Intensification of the Dimethyl Sulfide Precursor Conversion Reaction: A Retrospective Analysis of Pilot-Scale Brewer’s Wort Boiling Experiments Using Hydrodynamic Cavitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrodynamic Cavitation
2.2. HC Device
2.3. Brewer’s Wort Boiling Trials
2.4. SMM Conversion Reaction Model
2.5. Free DMS Removal Efficiency
2.6. Sampling and Analyses
- pH: Measurement at 20 °C with a potentiometric method. Reference: ANALYTICA EBC 8.17/1999 [36].
- Bitterness: Determination in units of International Bitterness Unit (IBU) by extraction with trimethylpentane and spectrophotometric reading in the ultraviolet. Reference: ANALYTICA EBC 8.8/2004 [37].
- Color: Measurement of the wort color on the EBC scale, performed using a spectrophotometric method after filtration. Reference: ANALYTICA EBC 8.5/2000 [38].
- Free amino nitrogen (FAN): Determination with a colorimetric reading by spectrophotometer and ninhydrin. Reference: ANALYTICA EBC 8.10.1/2015 [39].
- Viscosity: Determination with a falling-ball microviscometer. Reference: ANALYTICA EBC 8.4/2004 [40].
- Beta glucans: Determination of high-molecular-weight beta glucans with an R-Biopharm kit (R-Biopharm Italia Srl, Melegnano, Italy) and colorimetric reading with a spectrophotometer. Reference: ANALYTICA EBC 4.16.3/2005 [41].
3. Results
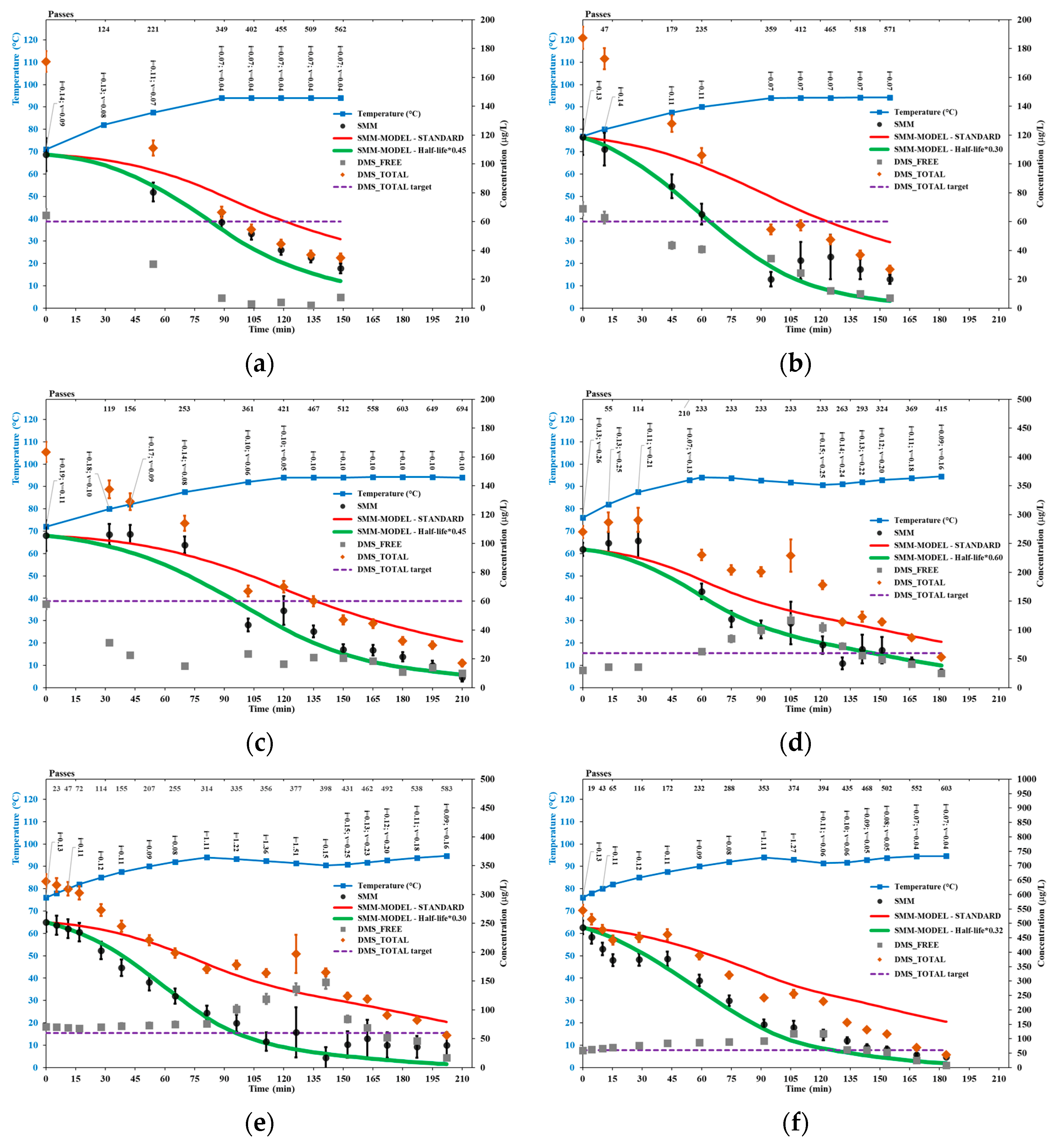
3.1. SMM Model Sensitivity to the Time Step
3.2. SMM and DMS in Standard Trials
3.3. SMM and DMS in HC Trials
3.4. Other Wort Properties in Selected HC Trials
4. Discussion
- The original gravity (HC2 < HC5 < HC6), with high gravity found to hinder the hop alpha-acid isomerization process [50];
