Evaluation of Fractions Obtained Through Steam Distillation and Hydroalcoholic Maceration of Wood Chips from Pinus mugo for Flavouring Italian Spirit grappa
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Treatments
2.2. Steam Distillation
2.3. Hydroalcoholic Maceration
2.4. Grappa Flavouring
2.5. Chemical Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results
3.1. Steam Distillation and Hydroalcoholic Extraction
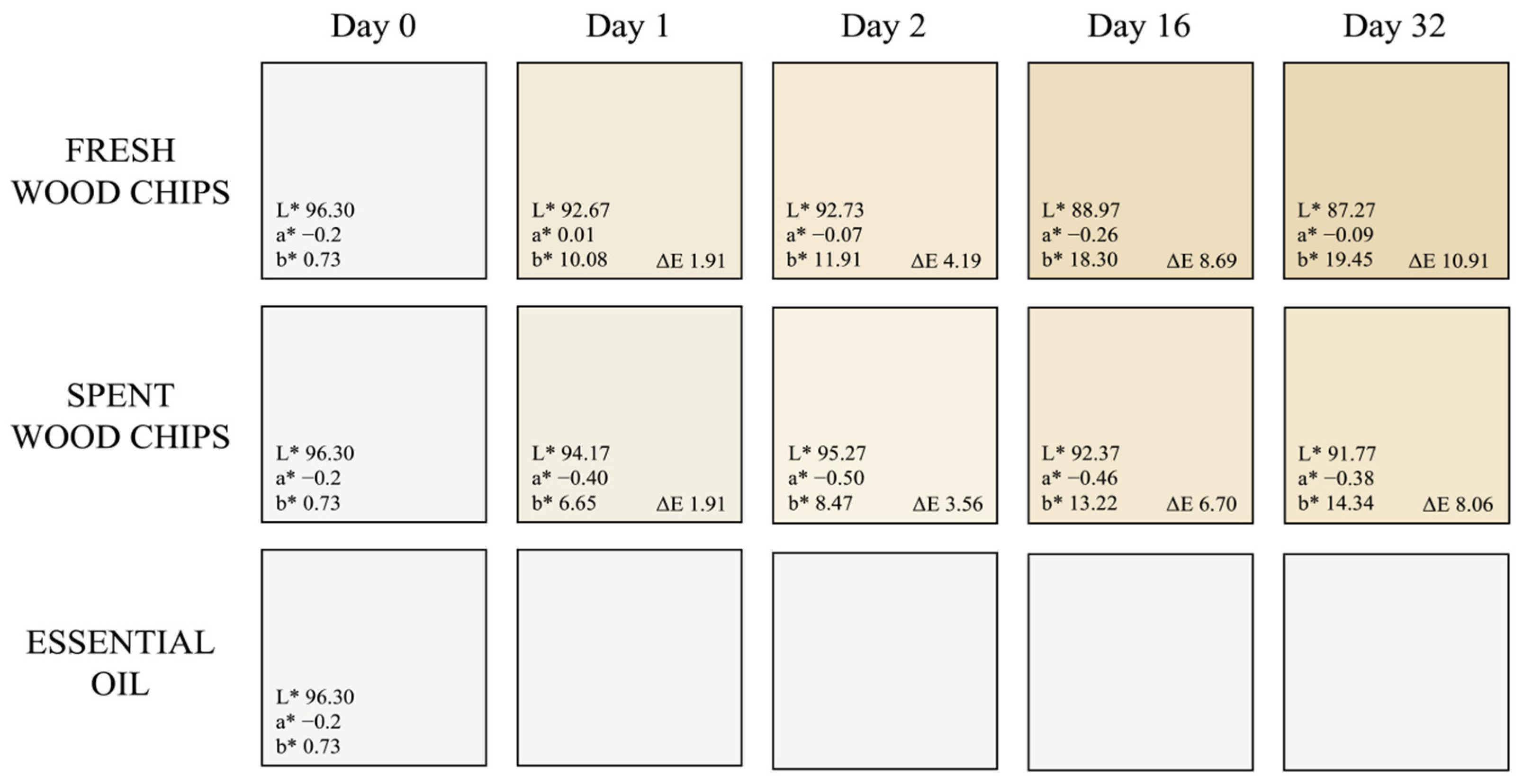
Extraction Kinetics
3.2. Analysis of Grappa Flavouring
3.2.1. Chemical Composition
3.2.2. Sensory Analysis
3.2.3. Safety Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Porto, C. Grappa: Production, Sensory Properties and Market Development. In Alcoholic Beverages: Sensory Evaluation and Consumer Research; Woodhead Publishing: Sawston, UK, 2011; pp. 299–314. [Google Scholar]
- Decreto Ministeriale n. 747, G.U. Scheda Tecnica della Grappa, Ministry of Agricultural, Food and Forestry Policies of Italy. 2016. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2016-02-08&atto.codiceRedazionale=16A00865 (accessed on 29 January 2016).
