1. Introduction
Greek viticulture and enology have a rich history, showcasing a diverse array of genetically distinct
Vitis vinifera L. grape varieties that have shaped unique wine styles from antiquity to the present. With over 300 indigenous grape varieties, Greece has maintained a strong winemaking tradition, cultivating a genetically rich population [
1,
2]. However, the globalization of the wine market has led to a significant reduction in biodiversity, as many local varieties were replaced by a few international cultivars prized for their phenolic and aromatic properties critical to vinification [
3]. Recently, there has been renewed global and local interest in native grape varieties to preserve rare native stocks at risk of extinction and to diversify the wine market. Minor grape varieties and newly identified genotypes are increasingly valued for their potential to offer vibrant, aromatic wines and to meet consumer demand while preserving genetic diversity and cultural heritage [
4,
5].
Wine polyphenols, a diverse group of secondary metabolites primarily derived from grape berries, are essential to wine’s sensory, antioxidant and nutraceutical properties [
6,
7]. These compounds have a crucial role in wine quality by contributing to color, taste and oxidative stability, as well as playing a significant role in plant metabolism. Their composition is shaped by genetic factors, environmental conditions and viticultural practices. Among polyphenols, anthocyanins are responsible for the red and purple hues in wine and undergo reactions during fermentation and aging that affect long-term color stability [
1]. Flavanols, another vital polyphenol subgroup, are particularly significant as they polymerize to form tannins, or proanthocyanidins, which are extracted from grape skins, seeds and stems during winemaking [
6,
7,
8]. These condensed tannins contribute to astringency by precipitating proteins, influence bitterness and stabilize red wine’s color through interactions with anthocyanins. The contribution of tannins to astringency is determined by their molecular size and subunit composition, both of which are influenced by winemaking practices such as fermentation and pressing. Additionally, extended maceration can modulate their content by either stabilizing or reducing it [
9,
10]. Polyphenols form the foundation of wine’s sensory and structural qualities while being integral to its appealing and health-related attributes [
7].
Astringency in wine, often described as a “tannic” sensation, is a tactile experience characterized by dryness, roughness and shrinking in the mouth, primarily caused by tannins–polymeric pigments formed from flavan-3-ol subunits like catechin and epicatechin [
4,
11]. These tannins interact with salivary proteins, forming complexes that reduce lubrication, leading to the distinct perception of astringency. While tannins are the primary contributors, other factors such as anthocyanins, acidity, ethanol concentration and polysaccharides also play roles, and imbalances can lead to an overly astringent wine, described as “harsh” or “unripe” [
12,
13]. The perception of astringency is influenced by the wine’s tannin content, pH, polyphenolic composition and alcohol level, with extensive research linking reduced astringency to adjustments in these elements. Winemakers can manage astringency by carefully balancing tannin extraction, enhancing polysaccharide content and optimizing production processes. Although newer methods to mitigate undesirable phenolic astringency are emerging, comprehensive evaluations of their effectiveness are still needed [
14,
15,
16].
Several viticultural practices are essential for modifying the grapevine microclimate to enhance fruit quality and address environmental challenges, with various strategies employed during grape cultivation or winemaking to influence the phenolic composition of red wines [
17,
18]. Adjusting the canopy, through techniques, like leaf removal and different pruning systems, regulates light exposure to grape clusters, affecting their microclimate and altering their chemical composition [
19]. Building on these approaches, further adaptation strategies are necessary to address climate change impacts in warm regions [
20,
21]. These include exploring new winemaking processes or the use of natural products to mitigate ripening imbalances, which take advantage of high radiation and temperatures. Techniques like post-harvest withering, involving the partial dehydration of grapes, induce metabolic changes that enhance their physical and chemical properties [
22,
23]. Thereby, over-ripening grapes through sunlight techniques naturally alters their composition and facilitates the creation of new styles of wines [
24].
Recent research has emphasized that vineyard and winery practices not only influence the phenolic and structural attributes of wines but also have a considerable impact on their volatile composition and aromatic profile. In particular, basal and cluster-zone defoliation have been shown to increase key varietal and fermentation-derived aromas such as β-damascenone and linalool, while simultaneously reducing undesired green notes like methoxypyrazines when applied early in the season [
25]. These changes contribute positively to wine’s complexity and sensory appeal. Additionally, air dehydration, by modulating the concentration of aroma precursors, and seed removal, by reducing astringent bitterness and influencing volatile release during fermentation, may further shape the wine’s aromatic expression. Therefore, it is essential to consider not only the structural and phenolic outcomes but also the volatile and sensory implications of these interventions when aiming to optimize wine quality.