- pH levels, which were slightly higher in trial HC2 than HC5 or HC6, with the isomerization reaction rate increasing with pH level [50];
- As reported in Section 3.3, trial HC6 was affected by excessive foaming in its early phase, which hindered the cavitation processes;
- The onset of a rest phase in trial HC5 that started after 80 min of process time, which likely hindered the isomerization process.
- The sensible heat loss in full-scale HC equipment would be comparatively much lower than the pilot scale device used in the considered trials, which would help save additional energy and process time. Indeed, the balance discussed in Section 3.3 between the power supplied by the pump, on the one hand, and the loss of sensible heat and latent heat of evaporation, on the other hand, would shift to higher temperatures than 94.4 ± 0.4 °C, in turn corresponding to higher SMM conversion rates while further reducing the process time and energy consumption.
- Larger centrifugal pumps used to drive the wort circulation in full-scale HC equipment would be more energy-efficient than the pump used in the experimental trials discussed in this study, which would help save additional energy.
- A large amount of latent heat from evaporation is available in full-scale equipment used for brewer’s wort boiling. It is common practice in industrial breweries to use such waste heat from previous boiling sessions to perform preliminary wort heating because all the desired processes in conventional wort boiling practically occur starting from a temperature around 95 °C. Heating the wort before HC processing, for example, raising the wort temperature to 85 or 90 °C, would help achieve further and substantial energy savings due to the steep increase in the SMM conversion reaction rate with the temperature.
