis” is in accordance with the “Declaration of Helsinki (DoH)” from October 2024, and was approved by the Research Ethics Committee of the School of Nutrition at the Federal University of Bahia, under protocol number 1,759,169. The approval was issued on 27 September 2016 and pertains to studies involving “Kefir–Grains and Beverages”. According to the Committee’s statement, “any and all research involving Kefir–Grains and Beverages is authorized for sensory evaluation in participants from the general population, provided they do not present pre-existing chronic pathologies”. Furthermore, the approval is granted with permanent validity, contingent upon strict adherence to the ethical principles governing sensory analysis research and for studies involving humans.
All participants completed an Informed Consent Statement prior to participation. The forms were filled out anonymously, and no personal identifiers were collected. As such, individual authorization for publication is not required since the confidentiality and anonymity of the participants were fully preserved, and no individual can be identified in the results.
2.8. Microbial Shelf Life Analysis of Fermented Non-Alcoholic Beers
Samples of fermented non-alcoholic beers were analyzed for microbial shelf life at times T0 (fermented non-alcoholic beers), T10, T20, and T30, according to Tavares et al. [2], Ferreira et al. [17], and APHA [18]. Different microorganism groups were enumerated using the surface spread technique [2,17,18]. Microbial enumeration was carried out in six culture media types. To characterize lactic acid bacteria (LAB) genera, De Man, Rogosa, and Sharpe Agar (MRS, Oxoid, Hampshire, UK) was used to enumerate Lactobacillus, Lacticaseibacillus, and Lentilactobacillus genera. M17 agar (Oxoid, Hampshire, UK) was used to enumerate Lactococcus genus, and LUSM medium (Sigma, St. Louis, MO, USA) was used to enumerate Leuconostoc genus. Edwards medium (Sigma, St. Louis, USA) was used to enumerate Streptococcus genus, and 254-medium (Sigma, St. Louis, MO, USA) was used to enumerate Acetobacter genus. All bacterial media were supplemented with 0.4 mg/mL nystatin (Sigma, St. Louis, MO, USA). For yeast enumeration, the Sabouraud Agar medium supplemented with 50 mg/L of chloramphenicol (Sigma, St. Louis, MO, USA) was used. After spreading, plates were incubated at 30 °C for 48 h for bacteria (aerobic cultivation except for MRS) (anaerobic cultivation in anaerobic chamber-Anaerobe Systems, Morgan Hill, CA, USA), and 120 h for yeasts (aerobic cultivation). Colony-forming units (CFU/mL) were quantified [2,17,18].
The Maldi-Tof MS proteomic technique was used to identify microbial isolates at the species level [19]. Microbial colonies were suspended in deionized water, treated with absolute ethanol for cell lysis, and centrifuged to remove alcohol. Cellular proteins were extracted using formic acid and acetonitrile, and the supernatant was analyzed. For identification, 1 µL of the sample was applied to a Maldi-Tof MS target plate, covered with a specific matrix, and dried. The plate was then inserted into the Maldi-Tof MS equipment to obtain spectra, which were analyzed using software for species-level proteomic identification [19].
2.9. Statistics
The results were evaluated using analysis of variance (ANOVA). All analyses were performed in triplicate, and Tukey’s test (p < 0.05) was applied for comparisons of means. Statistical analyses were conducted using GraphPad Prism, version 10.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Sugary Kefir Grains Adaptation
During a seven-day period, sugary kefir grains were adapted in a solution containing water and brown sugar at 12 °Brix in triplicate. A new substrate was changed every 24 h. Figure 2 shows that on “Day 6”, the Brix value was 3, and it remained the same on “Day 7”. Brix stability demonstrated adaptation of sugary kefir grains. As the microbial population increases daily, substrate consumption also intensifies. Over time, microbial growth tends to plateau due to substrate depletion. Consequently, the °Brix values begin to stabilize, indicating a balance between microbial activity and the availability of nutrients. This stability reflects a microbial population that is well-adapted and suited to the specific substrate conditions.

Figure 2.
Sugary kefir grains adaptation in 12 °Brix brown sugar solution.
3.2. Fermentation Kinetics
After the adaptation period, sugary kefir grains were inoculated at a rate of 10% of the volume of the non-alcoholic beers (corresponding to the volume of each commercial beer brand analyzed). The fermentation process took place at a temperature of 25 °C for a period of 24 h. After the fermentation process, the sugary kefir grains were recovered through a sieve, and the non-alcoholic beers were analyzed. The non-alcoholic beers (NABs) were denominated NAB1, NAB2, and NAB3. Commercial non-alcoholic beers presented a residual sugar value from the brewing process of ~5 °Brix.