In the light of the above, the aim of this study was a modification in the astringency of wines made exclusively from Mandilaria grapes, a red, indigenous variety cultivated on the Aegean islands, particularly under the environmental conditions of Paros Island. Mandilaria is known for its high tannic content, full-bodied profile and dense grape clusters, which often suffer from rot and present challenges for winemakers because of their pronounced bitterness and astringency. To achieve this goal, viticultural practices, improving phenolic ripening through grape exposure to sunlight and reducing cluster density, were employed. Additionally, dehydration techniques and tannin reduction methods were used. Finally, a complementary sensory analysis conducted by trained assessors was included to support the observed modification in astringency and to explore its potential correlation with other measured parameters.
2. Materials and Methods
2.1. Chemicals and Reagents
Methanol, ethanol, acetonitrile, ethyl acetate, water, acetone (HPLC grade), phloroglucinol and sodium metabisulfite were purchased from Merck (Zedelgem, Belgium). Methanol (HPLC-Ultra LC-MS grade) was also obtained from HiPerSolv CHROMANORM, (VWR Chemicals BDH, Amsterdam, The Netherlands) to produce standard solutions. L-ascorbic acid, formic acid (99%) and hydrochloric acid (37%) for the analysis were purchased from Carlo Erba (Val-de-Reuil, France), and the trifluoroacetic acid for the LC-MS was obtained from Fluka (Buchs, Switzerland). Sodium hydroxide, Folin–Ciocalteu phenol reagent, 2,2-diphenyl-picryl-hydrazyl radical (DPPH), methylcellulose (MCP) and bovine serum albumin (BSA, fraction V) were obtained from Sigma Aldrich (Darmstadt, Germany). The analytical standards of delphinidin-3-O-glucoside chloride (99.5%), cyanidin-3-O-glucoside chloride (96.2%), petunidin-3-O-glucoside chloride (97.5%), peonidin-3-O glucoside chloride (96.6%) and malvidin-3-O-glucoside chloride (96.7%) were obtained from Extrasynthese (Genay Cedex, France). The anthocyanin standards were diluted separately in methanol LC-MS with 0.1%HCl. Catechin (98%), epicatechin (97%), epigallocatechin (99.5%), epicatechin gallate (98%), gallocatechin (98%), epigallocatechin gallate (95%), procyanidin B1 (90%) and procyanidin B2 (90%) were acquired from Sigma-Aldrich (Darmstadt, Germany).
2.2. Experimental Design
On Paros Island (Greece), the experiments were conducted in linear vineyards to facilitate the application of treatments. The Mandilaria grapes were harvested in 2023, when the soluble solids content was 18.8 ± 1° Brix. Initially, viticultural practices were conducted to increase the grapes’ exposure to light, reducing the cluster density and berry size through complete (T-RIF) and partial defoliation (E-RIF), carried out in late May. Additionally, the distribution of the pruning load was modified to influence the phenolic content of the Mandilaria grapes. To achieve this goal, long pruning (Guyot system) was applied, retaining one cane with 10 buds (CANE).
Furthermore, the following three different dehydration techniques were used: sun-drying (D-SUN); dehydration in a closed room with shaded air circulation (D-AIR), which is comparable to the Amarone procedure; and pedicel crushing for extended ripening on the vine (D-VIN). The dehydration process was closely observed, measuring the sugar content and fruit weight every day.
Simultaneously, the following two interventions were tried consecutively during the winery phase: mechanically removing 20% (SE20) and 30% (SE30) of the seeds on the 8th day of maceration. The seeds were removed to reduce the extraction of seed-bound phenolics, which are mostly responsible for astringency.
2.3. Vinification Protocol
Experimental winemaking was carried out using 70 kg of harvested grapes for each treatment. Grapes were crushed, destemmed and supplemented with potassium metabisulfite (SO2) at 50 mg/L. Pectolytic enzymes at 40 mg/L (Safizym Pres, Fermentis, Marquette-Lez-Lille, France) (previously hydrated in water 15 min, 38 °C) and nutrients at 30 g/tn were also added. All vinifications for the different treatments were carried out in 100 L tanks to ensure stable and standardized conditions. In vineyard and seed removal experimental trials, spontaneous alcoholic fermentation was carried out, whereas with the vinification of the dehydrated grapes, because of the high initial density, a commercial strain of Saccharomyces cerevisiae Collezione Primavera ‘56 (Laffort, Bordeaux, France) at 300 mg/L was used to ensure the complete conversion of sugars into alcohol. Fermentation was monitored by twice daily punch-downs to enhance the extraction of the phenolic compounds, with the frequency reduced over time as the days passed. At the end of the maceration, the juice was separated from the pomace and allowed to completely ferment over the following six days, during which all fermentations were finalized. Wines were racked, supplemented with 40 mg/L SO2 and bottled after filtration or stabilization. No malolactic fermentation was performed. After the completion of alcoholic fermentation, the wines were stored at 18 ± 2 °C in the dark until the analysis.