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheuren, H.; Baldus, M.; Methner, F.-J.; Dillenburger, M. Evaporation Behaviour of DMS in an Aqueous Solution at Infinite Dilution—A Review. J. Inst. Brew. 2016, 122, 181–190. [Google Scholar] [CrossRef]
- Bamforth, C.W. Dimethyl Sulfide—Significance, Origins, and Control. J. Am. Soc. Brew. Chem. 2014, 72, 165–168. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Beer-Brewing Powered by Controlled Hydrodynamic Cavitation: Theory and Real-Scale Experiments. J. Clean. Prod. 2017, 142, 1457–1470. [Google Scholar] [CrossRef]
- Scheuren, H.; Tippmann, J.; Methner, F.J.; Sommer, K. Decomposition Kinetics of Dimethyl Sulphide. J. Inst. Brew. 2014, 120, 474–476. [Google Scholar] [CrossRef]
- Anness, B.J.; Bamforth, C.W. Dimethyl Sulphide—A Review. J. Inst. Brew. 1982, 88, 244–252. [Google Scholar] [CrossRef]
- Dickenson, C.J. Identification of the Dimethyl Sulphide Precursor in Malt. J. Inst. Brew. 1979, 85, 329–333. [Google Scholar] [CrossRef]
- Luo, D.; Tian, B.; Li, J.; Zhang, W.; Bi, S.; Fu, B.; Jing, Y. Mechanisms Underlying the Formation of Main Volatile Odor Sulfur Compounds in Foods during Thermal Processing. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13389. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Kim, J.S.; Kim, M.-H.; Park, J.-H.; Lee, J.-Y.; Lee, W.; Min, K.K.; Song, M.G.; Choi, C.-Y.; Kim, W.-S.; et al. Effect of Enhancers on in Vitro and in Vivo Skin Permeation and Deposition of S-Methyl-L-Methionine. Biomol. Ther. 2017, 25, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, C.J. The Relationship of Dimethyl Sulphide Levels in Malt, Wort and Beer. J. Inst. Brew. 1979, 85, 235–239. [Google Scholar] [CrossRef]
- Luo, D.; Pang, X.; Xu, X.; Bi, S.; Zhang, W.; Wu, J. Identification of Cooked Off-Flavor Components and Analysis of Their Formation Mechanisms in Melon Juice during Thermal Processing. J. Agric. Food Chem. 2018, 66, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Stafisso, A.; Marconi, O.; Perretti, G.; Fantozzi, P. Determination of Dimethyl Sulphide in Brewery Samples by Headspace Gas Chromatography Mass Spectrometry (HS-GC/MS). Ital. J. Food Sci. 2011, 23, 19–27. [Google Scholar]
- Pires, E.; Brányik, T. Biochemistry of Beer Fermentation; Springer International Publishing AG: Cham, Switzerland, 2015; ISBN 978-3-319-15188-5. [Google Scholar]
- Luo, D.; Xu, X.; Bi, S.; Liu, Y.; Wu, J. Study of the Inhibitors of Cooked Off-Flavor Components in Heat-Treated XiZhou Melon Juice. J. Agric. Food Chem. 2019, 67, 10401–10411. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Albanese, L.; Di Stefano, V.; Delisi, R.; Avellone, G.; Meneguzzo, F.; Pagliaro, M. Beer Produced via Hydrodynamic Cavitation Retains Higher Amounts of Xanthohumol and Other Hops Prenylflavonoids. LWT 2018, 91, 160–167. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Gluten Reduction in Beer by Hydrodynamic Cavitation Assisted Brewing of Barley Malts. LWT 2017, 82, 342–353. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, G.; Petkovšek, M.; Long, K.; Coutier-Delgosha, O. Intensity and Regimes Changing of Hydrodynamic Cavitation Considering Temperature Effects. J. Clean. Prod. 2022, 338, 130470. [Google Scholar] [CrossRef]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process Intensification Strategies for Lignin Valorization. Chem. Eng. Process.-Process Intensif. 