3.2.1. Sugar and Acidity Parameters
Table 1 shows the results found for the parameters of pH, acidity, total soluble solids (°Brix), and sucrose of the different commercial non-alcoholic beers in natura and with kefir inoculant. The results demonstrate that sugary kefir grains microorganisms used the residual sugar from the brewing process (~5 °Brix) as a substrate for fermentation. The pH and sucrose (g/L) and °Brix values were ~3.74 ± 0.02, ~0.35 ± 0.01, and 5.55 ± 0.01, respectively, for T0. After kefir inoculant addition, these values lowered in T24, as observed in Table 1, with ~3.52 ± 0.01 for pH, ~0.22 ± 0.01 for sucrose, and 3.80 ± 0.02. The acidity (% m/v) value for T0 was ~1.30 ± 0.02. After kefir inoculant addition, these values increased to ~2.30 ± 0.02 in T24, as observed in Table 1.

Table 1.
Sugar and acidity parameters, performed on commercial non-alcoholic beers.
3.2.2. Organic Acids and Ethanol Parameters
Table 2 shows the results found for the organic acid and ethanol parameters for the different commercial non-alcoholic beers in natura and with kefir inoculant. Regarding organic acid concentrations, the lactic acid and acetic acid values in g/L were ~0.55 ± 0.01 and ~0.08 ± 0.01, respectively, for T0. After kefir inoculant addition, these values increased slightly for T24 (~1.25 ± 0.02 g/L for lactic acid and ~0.14 ± 0.01 for acetic acid), as observed in Table 2. The ethanol concentrations quantified after 24 h of kefir inoculant addition did not modify the “non-alcoholic beers” classification for the commercial beers analyzed. All analyses were performed in triplicate under controlled conditions using calibrated instrumentation. The relative standard deviation (RSD%) for compound quantification remained below 5% for sucrose, lactic acid, and acetic acid, which is within the acceptable range for analytical precision in chromatographic techniques.

Table 2.
Organic acids and ethanol in commercial non-alcoholic beers.
3.2.3. Microbial Genetic Profile
The microbial genetic profile of prokaryotes and eukaryotes, as presented in Figure 3, was used to genetically determine the total microbial population in different commercial non-alcoholic beers inoculated with kefir. The genetic profiles remained consistent for both the inoculum and the beers inoculated with sugary kefir grains. The biodiversity of bacterial and yeast species, identified through genetic material amplification and DNA sequencing, is detailed in Table 3. The Saccharomyces cerevisiae yeast species, used in the production of various commercial non-alcoholic beers, was confirmed through molecular identification.

Figure 3.
Microbial genetic profile. (a) Prokaryotes. (b) Eukaryotes. Microbial species related to Bands A-N are shown in Table 3.

Table 3.
Microbial species based on prokaryotic/eukaryotic genetic profile.
3.2.4. Color Scale for Non-Alcoholic Beers
The color evaluation of non-alcoholic beers was conducted according to the EBC (European Brewing Convention) and SRM (Standard Reference Method) color scales, both of which are recognized by Brazilian legislation that establishes identity and quality standards for brewery products, as outlined in ‘Normative Instruction No. 65, dated 10 December 2019’. Figure 4 illustrates that no variation in color scale was observed when comparing T0 and T24. For NAB1, the values were 8 on the EBC scale and 4 on the SRM scale; for NAB2, the values were 39 on the EBC scale and 20 on the SRM scale; and for NAB3, the values were 4 on the EBC scale and 2 on the SRM scale (Figure 4).

Figure 4.
Color scale for non-alcoholic beers according to EBC (European Brewing Convention) and SRM (Standard Reference Method). Non-alcoholic beer—NAB.
3.2.5. Sensory Parameters
The sensory evaluation scores for commercial non-alcoholic beers (both control and kefir-inoculated) are presented in Figure 5. No significant sensory differences were observed between the non-alcoholic commercial beers (control vs. those with kefir inoculant). Testers rated the commercial non-alcoholic beers as having similar sensory characteristics across the attributes of ‘Appearance’, ‘Texture’, ‘Color’, ‘Flavor’, and ‘Overall Acceptability’. The sensory scores for these beers ranged from 6.9 to 8.4 on a 9-point hedonic scale, indicating a preference of ‘Like Moderately’ to ‘Like Extremely’.
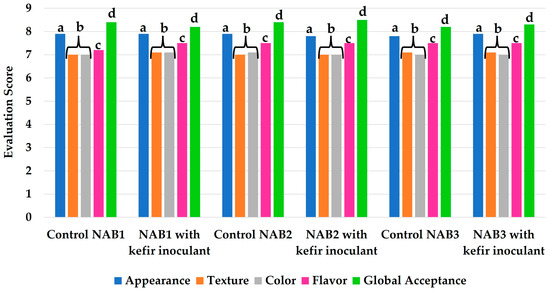
Figure 5.
Sensory evaluation score for commercial non-alcoholic beers (control and with kefir inoculant). Non-alcoholic beer—NAB. Different letters indicate statistically significant differences at p < 0.05.