2.4. Grape and Wine Analysis
Following the OIV standard procedures for wine analysis, samples were collected for each treatment’s grape maturity analysis [
26]. In particular, the measurements of pH, total soluble solids (Brix) and total titratable acidity (TA) were performed for samples of about 50 grapes. All analyses were performed in triplicate. Another sub-sample of 50 berries was weighed, and skins and seeds were manually separated to measure the average berry mass and analyze the distribution of the berry mass components. The percentage of skin and seed per berry weight ratio was assessed across the different treatments.
2.5. Chemical Analysis
The seeds and skins from 50 berries per replicate were manually separated, freeze-dried and ground into powder. The extraction of phenolics from skins and seeds was carried out followed previously established protocols [
27,
28] with slight modifications. Briefly, 0.3 g of powdered material was first extracted with 2.5 mL of acetone/water (80:20,
v/
v) for 3 h, followed by a second extraction using 2.5 mL of methanol/water (60:40,
v/
v) for 2.5 h. The resulting supernatants were combined, and the organic solvents were removed by evaporation under pressure at 30 °C. Finally, the residue was dissolved in water and lyophilized, yielding a crude phenolic extract. In the case of grapes, only total anthocyanins and extractability were measured in whole berries, while all other grape analyses were conducted on freeze-dried tissue. The phenolic compounds in the grapes and wines were analyzed utilizing the methods described in the following assays.
2.5.1. Color and Polyphenol Analyses
The color characteristics of the wine samples were assessed following the Glories method [
29], characterizing the color intensity and hue, with the absorbance recorded at 420, 520 and 620 nm using a UV-VIS spectrophotometer equipped with 1 mm quartz cells.
For the analysis of the total polyphenolic index (TPI), wine samples were first filtered and then diluted with distilled water at a 1:100 ratio before measuring absorbance at 280 nm. All absorbance measurements were performed on a Hitachi U-2000 spectrophotometer (Jasco, Victoria, BC, Canada).
2.5.2. Total Phenols and Antioxidant Activity
The total polyphenol concentration in grapes and wines was determined using the Folin–Ciocalteu assay, following a previously established microscale protocol with slight modifications [
30,
31]. The absorbance was recorded at 750 nm using a spectrophotometer, and the polyphenol levels are expressed as gallic acid equivalents (GAE) in mg/L for wines and mg/g for grapes.
The antioxidant activities of the wine samples and grape extracts were assessed using the DPPH radical scavenging method. The reaction was monitored at 515 nm over 30 min, while the absorbance measurements were recorded using a UV-VIS spectrophotometer. The antioxidant capacity is expressed as the Trolox equivalent antioxidant capacity (TEAC) in mmol Trolox, determined through interpolation in a Trolox standard curve.