2022, 171, 108732. [Google Scholar] [CrossRef]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-Thermal, Energy Efficient Hydrodynamic Cavitation for Food Processing, Process Intensification and Extraction of Natural Bioactives: A Review. Ultrason. Sonochem 2023, 98, 106504. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Natural Product Extraction via Hydrodynamic Cavitation. Sustain. Chem. Pharm. 2023, 33, 101083. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, J.; Rahimi, M.; Halim, R.; Shen, H.; Tiwari, B.K.; Zhao, L. Chemistry of Hydrodynamic Cavitation Technology. In Chemistry of Thermal and Non-Thermal Food Processing Technologies; Brijesh, K.T., Mysore, B.L., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 259–287. ISBN 9780443221828. [Google Scholar]
- Capocelli, M.; Musmarra, D.; Prisciandaro, M.; Lancia, A. Chemical Effect of Hydrodynamic Cavitation: Simulation and Experimental Comparison. AIChE J. 2014, 60, 2566–2572. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater Treatment Using Hybrid Treatment Schemes Based on Cavitation and Fenton Chemistry: A Review. Ultrason. Sonochem 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, C.; Mou, J.; Qian, H.; Duan, Z.; Zhang, S.; Zhou, P. Numerical Study of Rotating Cavitation and Pressure Pulsations in a Centrifugal Pump Impeller. AIP Adv. 2024, 14, 105024. [Google Scholar] [CrossRef]
- Shen, X.; Wu, H.; Yang, G.; Tang, R.; Chang, C.; Xu, B.; Lin, S.; Zhang, D. Experimental Study on the Classification and Evolution of the Tip Cavitation Morphology in Axial Waterjet Pumps with Two Different Blade Numbers. J. Mar. Sci. Eng. 2024, 12, 1898. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.; Xuan, X.; Manickam, S.; Boczkaj, G.; Wang, B. Assessing the Industrialization Progress of Hydrodynamic Cavitation Process Intensification Technology: A Review. Curr. Opin. Chem. Eng. 2024, 45, 101037. [Google Scholar] [CrossRef]
- Manoharan, D.; Radhakrishnan, M.; Tiwari, B.K. Cavitation Technologies for Extraction of High Value Ingredients from Renewable Biomass. TrAC—Trends Anal. Chem. 2024, 174, 117682. [Google Scholar] [CrossRef]
- Mathijssen, A.J.T.M.; Lisicki, M.; Prakash, V.N.; Mossige, E.J.L. Culinary Fluid Mechanics and Other Currents in Food Science. Rev. Mod. Phys. 2023, 95, 25004. [Google Scholar] [CrossRef]
- Iyer, G.; Pandit, A.B. Bridging Ingenuity and Utility in Cavitation─A Pioneer’s Predicament. Ind. Eng. Chem. Res. 2024, 63, 12265–12276. [Google Scholar] [CrossRef]
- EN 50470-1:2006. Electricity Metering Equipment (a.c.)—Part 1: General Requirements, Tests and Test Conditions—Metering Equipment (Class Indexes A, B and C). Available online: https://standards.iteh.ai/catalog/standards/clc/de32db50-81a9-4169-8710-6d969d07fe11/en-50470-1-2006 (accessed on 29 January 2025).
- EN 50470-3:2006. Electricity Metering Equipment (a.c.)—Part 1: General Requirements, Tests and Test Conditions—Metering Equipment (Class Indexes A, B and C). Available online: https://standards.iteh.ai/catalog/standards/clc/06ca5058-b825-47d1-bef4-85f652c79ee2/en-50470-3-2006 (accessed on 29 January 2025).
- General Requirements for the Competence of Testing and Calibration Laboratories (ISO/IEC 17025:2017). Available online: https://standards.iteh.ai/catalog/standards/cen/8284a587-bc96-4342-8585-8953be8f37a3/en-iso-iec-17025-2017 (accessed on 29 January 2025).
- ISO 9001:2015. Quality Management Systems — Requirements. Available online: https://standards.iteh.ai/catalog/standards/iso/9563cbca-c505-47c0-ba11-9c961f5a927e/iso-9001-2015 (accessed on 29 January 2025).