3.3. Microbial Shelf Life for Non-Alcoholic Beers Fermented with Sugary Kefir
Table 4 presents microbial growth data from the evaluation of microbial shelf life in non-alcoholic beers fermented with sugary kefir. The shelf life was assessed over 10, 20, and 30 days of storage at temperatures of 4 °C and 25 °C. Microbial enumeration was performed using six different culture media types to characterize the genera of lactic acid bacteria (LAB), Acetobacter, and yeasts. The microbial counts observed for the various storage times and temperatures were within the acceptable range for probiotic microorganisms, in compliance with Brazilian legislation (‘MAPA Normative Instruction No. 46, dated 23 October 2007’), which mandates a minimum of 107 CFU/mL for LAB and 104 CFU/mL for yeasts. Non-alcoholic beers without sugary kefir grains (control) were also analyzed for microbial shelf life. Only minimal yeast growth was observed in NAB1, NAB2, and NAB3 (~1.1 × 102 CFU ± 0.02 for all evaluated times).

Table 4.
Microbial shelf life for fermented non-alcoholic beers.
The Maldi-Tof MS proteomic technique was employed to identify microbial isolates at the species level (Figure 6). The microbial biodiversity (Figure 6) for prokaryotes consisted of Lacticaseibacillus paracasei, Lacticaseibacillus casei, Lentilactobacillus kefiri (75%), Lactococcus lactis (10%), Leuconostoc citreum (10%), and Acetobacter lovaniensis (5%). For eukaryotes, the biodiversity included Saccharomyces cerevisiae (60%), Kluyveromyces lactis (20%), Lachancea meyersii (10%), and Kazachstania aerobia (10%). The proteomic analysis technique could not discriminate microbial species at the subspecies level.

Figure 6.
Percentage of microbial species using the Maldi-Tof technique. Numerical values in percentage (%). (a) Prokaryote. (b) Eukaryote.
4. Discussion
In recent times, the global population has increasingly opted for foods and beverages with functional properties that contribute to overall well-being. Probiotics are among the diverse range of functional foods and beverages available [9,22,23,24,25,26]. Research has shown that probiotic microorganisms not only support gut microbiome balance but can also positively influence the gut–brain axis, enhancing the host’s immune system [24]. When this occurs, probiotics are classified as psychobiotics [24]. Psychobiotic microorganisms can regulate brain pathways and serotonin production, aiding in the management of symptoms related to anxiety and depression [24]. Additionally, probiotic microorganisms impact neuro-endocrine systems associated with sexual function [24]. A clinical study demonstrated that probiotic supplementation improved sexual satisfaction while also alleviating symptoms of anxiety, stress, and depression [24]. Probiotics can be administered through isolated microbial cultures or mixed symbiotic cultures, such as kefir [10,27,28,29,30,31,32,33,34,35,36,37]. Reflecting current trends in the search for innovative probiotic foods and beverages, scientific studies continue to expand our understanding of kefir and explore the potential for new, consumer-friendly probiotic products [10,27,28,29,30,31,32,33,34,35,36,37].
In this study, sugary kefir grains were used to enrich commercial non-alcoholic beers of Brazilian origin with nutritional value. Non-alcoholic beers are widely consumed in Brazil, appealing to a broad consumer base that includes young people, adults, and even the elderly. Therefore, incorporating nutritional and probiotic value into these beverages, without altering their organoleptic characteristics, could enhance consumer interest [38]. Probiotic non-alcoholic beers may offer several benefits, including lower turbidity, no impact on the color of the final product, and reduced pH levels, which help prevent spoilage and extend shelf life. Additionally, an increased concentration of volatile compounds can improve the flavor profile of the beverage [38].
Table 1 presents the results for pH, acidity, total soluble solids (°Brix), and sucrose in different commercial non-alcoholic beers, both in their natural state and with kefir inoculant. A reduction in soluble solids was observed during the 24 h fermentation process, which can be attributed to the metabolism of kefir microorganisms, which are capable of utilizing the sugars present in the beers. These kefir microorganisms release metabolites such as acetic and lactic acids into the fermentative medium. The production of acids by kefir microorganisms plays a crucial role in the beverage’s stability, as these acids have an inhibitory effect on spoilage and even pathogenic microorganisms [11,12,13,14]. The microbial consortia found in sugary kefir are naturally adapted to acidic environments, which is a characteristic of traditional kefir beverages. This acid tolerance, along with their probiotic potential, was one of the main reasons for selecting kefir grains for this study.