- Motti, R.; Bonanomi, G.; De Falco, B. Wild and Cultivated Plants Used in Traditional Alcoholic Beverages in Italy: An Ethnobotanical Review. Eur. Food Res. Technol. 2022, 248, 1089–1106. [Google Scholar] [CrossRef]
- Stevanovic, T.; Garneau, F.; Jean, F.; Gagnon, H.; Vilotic, D.; Petrovic, S.; Ruzic, N.; Pichette, A. The Essential Oil Composition of Pinus Mugo Turra from Serbia. Flavour Fragr. J. 2005, 20, 96–97. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Karanfilova, I.C.; Panovska, T.K.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Chemical Characterization and Antioxidant Activity of Mountain Pine (Pinus Mugo Turra, Pinaceae) from Republic of Macedonia. Rec. Nat. Prod. 2018, 13, 50–63. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, E.; Pentimalli, D.; et al. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oils of Different Pinus Species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Lis, A.; Lukas, M.; Mellor, K. Comparison of Chemical Composition of the Essential Oils from Different Botanical Organs of Pinus Mugo Growing in Poland. Chem. Biodivers. 2019, 16, e1900397. [Google Scholar] [CrossRef]
- Garzoli, S.; Vaglia, V.; Iriti, M.; Vitalini, S. Vapor and Liquid Phase Profiles of Essential Oils from Abies, Picea and Pinus Species and Their Phytotoxic Interactions with Weed Growth in Pre- and Post-Emergence Conditions. Plants 2023, 12, 1172. [Google Scholar] [CrossRef]
- Valussi, M. Materia Aromatica; Tecniche Nuove: Milan, Italy, 2023. [Google Scholar]
- Semerdjieva, I.; Zheljazkov, V.D.; Cantrell, C.L.; Koleva-Valkova, L.; Maneva, V.; Radoukova, T.; Astatkie, T.; Kačániová, M.; Slavov, S.B.; Atanasova, D.; et al. Phytochemical Composition and Biopesticidal Potential of Pinus Mugo Turra Essential Oil. Ind. Crops Prod. 2024, 209, 118019. [Google Scholar] [CrossRef]
- Tolvaj, L.; Papp, G.; Varga, D.; Lang, E. Effect of Steaming on the Colour Change of Softwoods. BioResources 2012, 7, 2799–2808. [Google Scholar] [CrossRef]
- Wickramasinghe, Y.W.H.; Wickramasinghe, I.; Wijesekara, I. Effect of Steam Blanching, Dehydration Temperature & Time, on the Sensory and Nutritional Properties of a Herbal Tea Developed from Moringa oleifera Leaves. Int. J. Food Sci. 2020, 2020, 5376280. [Google Scholar] [CrossRef]
- Saikumar, A.; Singh, A.; Dobhal, A.; Arora, S.; Junaid, P.M.; Badwaik, L.S.; Kumar, S. A Review on the Impact of Physical, Chemical, and Novel Treatments on the Quality and Microbial Safety of Fruits and Vegetables. Syst. Microbiol. Biomanuf. 2024, 4, 575–597. [Google Scholar] [CrossRef]
- Cavalli, R.; Pellegrini, M.; Grigolato, S.; Bietresato, M. A Strategy for the Management of Abandoned Mountain Pasture Land Colonised by Dwarf Pine. L’Italia For. E Mont. 2011, 66, 383–393. [Google Scholar] [CrossRef][Green Version]
- Friso, D.; Grigolato, S.; Cavalli, R. Energetic and Exergetic Analysis of Steam Production for the Extraction of Coniferous Essential Oils. Biomass Bioenergy 2011, 35, 4045–4056. [Google Scholar] [CrossRef]
- ISO 18134-2:2017; Solid Biofuels-Determination of Moisture Content-Oven Dry Method Part 2: Total Moisture-Simplified Method. International Standard Organization: Geneva, Switzerland, 2017.