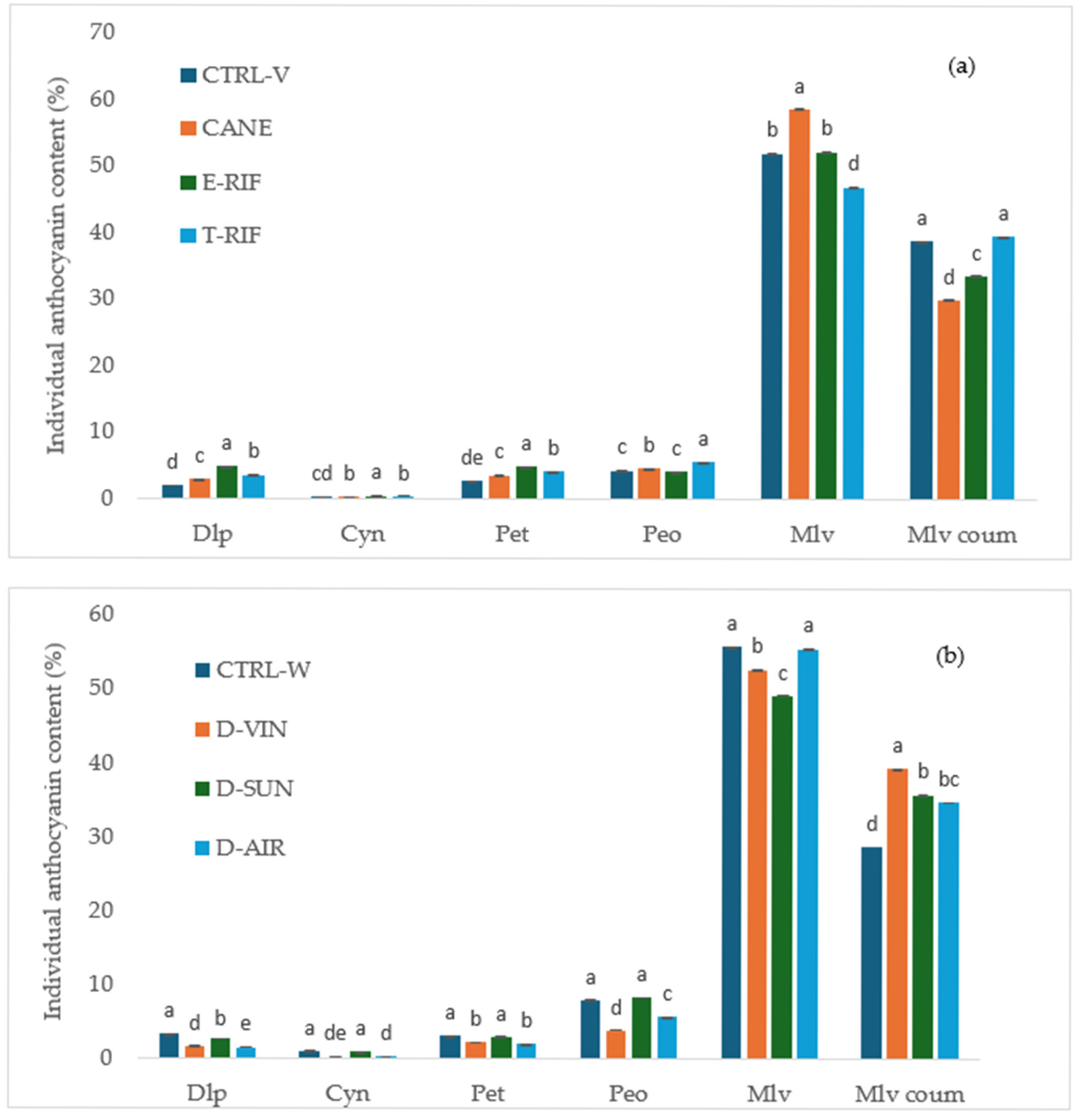
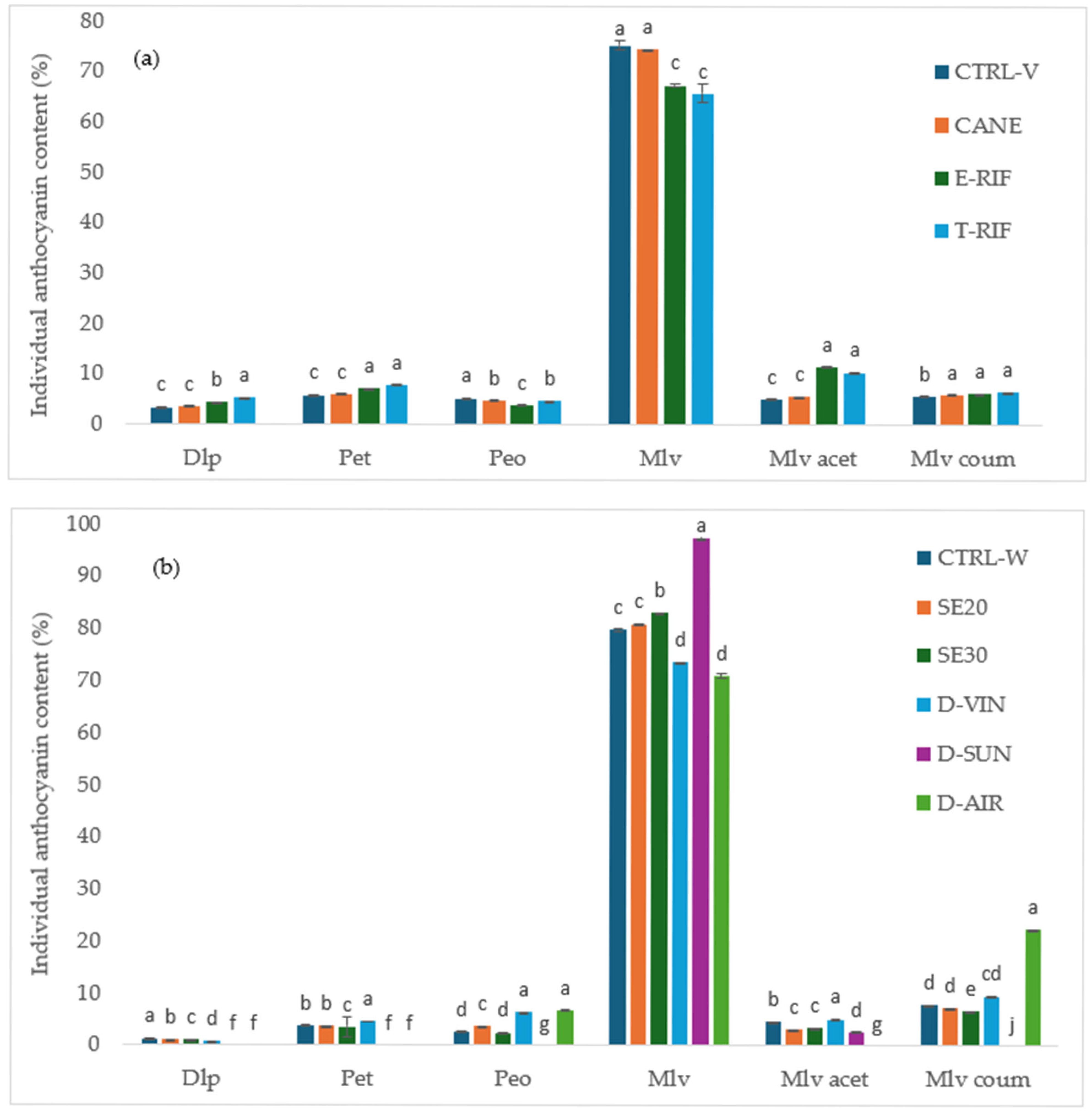
2.5.3. Analysis of Anthocyanins
The total anthocyanin content of the grape berries was assessed by spectrophotometry using the Iland method [
32], while extractable anthocyanins were quantified following Glories’ method [
29]. To further characterize the monomeric anthocyanins in grapes and wines, high-performance liquid chromatography (HPLC) was used, following previously established methods [
33]. The identification and quantification of the individual anthocyanins were achieved by comparing retention times with those of the authentic standards. Chromatographic analysis was performed using a SpectraSYSTEM HPLC system (Thermo Separation Products, Austin, TX, USA), which included a P2000 secondary solvent pump, an AS3000 autosampler with a 100 μL injection loop and a UV6000LP diode array detector. The chromatographic separation was performed on a Nucleosil 100-5 C18, 250 × 4.6 mm, 5-μm and reversed-phase (RP) column (Macherey–Nagel, Düren, Germany). The column oven temperature was 40 °C, the injection volume was 5 μL and the total runtime was 40 min. The mobile phases were aqueous formic acid 5% (Solvent A) and methanol (Solvent B) at a flow rate of 1 mL/min. The gradient elution program was applied, starting with 10% Solvent B (90% A) and held for 22 min, at which point the ratio was adjusted to 50:50. This was followed by a sharp increase to 95% B at 32 min, maintained briefly until 34 min and re-equilibrated to 10% B (90% A) by 35 min, which was maintained until the end at 40 min.