Table 2 presents the results for organic acid and ethanol concentrations in different commercial non-alcoholic beers, both in their natural state and with kefir inoculant. Regarding organic acids (lactic acid and acetic acid), their concentrations increased at T24 in the non-alcoholic beers. The presence of organic acids in fermented beverages is beneficial for enhancing the antioxidant profile, as the bioavailability and bioaccessibility of various compounds—including antioxidant compounds like polyphenols and vitamins—are improved by the activity of different enzymes following the substrate’s acidification [11,12,13,14,30,31,32,33,34,36]. This is an important factor in characterizing a food or beverage as functional and probiotic [10,34,36]. No significant differences in ethanol concentrations were observed after 24 h of kefir fermentation. The fermentation process did not affect the classification of the beverages as “non-alcoholic”, ensuring that the commercial beers analyzed retained their status. This supports the use of sugary kefir grains to add nutritional, functional, and probiotic value to commercial non-alcoholic beers [11,38]. According to Brazilian legislation, as defined by Normative Instruction No. 65, of 10 December 2019 (Ministry of Agriculture, Livestock and Supply—MAPA), a “non-alcoholic beer” (cerveja sem álcool in Brazil) is a beer that contains an ethanol content of up to 0.5% v/v. This classification aligns with international standards and has been observed throughout our study. The ethanol levels in all experimental samples remained within this legal threshold, ensuring compliance with Brazilian regulations for non-alcoholic beverages.
Sugary kefir grains and commercial non-alcoholic beers inoculated with kefir displayed a stable microbial population throughout the fermentation process (Figure 3), indicating that the commercial non-alcoholic beers remained free of microbial contamination. The microbial population consisted of bacteria such as Lacticaseibacillus paracasei, Lacticaseibacillus casei, Lacticaseibacillus paracasei subsp. paracasei, Lacticaseibacillus paracasei subsp. tolerans, Lactobacillus delbrueckii subsp. lactis, Lentilactobacillus parabuchneri, Lentilactobacillus kefiri, Lactococcus lactis, Leuconostoc citreum, and Acetobacter lovaniensis. The yeast species identified included Saccharomyces cerevisiae, Kluyveromyces lactis, Lachancea meyersii, and Kazachstania aerobia (Table 3). The NCBI-BLAST identification process revealed an excellent similarity percentage, exceeding 98%.
The microbial species present in sugary kefir grains exhibit probiotic activity, primarily through their ability to enhance immune function and defend against pathogenic microorganisms [10,27,28,29,30,31,32,33]. Species from the genera Lactobacillus, Lacticaseibacillus, and Lentilactobacillus produce vitamins A and B complexes during the fermentation process. In addition to their probiotic functions, these species also nutritionally enrich foods and beverages [10,14,20]. Lactococcus spp. are capable of synthesizing folate, a vitamin with antioxidant properties [10,14,20], while Leuconostoc spp. can convert glucose into energy for synthesizing exopolysaccharides (such as dextran and levan), bacteriocins, and water-soluble vitamins (including vitamin C and B-complex vitamins). Water-soluble vitamins are stored in the body for only a short period and must, therefore, be consumed daily to prevent deficiencies [10,14,20]. The Saccharomyces cerevisiae yeasts possess antioxidant and immunomodulatory potential, along with antimicrobial properties against pathogenic microorganisms [10,33]. Consequently, kefir serves as an alternative source of vitamins and other essential nutritional compounds [10,33].
The color evaluation of non-alcoholic beers was conducted according to the EBC (European Brewing Convention) and SRM (Standard Reference Method) color scales, as specified by Brazilian legislation, which establishes identity and quality standards for brewery products (Normative Instruction No. 65, 10 December 2019) (Figure 4). Beer color plays a crucial role in both sensory appeal and commercial value [39,40,41,42]. In this study, non-alcoholic beers fermented with kefir maintained the visual quality standards accepted in the commercial market. This can be attributed to the fact that no changes were observed in the color scale of any of the non-alcoholic beers after fermentation with kefir (Figure 4). These results demonstrate that sugary kefir grains successfully enhanced the nutritional and probiotic value of the non-alcoholic beers without altering their visual organoleptic characteristics.
The sensory evaluation scores for commercial non-alcoholic beers (control and kefir-inoculated) are shown in Figure 5. No significant sensory differences were observed between the commercial non-alcoholic beers (control and those with kefir inoculant). The sensory attributes of these beers were scored between 6.9 and 8.4 on a 9-point hedonic scale, indicating a preference of “Like Moderately or Extremely”. These results suggest that the kefir grain fermentation process did not affect the organoleptic characteristics of the non-alcoholic beers. Sugary kefir grains enriched the beers with probiotic and nutritional value, offering health benefits. These findings demonstrate that sugary kefir grains could serve as a commercial alternative to enhance the functional value of non-alcoholic beers. The growing consumer interest in functional and probiotic products highlights an expanding market [43]. This trend has sparked research into food and beverage products fermented with probiotic microorganisms, leading to the development of new functional products for the market [27,29,30,31,32,33,34,36,37,38,39,40,41,42].
Table 4 presents the microbial growth data for the evaluation of microbial shelf life in fermented non-alcoholic beers stored for 10, 20, and 30 days at temperatures of 4 °C and 25 °C. These analyses were performed after removing the sugary kefir grains from non-alcoholic beers that had been fermented for 24 h. The microbial counts recorded for the different days and temperatures were within the acceptable range for probiotic microorganisms, in accordance with Brazilian legislation (MAPA Normative Instruction No. 46 of 23 October 2007), with a minimum of 107 CFU/mL for bacteria and 104 CFU/mL for yeast. The Maldi-Tof MS proteomic technique was used to identify the microorganisms isolated during the shelf life analysis at the species level (Figure 6). The viable microbial species (cultured) aligned with the results of the microbial genetic profile carried out in this study, confirming the viability of kefir probiotic microorganisms in non-alcoholic beers. Therefore, the incorporation of probiotic kefir into non-alcoholic beers adds functional value, potentially enhancing the nutritional properties of the products [44,45].