- Internationale Beleuchtungskommission (Ed.) Colorimetry, 3rd ed.; Commission International de l’éclairage (CIE): Wien, Austria, 2004; ISBN 978-3-901906-33-6. [Google Scholar]
- Hirschler, R. Whiteness, Yellowness, and Browning in Food Colorimetry: A Critical Review. In Color in Food; Caivano, J.L., Del Pilar Buera, M., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 118–129. ISBN 978-0-429-11069-6. [Google Scholar]
- Lomolino, G.; Crapisi, A.; Cagnin, M. Study of Elements Concentrations of European Seabass (Dicentrarchus Labrax) Fillets after Cooking on Steel, Cast Iron, Teflon, Aluminum and Ceramic Pots. Int. J. Gastron. Food Sci. 2016, 5–6, 1–9. [Google Scholar] [CrossRef]
- Kemp, S.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-4051-6210-4. [Google Scholar]
- ISO 4120:2021; Sensory Analysis-Methodology-Triangle Test. International Standard Organization: Geneva, Switzerland, 2017.
- Schimitberger, V.M.B.; Pratti, D.L.D.A.; Cavalcanti, L.C.; Ramalho, V.F.; Costa, A.P.F.D.; Scherer, R.; Kuster, R.M.; Ramos, A.C.; Silva, A.G.D. Volatile Compounds Profile Changes from Unripe to Ripe Fruits of Brazilian Pepper (Schinus Terebinthifolia Raddi). Ind. Crops Prod. 2018, 119, 125–131. [Google Scholar] [CrossRef]
- Delgado-González, M.J.; García-Moreno, M.V.; Sánchez-Guillén, M.M.; García-Barroso, C.; Guillén-Sánchez, D.A. Colour Evolution Kinetics Study of Spirits in Their Ageing Process in Wood Casks. Food Control. 2021, 119, 107468. [Google Scholar] [CrossRef]
- Psarra, C.; Gortzi, O.; Makris, D.P. Kinetics of Polyphenol Extraction from Wood Chips in Wine Model Solutions: Effect of Chip Amount and Botanical Species: Polyphenol Extraction from Wooden Chips in Wine Model. J. Inst. Brew. 2015, 121, 207–212. [Google Scholar] [CrossRef]
- Canas, S. Phenolic Composition and Related Properties of Aged Wine Spirits: Influence of Barrel Characteristics. A Review. Beverages 2017, 3, 55. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Pelliccia, D.; Plozza, T.; Frisina, C.; Chandra, S.; Goodwin, I. Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour. Horticulturae 2021, 7, 255. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Islam, M.S.; Goodwin, I. A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards. Horticulturae 2022, 8, 459. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Mrvčić, J.; Srečec, S.; Filipan, K.; Blažić, M.; Stanzer, D. Physicochemical and Aromatic Characterization of Carob Macerates Produced by Different Maceration Conditions. Food Sci. Nutr. 2020, 8, 942–954. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Lukin, P.; Derewiaka, D.; Mrvčić, J.; Stanzer, D. Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album). Beverages 2022, 8, 46. [Google Scholar] [CrossRef]
- Gavahian, M.; Ratchaneesiripap, P.; Lin, Y. Bioactive Compounds Extraction from Oak Chips into Rice Spirit: New Application of Ultrasound. J. Food Process. Eng. 2023, 46, e14213. [Google Scholar] [CrossRef]
- ISO 21093:2003; Oil of Dwarf Pine (Pinus Mugo Turra). International Standard Organization: Geneva, Switzerland, 2003.
- Hassan, B.; Mankowski, M.E.; Kirker, G.; Ahmed, S. Effects of Heartwood Extractives on Symbiotic Protozoan Communities and Mortality in Two Termite Species. Int. Biodeterior. Biodegrad. 2017, 123, 27–36. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Vázquez-Araújo, L.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Optimization of the Process of Aromatic and Medicinal Plant Maceration in Grape Marc Distillates to Obtain Herbal Liqueurs and Spirits. J. Sci. Food Agric. 2016, 96, 4760–4771. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance); European Commission: Luxembourg, 2023. [Google Scholar]
- Decreto Legislativo n. 31, G.U. Scheda Tecnica della Grappa, Ministry of Agricultural, Food and Forestry Policies of Italy. 2001. Available online: https://www.gazzettaufficiale.it/eli/id/2001/03/03/001G0074/sg (accessed on 3 March 2001).