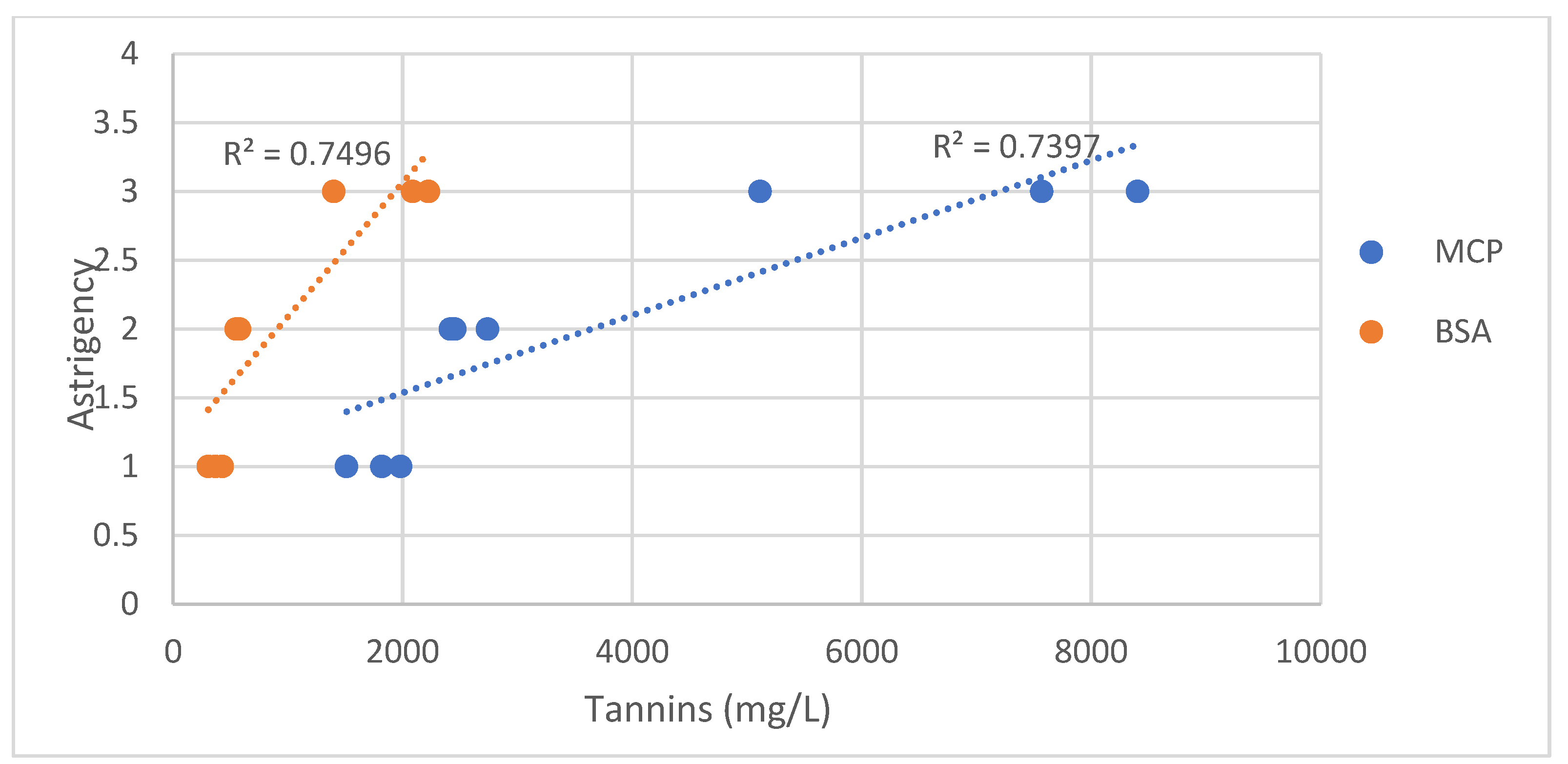
2.5.4. Analysis of Tannins
The tannin contents in the grape and wine samples were evaluated using protein precipitation assays with bovine serum albumin (BSA), following an established methodology [
34]. The absorbance at 510 nm was recorded using a UV-/VIS spectrophotometer. Additionally, tannins were measured with an alternative method developed by Sarneckis et al. [
35], and the estimated tannin levels were compared to those of a control sample, with a methylcellulose-precipitated sample, and the absorbance measured at 280 nm. For both methods, the tannin concentration was determined based on a catechin standard curve, and the results are expressed in mg catechin equivalents per liter.