Beer is the most widely consumed alcoholic beverage globally, appreciated not only for its sociocultural significance but also for its distinctive organoleptic and sensory characteristics, such as flavor, aroma, and mouthfeel, which play a crucial role in consumer acceptance and commercial success [44,46]. However, non-alcoholic beer has been gaining ground worldwide. Non-alcoholic beer is a type of beer that contains little to no alcohol, usually less than 0.5% ABV (alcohol by volume). It is made using a similar brewing process to that of regular beer, but the alcohol is removed or reduced afterward. Non-alcoholic beer is a popular option for people who want to enjoy the taste of beer without the effects of alcohol. It is often chosen for health, religious, or personal reasons and is available in many styles, such as lager, ale, and stout. In recent years, growing consumer demand for functional foods has encouraged the development of innovative non-alcoholic beverages with added health benefits. In this context, the incorporation of probiotic microorganisms into non-alcoholic beer has emerged as a promising strategy, potentially combining the hedonic appeal of beer with the functional advantages of probiotics [44].
According to Santos et al. [44], probiotic beers may offer health benefits such as improved gut health, immune modulation, and enhanced nutrient absorption—attributes not typically associated with conventional beers. However, the development of probiotic beers presents significant technological challenges. Many probiotic strains are highly sensitive to environmental conditions such as low pH, oxygen exposure, and storage temperature, which can compromise their viability during production and shelf life.
Therefore, the selection of robust, beer-tolerant probiotic strains is essential. Strategies such as microencapsulation, co-fermentation, and post-fermentation addition of probiotics have been explored to enhance microbial survival without adversely affecting the beer’s sensory quality. Furthermore, the choice of fermentation substrate, brewing process parameters, and packaging methods must be carefully optimized to balance probiotic viability with the desired flavor profile and stability of the final product.
Ultimately, the successful development of probiotic beer depends on an interdisciplinary approach that integrates microbiology, food technology, and sensory science. Such innovations could open new markets within the craft and functional beverage industries, appealing to health-conscious consumers while contributing to product diversification in the brewing sector.
5. Conclusions
The results of this study indicate that the incorporation of sugary kefir into non-alcoholic beers offers promising commercial and health-related opportunities. From a commercial standpoint, the successful integration of probiotics into an already popular beverage category could diversify product lines, add value to existing brands, and meet the growing consumer demand for functional and health-enhancing products. The high sensory acceptance observed suggests market feasibility without compromising the original organoleptic characteristics of the beer.
From a health perspective, the presence of viable probiotic strains and the production of beneficial organic acids, such as lactic and acetic acid, point to potential contributions to gut health, immune modulation, and overall well-being. These benefits align with current trends in preventive health and functional nutrition.
The incorporation of kefir into brewing processes could be adapted as a post-fermentation step using static aerobic fermentation, as demonstrated in this study. This method requires minimal modification of existing production infrastructure, making it a practical approach for craft and industrial breweries interested in functional product innovation.
However, regulatory considerations must be addressed before commercial implementation. These include labeling requirements for probiotic claims, stability and viability standards during shelf life, and compliance with national and international food safety regulations regarding the use of live cultures in beverages. Further collaboration with food safety agencies and standardization bodies will be necessary to ensure legal and safe market entry.
In summary, this study opens the door for the development of probiotic non-alcoholic beers using sugary kefir, representing a novel and feasible strategy to unite health benefits with consumer preferences in the functional beverage sector.
6. Limitations of the Study and Future Prospects
While the findings of this study are promising and highlight the potential of sugary kefir to enhance the functional value of non-alcoholic beers, several limitations should be acknowledged. Firstly, the sample size, both in terms of the number of beer brands tested and the scale of fermentation trials, was relatively limited. Variations in the composition of commercial non-alcoholic beers—such as sugar content, acidity, and flavoring agents—could influence the fermentation behavior and probiotic viability, which warrants further exploration.
Secondly, the sensory evaluation, although positive, was conducted with a semi-trained panel. The lack of extensive training or calibration among panelists may introduce variability in perception and limit the generalizability of the sensory results. Future studies could benefit from a larger and more rigorously trained panel, including a broader demographic range of consumers to better reflect market acceptance.
Another important consideration is the duration and stability of probiotic viability over time. This study focused on the short-term effects of fermentation (24 h), but it did not assess the long-term survival of probiotic strains during storage or their behavior under different packaging and distribution conditions.