- Ministero delle Politiche Agricole Alimentari e Forestali (MIPAAF). Decreto Ministeriale n. 238, G.U. 29 August 2017; MIPAAF: Rome, Italy, 2017. [Google Scholar]
- OIV. Determination of Chromatic Characteristics According to CIELab. In Compendium of International Analysis of Methods; OIV-MA-AS2-11:2006 1-16; OIV: Paris, France, 2006. [Google Scholar]
- Carvalho, F.R.; Moors, P.; Wagemans, J.; Spence, C. The Influence of Color on the Consumer’s Experience of Beer. Front. Psychol. 2017, 8, 2205. [Google Scholar] [CrossRef] [PubMed]
- Jaud, D.A.; Lorey, T.; Pouzalgues, N.; Masson, G. The Effect of Rosé Wine Colors on Expected Flavor and Tastiness: A Cross-Modal Correspondence Explanation. Food Qual. Prefer. 2025, 123, 105308. [Google Scholar] [CrossRef]



| Heating time (min) | 28.00 ± 5.29 |
| Condensation time (min) | 65.16 ± 2.70 |
| Total operating time (min) | 93.16 ± 7.94 |
| Water flow rate (dm3/min) | 1.81 ± 0.12 |
| Total water use (dm3) | 117.44 ± 3.17 |
| Initial temperature (°C) | 13.67 ± 2.08 |
| Essential oil yield (cm3/kg) | 0.36 ± 0.12 |
| Fresh | Spent | ||
|---|---|---|---|
| pH | 5.51 ± 0.04 | 6.02 ± 0.16 | p = 0.01 |
| ORP (mV) | 72.67 ± 2.52 | 44.67 ± 8.96 | p = 0.01 |
| Fresh Wood Chips | Spent Wood Chips | |||||
|---|---|---|---|---|---|---|
| m | q | R2 | m | q | R2 | |
| b* | −6.53 ± 3.06 × 10−4 | 9.64 ± 1.31 × 100 | 7.49 ± 2.45 × 10−2 | −6.11 ± 4.05 × 10−4 | 1.36 ± 0.58 × 101 | 7.58 ± 1.46 × 10−2 |
| Chroma | −6.51 ± 3.10 × 10−4 | 9.65 ± 1.31 × 100 | 7.49 ± 2.48 × 10−2 | −6.10 ± 4.04 × 10−4 | 1.36 ± 0.58 × 101 | 7.58 ± 1.43 × 10−2 |
| ΔE | −1.11 ± 3.51 × 10−3 | 4.14 ± 2.63 × 100 | 8.07 ± 6.18 × 10−2 | −1.22 ± 5.73 × 10−3 | 5.14 ± 0.64 × 100 | 8.10 ± 9.26 × 10−3 |
| YI | −7.09 ± 2.58 × 10−4 | 1.46 ± 2.14 × 101 | 0.84 ± 3.82 × 10−2 | −6.85 ± 3.94 × 10−4 | 2.11 ± 1.04 × 101 | 7.97 ± 1.64 × 10−2 |
| WI | 1.19 ± 0.16 × 10−4 | 8.95 ± 1.74 × 101 | 8.71 ± 5.70 × 10−2 | 1.68 ± 0.04 × 10−4 | 8.50 ± 0.87 × 101 | 8.64 ± 1.52 × 10−2 |
| Compound | Concentration (%) | |||
|---|---|---|---|---|
| Essential Oil | Fresh | Spent | ||
| 2-Butenal, 3-methyl- | 0.01 ± 0.02 | <LOD | <LOD | n.s. |
| Hexanal | 1.05 ± 0.25 a | 1.04 ± 0.07 b | 0.21 ± 0.04 b | ** |
| Furfural | <LOD | <LOD | <LOD | n.s. |
| 1-Hexanol | 0.12 ± 0.03 a | 1.01 ± 0.21 a | 0.03 ± 0.00 b | *** |