- Floridi, S.; Miniati, E.; Montanari, L.; Fantozzi, P. Carbohydrate Determination in Wort and Beer by HPLC-ELSD. Monatsschrift Fü Brauwiss. 2001, 54, 209–215. [Google Scholar]
- Rosendal, I.; Schmidt, F. The Alcohol Table for Beer Analysis and Polynomials for Alcohol and Extract. J. Inst. Brew. 1987, 93, 373–377. [Google Scholar] [CrossRef]
- 8.3—Extract of Wort. Available online: https://brewup.eu/ebc-analytica/wort/extract-of-wort/8.3 (accessed on 29 January 2025).
- 8.1—Sampling of Wort. Available online: https://brewup.eu/ebc-analytica/wort/sampling-of-wort/8.1 (accessed on 29 January 2025).
- 8.8—Bitterness of Wort. Available online: https://brewup.eu/ebc-analytica/wort/bitterness-of-wort/8.8 (accessed on 29 January 2025).
- 8.5—Colour of Wort: Spectrophotometric Method (IM). Available online: https://brewup.eu/ebc-analytica/wort/colour-of-wort-spectrophotometric-method-im/8.5 (accessed on 29 January 2025).
- 8.10.1—Free Amino Nitrogen in Wort by Spectrophotometry—Manual Method (IM). Available online: https://brewup.eu/ebc-analytica/wort/free-amino-nitrogen-in-wort-by-spectrophotometry-manual-method-im/8.10.1 (accessed on 29 January 2025).
- 8.4—Viscosity of Wort (IM). Available online: https://brewup.eu/ebc-analytica/wort/viscosity-of-wort-im/8.4 (accessed on 29 January 2025).
- 4.16.3—High Molecular Weight β-Glucan Content of Malt Wort: Spectrophotometric Method. Available online: https://brewup.eu/ebc-analytica/malt/high-molecular-weight-glucan-content-of-malt-wort-spectrophotometric-method/4.16.3 (accessed on 29 January 2025).