To address these limitations, future research should include the following:
- (1)
- A comparative analysis using multiple commercial non-alcoholic beer brands to evaluate matrix variability;
- (2)
- Longitudinal studies assessing probiotic viability, physicochemical stability, and sensory quality over shelf life;
- (3)
- In-depth characterization of sensory profiles using trained and consumer panels;
- (4)
- Exploration of encapsulation or stabilization techniques to enhance the survival of probiotics in beer matrices.
These steps will strengthen the practical applicability and commercial scalability of using sugary kefir as a probiotic inoculant in non-alcoholic beers.
Author Contributions
Conceptualization, Conceptualization, A.S.M.d.N., R.N.A.d.S., A.K.d.C.L.L. and K.T.M.-G.; Data Curation, A.S.M.d.N., P.P.L.G.T., A.S.B., M.P.S.C. and K.T.M.-G.; Formal Analysis, R.N.A.d.S., P.P.L.G.T., M.P.S.C., A.K.d.C.L.L., R.C.d.C.A. and K.T.M.-G.; Funding Acquisition, K.T.M.-G.; Investigation, A.S.M.d.N., R.N.A.d.S., P.P.L.G.T., A.S.B. and K.T.M.-G.; Project Administration, A.K.d.C.L.L., R.C.d.C.A. and K.T.M.-G.; Supervision, K.T.M.-G.; Validation, A.S.M.d.N., P.P.L.G.T., A.S.B. and K.T.M.-G.; Writing—Original Draft Preparation, A.S.M.d.N., R.N.A.d.S., P.P.L.G.T., A.S.B., M.P.S.C. and K.T.M.-G.; Writing—Original Draft, A.S.M.d.N., R.N.A.d.S., A.K.d.C.L.L., R.C.d.C.A. and K.T.M.-G.; Writing—Review and Editing, P.P.L.G.T., A.K.d.C.L.L., R.C.d.C.A. and K.T.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the support provided by the student grants “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the School of Nutrition at the Federal University of Bahia (UFBA-Brazil), protocol code 1.759.169 on 16 August 2016.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data are available upon request.
Acknowledgments
The authors would like to dedicate the article to Ferlando Lima Santos (in memoriam), a professor at the Federal University of Recôncavo da Bahia, responsible for the donation of the sugary kefir grains cultures and who was always available to share her immeasurable knowledge. The authors thank the following Brazilian agencies: CNPq, CAPES e FAPESB.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yerlikaya, O.; Akan, E.; Kinik, O. The metagenomic composition of water kefir microbiota. Int. J. Gastron. Food Sci. 2022, 30, 100621. [Google Scholar] [CrossRef]
- Tavares, P.P.L.G.; dos Anjos, E.A.; Nascimento, R.Q.; da Silva Cruz, L.F.; Lemos, P.V.F.; Druzian, J.I.; de Oliveira, T.T.B.; de Andrade, R.B.; da Costa Souza, A.L.; Magalhães-Guedes, K.T.; et al. Chemical, Microbiological and Sensory Viability of Low-Calorie, Dairy-Free Kefir Beverages from Tropical Mixed Fruit Juices. CYTA J. Food 2021, 19, 457–464. [Google Scholar] [CrossRef]
- Klimczak, K.; Cioch-Skoneczny, M.; Ciosek, A.; Poreda, A. Application of Non-Saccharomyces Yeast for the Production of Low-Alcohol Beer. Foods 2024, 13, 3214. [Google Scholar] [CrossRef]
- Kokole, D.; Jané Llopis, E.; Anderson, P. Non-alcoholic beer in the European Union and UK: Availability and apparent consumption. Drug Alcohol. Rev. 2022, 41, 550–560. [Google Scholar] [CrossRef]
- Jackowski, M.; Czepiela, W.; Hampf, L.; Żuczkowski, W.; Dymkowski, T.; Trusek, A. Comparison of Two Commercially Available Strains, Saccharomycodes ludwigii and Torulaspora delbrueckii, for the Production of Low-Alcohol Beer. Beverages 2023, 9, 66. [Google Scholar] [CrossRef]
- Hernández-Mora, Y.N.; Verde-Calvo, J.R.; Malpica-Sánchez, F.P.; Escalona-Buendía, H.B. Consumer Studies: Beyond Acceptability—A Case Study with Beer. Beverages 2022, 8, 80. [Google Scholar] [CrossRef]
- Ożga, K.; Stepuch, P.; Maciejewski, R.; Sadok, I. Promising Gastric Cancer Biomarkers—Focus on Tryptophan Metabolism via the Kynurenine Pathway. Int. J. Mol. Sci. 2025, 26, 3706. [Google Scholar] [CrossRef]
- Kumar, M.R.; Yeap, S.K.; Mohamad, N.E.; Abdullah, J.O.; Masarudin, M.J.; Khalid, M.; Leow, A.T.C.; Alitheen, N.B. Metagenomic and Phytochemical Analyses of Kefir Water and Its Subchronic Toxicity Study in BALB/c Mice. BMC Complement. Med. Ther. 2021, 21, 183. [Google Scholar] [CrossRef]