| Cyclopentane, 1,2,3,4,5-pentamethyl- | 2.21 ± 1.12 | 11.09 ± 3.99 | 10.95 ± 2.67 | n.s. |
| Methyl hexanoate | 0.12 ± 0.09 | <LOD | <LOD | *** |
| α-Pinene | 14.53 ± 2.27 | <LOD | <LOD | * |
| Ethanone, 1-(1-methylcyclohexyl)- | 0.94 ± 0.47 | 5.32 ± 1.05 | 4.89 ± 1.07 | n.s. |
| Octane, 3-methyl-6-methylene- | 0.11 ± 0.06 | 1.1 ± 0.43 | 0.81 ± 0.18 | n.s. |
| Benzaldehyde | 0.1 ± 0.05 b | 1.17 ± 0.19 a | 0.45 ± 0.05 b | *** |
| β-Cymene | 2.02 ± 0.39 | <LOD | <LOD | ** |
| β-Pinene | 5.06 ± 0.54 a | 0.24 ± 0.05 b | 0.22 ± 0.06 b | * |
| β-Myrcene | 1.89 ± 0.33 | <LOD | <LOD | * |
| Ethyl hexanoate | <LOD b | 0.54 ± 0.13 a | 0.03 ± 0.01 b | *** |
| 3-Carene | 24.74 ± 1.47 a | 2.24 ± 0.44 b | 1.57 ± 0.28 b | ** |
| o-Cymene | 4.71 ± 0.42 a | 0.96 ± 0.18 b | 0.43 ± 0.07 b | ** |
| D-Limonene | 17.13 ± 1.86 | <LOD | <LOD | * |
| m-Mentha-6,8-diene, (R)-(+)- | <LOD | 0.85 ± 0.2 | 0.83 ± 0.18 | ** |
| Benzeneacetaldehyde | 7.36 ± 1.48 a | 0.06 ± 0.01 b | 0.03 ± 0.01 b | * |
| Eucalyptol | 0.01 ± 0.00 | <LOD | <LOD | *** |
| p-Cresol | 0.02 ± 0.02 | 0.06 ± 0.01 | 0.09 ± 0.02 | n.s. |
| p-Cymene | 0.25 ± 0.06 | <LOD | <LOD | *** |
| Isoterpinolene | <LOD | 0.02 ± 0.00 b | 0.05 ± 0.01 a | ** |
| Camphene hydrate | 0.18 ± 0.06 | <LOD | <LOD | *** |
| 4-Carene | 1.19 ± 0.27 | <LOD | <LOD | ** |
| Benzene, (2-methyl-1-propenyl)- | 0.70 ± 0.17 a | 0.23 ± 0.03 b | 0.16 ± 0.06 b | *** |
| α-Pinene oxide | <LOD | 0.01 ± 0.00 b | 0.06 ± 0.01 a | *** |
| Ethanone, 1-(2-methylphenyl)- | 0.01 ± 0.01 | <LOD | <LOD | ** |
| Phenol, 2-methoxy- | <LOD | <LOD | <LOD | * |
| Nonanal | 0.08 ± 0.01 | <LOD | <LOD | * |
| 4,5-Dimethylnonane | 0.01 ± 0 | <LOD | <LOD | *** |
| Camphene hydrate | 0.07 ± 0.03 | <LOD | <LOD | *** |
| trans-p-Menth-2-en-1-ol | 0.05 ± 0.02 | <LOD | <LOD | *** |
| cis-β-Terpineol | <LOD | <LOD | <LOD | ** |
| α-Campholenal | 0.25 ± 0.06 | <LOD | <LOD | ** |
| allo-Ocimene | 0.13 ± 0.03 | <LOD | <LOD | * |
| Pinocarveol | 0.16 ± 0.09 b | 0.28 ± 0.05 a | 0.07 ± 0.01 a | *** |
| Cyclohexanol, 4-(1-methylethyl)- | 0.30 ± 0.13 b | 0.74 ± 0.12 a | 0.45 ± 0.26 a | ** |
| 4-Isopropylcyclohexanone | 0.03 ± 0.01 c | 0.03 ± 0.01 b | 0.05 ± 0.01 a | *** |
| Phenol, 4-ethyl- | 0.04 ± 0.04 | <LOD | <LOD | * |