- Štěrba, J.; Punčochář, M.; Brányik, T. The Effect of Hydrodynamic Cavitation on Isomerization of Hop Alpha-Acids, Wort Quality and Energy Consumption during Wort Boiling. Food Bioprod. Process. 2024, 144, 214–219. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Wastewater Remediation via Controlled Hydrocavitation. Environ. Rev. 2017, 25, 175–183. [Google Scholar] [CrossRef]
- Carpenter, J.; Badve, M.; Rajoriya, S.; George, S.; Saharan, V.K.; Pandit, A.B. Hydrodynamic Cavitation: An Emerging Technology for the Intensification of Various Chemical and Physical Processes in a Chemical Process Industry. Rev. Chem. Eng. 2017, 33, 433–468. [Google Scholar] [CrossRef]
- Ding, W.; Hong, F.; Ying, D.; Huang, Y.; Nawaz Khan, S.; Jia, J. A Comprehensive Study on the Effects of Annular Protrusion for Cavitation Intensification in Venturi Tubes. Chem. Eng. J. 2024, 498, 155306. [Google Scholar] [CrossRef]
- Folden, T.S.; Aschmoneit, F.J. A Classification and Review of Cavitation Models with an Emphasis on Physical Aspects of Cavitation. Phys. Fluids 2023, 35, 81301. [Google Scholar] [CrossRef]
- Luo, J.; Fu, G.; Xu, W.; Zhai, Y.; Bai, L.; Li, J.; Qu, T. Experimental Study on Attenuation Effect of Liquid Viscosity on Shockwaves of Cavitation Bubbles Collapse. Ultrason. Sonochem 2024, 111, 107063. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, W.G.; Han, X.D. Effects of Solid Particles at Varying Concentrations on Hydrodynamic Cavitation Evolution in a Nozzle. J. Appl. Fluid. Mech. 2025, 18, 485–503. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Liu, C. Beers. In Current Developments in Biotechnology and Bioengineering: Food and Beverages Industry; Pandey, A., Sanromán, M.Á., Du, G., Soccol, C.R., Dussap, C.-G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–351. ISBN 9780444636775. [Google Scholar]