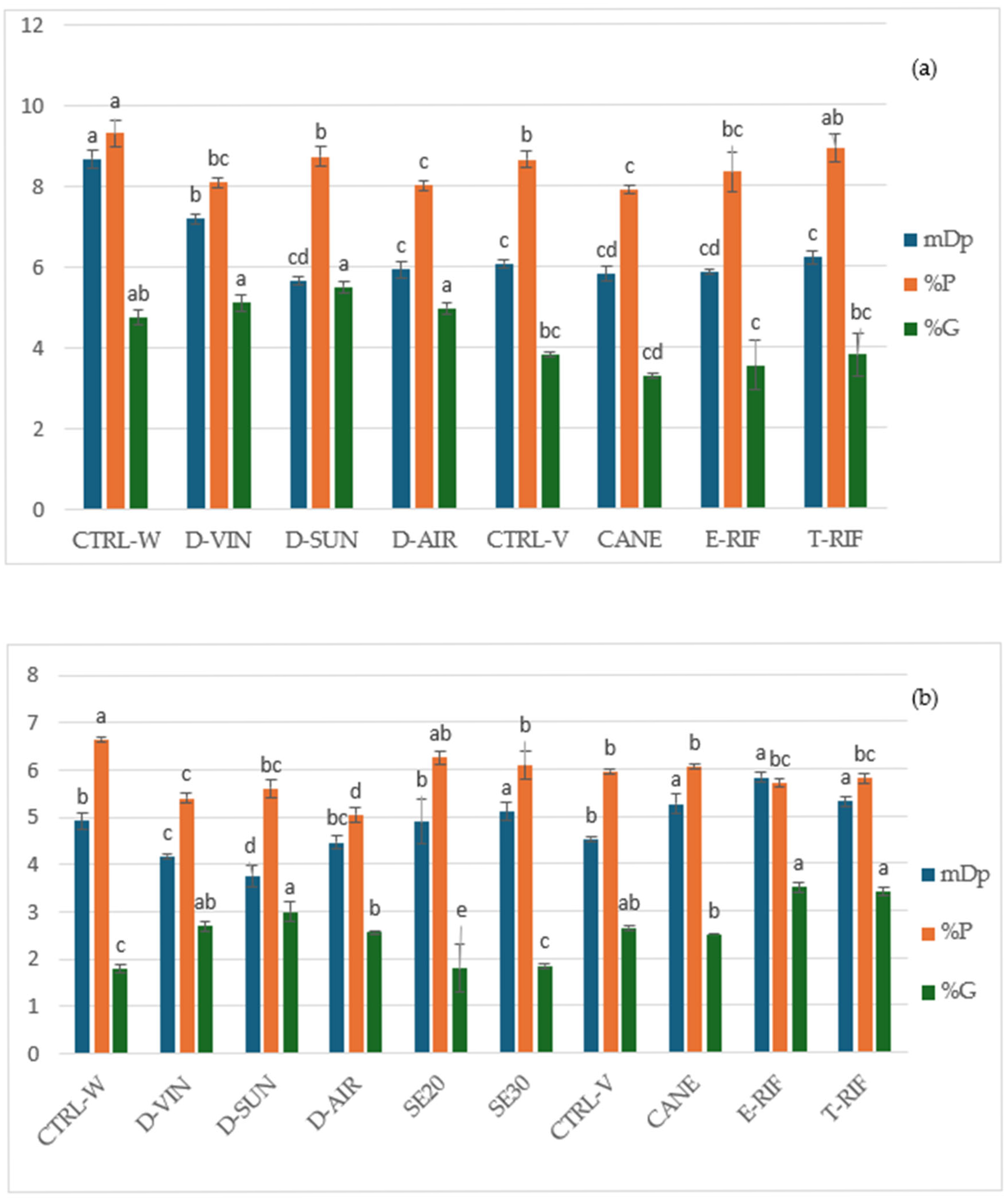
The mean degree of polymerization (mDP), percentage of galloylation (%G) and prodelphinidin (%P) of the tannins were determined using acid-catalyzed cleavage in the presence of excess phloroglucinol. No fractionation was performed to differentiate oligomeric and polymeric proanthocyanidins, and the total tannin extracts were dissolved in methanol and reacted with phloroglucinol reagent, followed by quenching with aqueous sodium acetate, according to modifications of previously described methods [
27,
36,
37]. The reaction products were analyzed by LC/MS on a Shimadzu 2010A (Shimatzu Corporation, Tokyo, Japan) coupled to a single-quadrupole mass spectrometer equipped with an electrospray ion source, according to the method described by Kyraleou et al. [
18]. The monomers (+)-catechin, (-)-epicatechin, (-)-epicatechin-3-
O-gallate and (--)-epigallocatechin were identified by comparing their retention times with those of the pure compounds. Separation was achieved on a reversed-phase Waters XTerra RR C18 (100 × 4.6 mm, 3.5 μm) column, at a flow rate of 0.5 mL/min, and the column oven temperature was 30 °C with an injection volume of 20 μL. The elution program involved decreasing eluent A from 80% to 40% over 20 min, maintaining this composition isocratically for an additional 10 min and then increasing it back to 80% over 2 min. Eluent A consisted of 0.1% acetic acid in water, while eluent B was methanol. Each sample was analyzed in triplicate. The elution performed under previously established conditions [
38].