- Santos, E.N.; Magalhães-Guedes, K.T.; Borges, F.E.d.M.; Ferreira, D.D.; da Silva, D.F.; Conceição, P.C.G.; Lima, A.K.d.C.; Cardoso, L.G.; Umsza-Guez, M.A.; Ramos, C.L. Probiotic Microorganisms in Inflammatory Bowel Diseases: Live Biotherapeutics as Food. Foods 2024, 13, 4097. [Google Scholar] [CrossRef]
- da Anunciação, T.A.; Guedes, J.D.S.; Tavares, P.P.L.G.; de Melo Borges, F.E.; Ferreira, D.D.; Costa, J.A.V.; Umsza-Guez, M.A.; Magalhães-Guedes, K.T. Biological Significance of Probiotic Microorganisms from Kefir and Kombucha: A Review. Microorganisms 2024, 12, 1127. [Google Scholar] [CrossRef]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Dimitreli, G.; Exarhopoulos, S.; Apidopoulou, P.; Groztidou, O.; Georgiou, D.; Kalogianni, E.P.; Goulas, A. Effect of Final Fermentation pH and Pre-Drying Storage Temperature on Properties of Kefir Powder Produced by Kefir Grains. Appl. Sci. 2025, 15, 2509. [Google Scholar] [CrossRef]
- Teijeiro, M.; Pérez, P.F.; De Antoni, G.L.; Golowczyc, M.A. Suitability of kefir powder production using spray drying. Food Res. Int. 2018, 112, 169–174. [Google Scholar] [CrossRef]
- Newbold, D.; Koppel, K. Carbonated Dairy Beverages: Challenges and Opportunities. Beverages 2018, 4, 66. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Chondrou, P.; Galanis, A.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Application of A Novel Potential Probiotic Lactobacillus paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms 2018, 6, 121. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P. A Big World in Small Grain: A Review of Natural Milk Kefir Starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef]
- Vitali, M.; Gandía, M.; Garcia-Llatas, G.; González-Sarrías, A.; Vallejo, F.; Cilla, A.; Gamero, A. Modulation of Antioxidant Capacity, Nutritional Composition, Probiotic Viability After Digestion and Sensory Attributes of Plant-Based Beverages Through Lactic Acid Fermentation. Foods 2025, 14, 1447. [Google Scholar] [CrossRef]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 5th ed.; Salfinger, Y., lou Tortorello, M., Eds.; APHA: Washington, DC, USA, 2015; Available online: https://ajph.aphapublications.org/doi/abs/10.2105/MBEF.0222 (accessed on 12 December 2024).
- da Silva, R.N.A.; Magalhães-Guedes, K.T.; de Oliveira Alves, R.M.; Souza, A.C.; Schwan, R.F.; Umsza-Guez, M.A. Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the Maldi-Tof MS/Genbank Proteomic Technique. Microorganisms 2024, 12, 678. [Google Scholar] [CrossRef]
- La Torre, C.; Caputo, P.; Fazio, A. Effect of Milk and Water Kefir Grains on the Nutritional Profile and Antioxidant Capacity of Fermented Almond Milk. Molecules 2025, 30, 698. [Google Scholar] [CrossRef]
- National Library of Medicine BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 December 2024).
- Ziarno, M.; Zaręba, D.; Kowalska, E.; Florowski, T. A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages. Appl. Sci. 2025, 15, 3714. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Tan, L.T.; Heng, S.W.Q.; Liu, S.Q. Effect of Co-Fermentation of Saccharomyces boulardii CNCM-I745 with Four Different Probiotic Lactobacilli in Coffee Brews on Cell Viabilities and Metabolic Activities. Fermentation 2023, 9, 219. [Google Scholar] [CrossRef]
- Yang, S.-J.; Nguyen, T.T.M.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yi, T.-H. A PRISMA Systematic Review of Sexual Dysfunction and Probiotics with Pathophysiological Mechanisms. Biology 2025, 14, 286. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and Antioxidant Activities of Saccharomyces cerevisiae IFST062013, a Potential Probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Magalhães-Guedes, K.T. Psychobiotic Therapy: Method to Reinforce the Immune System. Clin. Psychopharmacol. Neurosci. 2022, 20, 17–25. [Google Scholar] [CrossRef]
- Destro, T.M.; Prates, D.d.F.; Watanabe, L.S.; Garcia, S.; Biz, G.; Spinosa, W.A. Organic Brown Sugar and Jaboticaba Pulp Influence on Water Kefir Fermentation. Cienc. Agrotec. 2019, 43, e005619. [Google Scholar] [CrossRef]