- Mudura, E.; Muste, S. Improving the Hop Utilization in the Beer Biotechnology. Bull. UASVM 2008, 65, 281–286. [Google Scholar]
- Tienaho, J.; Liimatainen, J.; Myllymäki, L.; Kaipanen, K.; Tagliavento, L.; Ruuttunen, K.; Rudolfsson, M.; Karonen, M.; Marjomäki, V.; Hagerman, A.E.; et al. Pilot Scale Hydrodynamic Cavitation and Hot-Water Extraction of Norway Spruce Bark Yield Antimicrobial and Polyphenol-Rich Fractions. Sep. Purif. Technol. 2024, 360, 130925. [Google Scholar] [CrossRef]
- Wright, C.; Nyberg, D. Corporations and Climate Change: An Overview. WIREs Clim. Change 2024, 15, e919. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L. A Method and Relative Apparatus for the Production of Beer 2016. U.S. Patent 11,261,411, 18 May 2022. [Google Scholar]
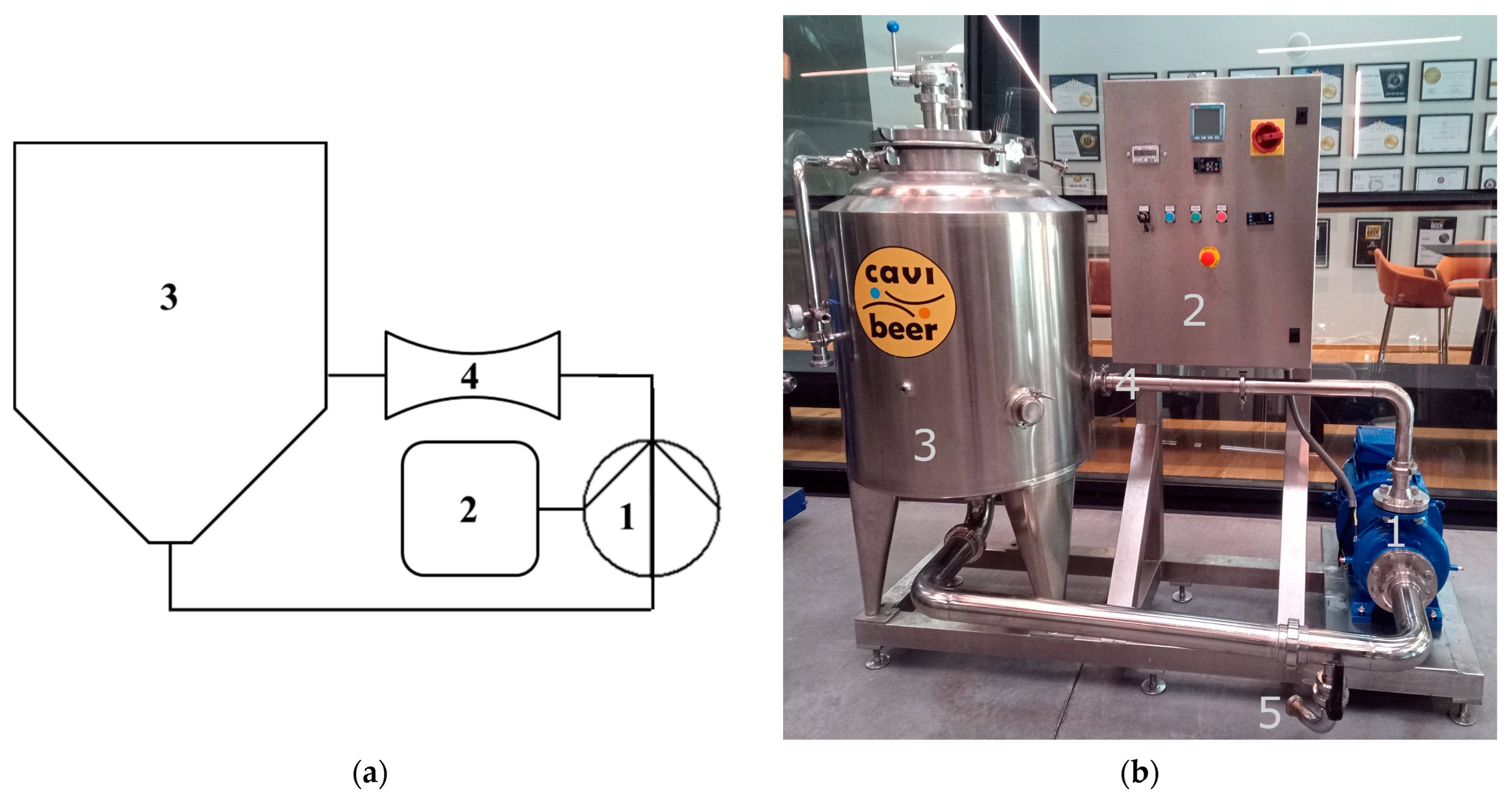
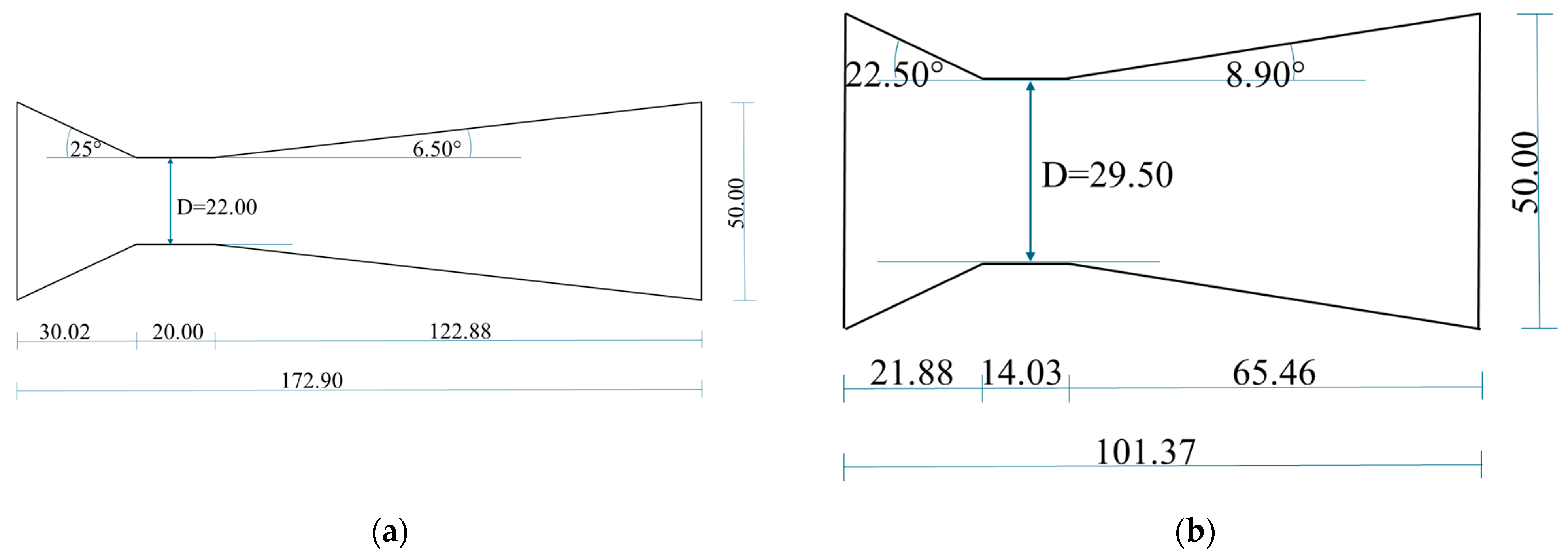

| Trial ID | Wort Volume (L) | Venturi a | Temperature (°C) | pH | Viscosity b (mPa·s) | Plato | Process Time (Minutes) |
|---|---|---|---|---|---|---|---|
| STD1 | 5000 | / | 88.8 | 5.40 ± 0.08 | 13.2 ± 0.1 | 81 | |
| STD2 | 180 | / | 75.0 | 5.35 ± 0.08 | 2.45 ± 0.04 | 19.6 ± 0.1 | 87 |
| HC1 | 210 | V22.0 | 71.0 | 5.30 ± 0.08 | 13.0 ± 0.1 | 149 | |
| HC2 | 210 | / | 76.8 | 5.45 ± 0.08 | 1.82 ± 0.04 | 13.8 ± 0.1 | 155 |
| HC3 | 210 | V22.0 | 72.0 | 5.26 ± 0.08 | 13.7 ± 0.1 | 210 | |
| HC4 | 210 | V29.5 | 76.0 | 5.29 ± 0.08 | 17.8 ± 0.1 | 181 | |
| HC5 | 210 | V29.5 | 76.0 | 5.16 ± 0.08 | 2.10 ± 0.04 | 17.0 ± 0.1 | 202 |
| HC6 | 210 | V22.0 | 76.0 | 5.42 ± 0.08 | 2.60 ± 0.04 | 20.8 ± 0.1 | 183 |
| Test ID | Process Time (Minutes) | Passes | Temperature (°C) | pH | IBU a | Hop Utilization (%) | Color (EBC) |