2.6. Sensory Analysis
Fifteen healthy participants from the Laboratory of Oenology of the Agricultural University of Athens took part in this study. All participants were skilled wine assessors who had undergone prior training in evaluating astringency. The experiment was conducted over three weeks, comprising six sessions (three replications). The assessments took place between 11:00 a.m. and 1:00 p.m. in individual booths.
A balanced block design was implemented to counteract the progressive accumulation of astringency perception and to equalize the impact of presentation order. The panelists were given 10 mL samples at room temperature and instructed to assess astringency intensity on a scale from 0 to 7. To prevent any lingering effects from previous samples, a five-minute break was observed between tastings, during which panelists rinsed their mouths with water. This ensured the restoration of normal oral lubrication and minimized any carryover effects. To comply with the research code of ethics, the sensorial evaluation of the wines received approval from the Research Ethics Commission of Agricultural University of Athens.
2.7. Statistical Analysis
A statistical analysis was conducted to evaluate differences at a 95% confidence level (p < 0.05). The analysis included one-way ANOVA performed using Microsoft Excel (Microsoft Excel 2019, Excel for Microsoft 365) with the Data Analysis tool, followed by Tukey’s HSD test for mean comparison when significant differences were observed. All analyses were conducted in triplicate to ensure reliability.
4. Conclusions
This study provides clear and quantifiable evidence that targeted vineyard interventions significantly impact grape and wine quality characteristics of the Mandilaria variety. Among these, total defoliation proved most effective, resulting in the highest anthocyanin content per berry (1.47 mg/berry), increased pigment concentration and superior wine color intensity (20.72 AU), alongside a lower hue value (5) indicative of a more vibrant red color. Long pruning enhanced the anthocyanin extractability (53.04%), facilitating more efficient pigment transfer during maceration. These interventions also influenced anthocyanin composition, favoring delphinidin, petunidin and malvidin derivatives, with the T-RIF treatments showing enriched malvidin acetates, known markers of phenolic maturity and stability. While pruning increased acidity without altering sugar levels—potentially supporting longer aging—defoliation improved sugar accumulation and lowered acidity, optimizing the balance for red wine production. Although the phenolic content and antioxidant activity changes in the grapes were modest, wines from treated vines—particularly under the T-RIF—exhibited significantly elevated levels, confirming that the combined effects of these viticultural practices substantially enhance wine composition, color vibrancy and aging potential.
Complementing the vineyard results, the study also demonstrates that post-harvest winery techniques play a decisive role in shaping the phenolic and sensory profile of Mandilaria wines. Air dehydration emerged as particularly effective, yielding wines with elevated phenolic content, improved tannin structure and higher antioxidant activity, while simultaneously reducing the harsh tannin characteristics associated with the grape skins of the variety. Early seed removal during maceration reduced astringency without compromising wine structure, a result tied to a shift toward more polymerized skin-derived tannins (high mDp). In contrast, dehydration treatments exhibited lower mDp values, suggesting a disruption in polymerization processes. Although galloylation is typically associated with seed tannins, in the seed removal experiment no significant changes were observed, whereas all dehydration techniques led to an increase in the degree of galloylation. Prodelphinidin percentages (%P) remained stable with seed removal, as expected, but declined slightly with dehydration, potentially due to oxidative and enzymatic degradation.
The findings of this study demonstrate that both vineyard interventions and post-harvest dehydration techniques applied to Mandilaria grapes led to an increase in perceived astringency, while seed removal significantly reduced it. Although dehydration intensified the astringent character, it simultaneously contributed to the concentration of sugars and the enhancement of certain structural and sensory attributes of the wines. Importantly, the results highlight that the sensory perception of astringency was influenced not merely by the total tannin concentration but also by the quality and origin of tannins, with the seed-derived tannins playing a critical role in harsher sensations. The modulation of phenolic composition, berry morphology and sugar levels, as well as their combined impact on the sensory perception of astringency, were key factors shaping the final wine profile. In Mandilaria, a traditionally highly tannic variety, the application of these strategies allows for a more precise management of astringency, supporting the production of wines with greater balance, refined tannic structure and enhanced consumer appeal. These results underline the potential of integrated vineyard and winemaking practices to optimize the sensory characteristics of Mandilaria and promote its repositioning as a premium Greek variety in the modern enological landscape.