- Dentice Maidana, S.; Argañaraz Aybar, J.N.; Albarracin, L.; Imamura, Y.; Arellano-Arriagada, L.; Namai, F.; Suda, Y.; Nishiyama, K.; Villena, J.; Kitazawa, H. Modulation of the Gut–Lung Axis by Water Kefir and Kefiran and Their Impact on Toll-like Receptor 3-Mediated Respiratory Immunity. Biomolecules 2024, 14, 1457. [Google Scholar] [CrossRef]
- Zannini, E.; Lynch, K.M.; Nyhan, L.; Sahin, A.W.; O’ Riordan, P.; Luk, D.; Arendt, E.K. Influence of Substrate on the Fermentation Characteristics and Culture-Dependent Microbial Composition of Water Kefir. Fermentation 2023, 9, 28. [Google Scholar] [CrossRef]
- Arroum, S.; Sboui, A.; Fguiri, I.; Dbara, M.; Ayeb, N.; Hammadi, M.; Khorchani, T. Influence of Kefir Grain Concentration on the Nutritional, Microbiological, and Sensory Properties of Camel Milk Kefir and Characterization of Some Technological Properties. Fermentation 2025, 11, 170. [Google Scholar] [CrossRef]
- Çevik, T.; Aydoğdu, N.S.; Özdemir, N.; Kök Taş, T. The Effect of Different Sugars on Water Kefir Grains. Turk. J. Agric. Food Sci. Technol. 2019, 7, 40–45. [Google Scholar] [CrossRef]
- Tzavaras, D.; Papadelli, M.; Ntaikou, I. From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages. Fermentation. 2022, 8, 135. [Google Scholar] [CrossRef]
- Agarbati, A.; Ciani, M.; Canonico, L.; Galli, E.; Comitini, F. Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium. Fermentation 2022, 8, 9. [Google Scholar] [CrossRef]
- Laureys, D.; de Vuyst, L. The Water Kefir Grain Inoculum Determines the Characteristics of the Resulting Water Kefir Fermentation Process. J. Appl. Microbiol. 2017, 122, 719–732. [Google Scholar] [CrossRef]
- de Almeida, K.V.; Sant’ Ana, C.T.; Wichello, S.P.; Louzada, G.E.; Verruck, S.; Teixeira, L.J.Q. Water Kefir: Review of Microbial Diversity, Potential Health Benefits, and Fermentation Process. Processes 2025, 13, 885. [Google Scholar] [CrossRef]
- Ströher, J.A.; Oliveira, W.d.C.; Freitas, A.S.d.; Salazar, M.M.; Flôres, S.H.; Malheiros, P.d.S. Microbial Dynamics and Volatile Compound Profiles in Artisanal Kefir During Storage. Fermentation 2025, 11, 105. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An Update on Water Kefir: Microbiology, Composition and Production. Int. J. Food. Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Díaz, A.B.; Durán-Guerrero, E.; Valiente, S.; Castro, R.; Lasanta, C. Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast. Foods 2023, 12, 2912. [Google Scholar] [CrossRef]
- Simón, D.; Palet, C.; Cristóbal, A. Cadmium Removal by Adsorption on Biochars Derived from Wood Industry and Craft Beer Production Wastes. Water 2024, 16, 1905. [Google Scholar] [CrossRef]
- Pater, A.; Januszek, M.; Satora, P. Comparison of the Chemical and Aroma Composition of Low-Alcohol Beers Produced by Saccharomyces cerevisiae var. chevalieri and Different Mashing Profiles. Appl. Sci. 2024, 14, 4979. [Google Scholar] [CrossRef]
- Vaštík, P.; Sulo, P.; Rosenbergová, Z.; Klempová, T.; Dostálek, P.; Šmogrovičová, D. Novel Saccharomyces cerevisiae × Saccharomyces mikatae Hybrids for Non-alcoholic Beer Production. Fermentation 2023, 9, 221. [Google Scholar] [CrossRef]
- Telini, B.d.P.; Villa, L.C.; Vainstein, M.H.; Lopes, F.C. From Vineyard to Brewery: A Review of Grape Pomace Characterization and Its Potential Use to Produce Low-Alcohol Beverages. Fermentation 2025, 11, 57. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Hussain, M.A. Functional Probiotic Foods Development: Trends, Concepts, and Products. Fermentation 2023, 9, 249. [Google Scholar] [CrossRef]
- Santos, D.; Barreiros, L.; Jesus, Â.; Silva, A.L.; Martins, J.P.; Oliveira, A.I.; Pinho, C. Beer with Probiotics: Benefits and Challenges of Their Incorporation. Beverages 2024, 10, 109. [Google Scholar] [CrossRef]
- Fu, X.; Guo, L.; Li, Y.; Chen, X.; Song, Y.; Li, S. Transcriptional Analysis of Mixed-Culture Fermentation of Lachancea thermotolerans and Saccharomyces cerevisiae for Natural Fruity Sour Beer. Fermentation 2024, 10, 180. [Google Scholar] [CrossRef]
- Anderson, P.; Kokole, D.; Jané Llopis, E.; Burton, R.; Lachenmeier, D.W. Lower Strength Alcohol Products—A Realist Review-Based Road Map for European Policy Making. Nutrients 2022, 14, 3779. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).