|---|---|---|---|---|---|---|---|
| HC2 | 0 | 0 | 76.8 | 5.45 ± 0.08 | 3.0 ± 1.0 | 7.1 ± 1.9 | |
| 95 | 359 | 94.0 | 5.38 ± 0.08 | 25.0 ± 3.0 | 62 ± 27 | 9.3 ± 2.1 | |
| HC5 | 0 | 0 | 76.0 | 5.16 ± 0.08 | 2.0 ± 0.1 | 7.4 ± 1.9 | |
| 141 b | 398 | 90.5 | 5.08 ± 0.08 | 17.1 ± 0.1 | 42 ± 11 | 9.6 ± 2.2 | |
| 202 | 583 | 94.8 | 5.04 ± 0.08 | 18.0 ± 2.0 | 45 ± 17 | 12.0 ± 2.4 | |
| HC6 | 0 | 0 | 76.0 | 5.42 ± 0.08 | 7.0 ± 0.1 | 10.2 ± 2.2 | |
| 168 | 552 | 94.5 | 5.26 ± 0.08 | 23.0 ± 1.0 | 45 ± 14 | 15.3 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneguzzo, F.; Albanese, L. Intensification of the Dimethyl Sulfide Precursor Conversion Reaction: A Retrospective Analysis of Pilot-Scale Brewer’s Wort Boiling Experiments Using Hydrodynamic Cavitation. Beverages 2025, 11, 22. https://doi.org/10.3390/beverages11010022
Meneguzzo F, Albanese L. Intensification of the Dimethyl Sulfide Precursor Conversion Reaction: A Retrospective Analysis of Pilot-Scale Brewer’s Wort Boiling Experiments Using Hydrodynamic Cavitation. Beverages. 2025; 11(1):22. https://doi.org/10.3390/beverages11010022
Chicago/Turabian StyleMeneguzzo, Francesco, and Lorenzo Albanese. 2025. "Intensification of the Dimethyl Sulfide Precursor Conversion Reaction: A Retrospective Analysis of Pilot-Scale Brewer’s Wort Boiling Experiments Using Hydrodynamic Cavitation" Beverages 11, no. 1: 22. https://doi.org/10.3390/beverages11010022
APA StyleMeneguzzo, F., & Albanese, L. (2025). Intensification of the Dimethyl Sulfide Precursor Conversion Reaction: A Retrospective Analysis of Pilot-Scale Brewer’s Wort Boiling Experiments Using Hydrodynamic Cavitation. Beverages, 11(1), 22. https://doi.org/10.3390/beverages11010022