| Ethyl benzoate | 0.01 ± 0.00 b | 0.50 ± 0.07 a | 0.03 ± 0.01 b | *** |
| Borneol | 0.33 ± 0.17 b | 1.23 ± 0.35 a | 0.34 ± 0.16 a | ** |
| p-Mentha-1,5-dien-8-ol | 0.10 ± 0.05 b | 0.16 ± 0.03 a | 0.17 ± 0.04 a | ** |
| trans-Pinocamphone | 0.10 ± 0.04 | 1.12 ± 0.11 | 0.90 ± 0.38 | n.s. |
| (-)-4-Terpineol | 0.70 ± 0.31 | <LOD | <LOD | *** |
| Octanoic Acid | 0.04 ± 0.02 | 0.32 ± 0.03 | 0.2 ± 0.1 | n.s. |
| p-Cymen-8-ol | 0.65 ± 0.27 | 4.86 ± 0.52 | 2.33 ± 1.09 | n.s. |
| α-Terpineol | 1.30 ± 0.95 | <LOD | <LOD | * |
| Myrtenol | 0.30 ± 0.17 b | 0.57 ± 0.11 a | 0.16 ± 0.06 a | *** |
| Ethyl octanoate | 0.01 ± 0.00 b | 0.18 ± 0.01 a | 0.07 ± 0.04 ab | * |
| Verbenone | 0.29 ± 0.14 b | 0.80 ± 0.09 a | 0.42 ± 0.17 a | *** |
| cis-Carveol | 0.05 ± 0.02 | <LOD | <LOD | *** |
| Caron | 0.12 ± 0.05 | <LOD | <LOD | *** |
| Benzenemethanol, α-(1-methylethyl)-, (R)- | 0.06 ± 0.01 | <LOD | <LOD | ** |
| (R)-citronellol | 0.10 ± 0.05 b | 0.34 ± 0.09 ab | 0.06 ± 0.02 a | ** |
| Thymol methyl ether | <LOD | 1.12 ± 0.12 | 0.53 ± 0.18 | ** |
| 3-Isopropylbenzaldehyde | 0.12 ± 0.05 a | 0.09 ± 0.00 b | 0.03 ± 0.01 b | *** |
| Carvotanacetone | 0.13 ± 0.06 | <LOD | <LOD | *** |
| 3-Carvomenthenone | 0.15 ± 0.07 b | 0.33 ± 0.02 a | 0.16 ± 0.11 a | ** |
| Phellandral | 0.1 ± 0.05 | <LOD | <LOD | *** |
| cis-Anethol | 0.38 ± 0.17 a | 0.03 ± 0.00 c | 0.14 ± 0.01 b | *** |
| Thymol | 0.06 ± 0.02 | <LOD | <LOD | n.s. |
| Thymoquinon | <LOD c | 0.33 ± 0.05 a | 0.10 ± 0.00 b | *** |
| Limonene-1,2-diol | <LOD b | 0.46 ± 0.02 a | 0.36 ± 0.10 a | ** |
| α-Terpinyl acetate | 0.24 ± 0.01 | 0.73 ± 0.17 | 0.55 ± 0.16 | n.s. |
| Triacetin | 0.01 ± 0.01 c | 0.95 ± 0.35 b | 2.9 ± 0.76 a | *** |
| Copaene | 0.09 ± 0.03 | <LOD | <LOD | . |
| β-Elemene | 0.17 ± 0.07 | <LOD | <LOD | . |
| Ethyl decanoate | 0.04 ± 0.02 | <LOD | <LOD | *** |
| Vanillin | <LOD b | 3.88 ± 0.52 a | 2.22 ± 0.4 a | ** |
| Carvone hydrate | <LOD b | 0.13 ± 0.02 a | 0.06 ± 0.01 a | ** |
| Carvenone oxide | 0.05 ± 0.00 b | 0.19 ± 0.03 ab | 0.05 ± 0.00 a | * |
| Isoeugenol | <LOD | 0.22 ± 0.05 b | 0.26 ± 0.06 a | *** |
| 2′,4′-Dihydroxypropiophenone | 0.03 ± 0.01 | <LOD | <LOD | . |
| Ethyl cinnamate | 0.01 ± 0.00 b | 0.18 ± 0.04 a | 0.02 ± 0.01 b | ** |
| γ-Cadinene | 1.05 ± 0.38 | <LOD | <LOD | . |
| Acetoisovanillone | <LOD b | 0.17 ± 0.02 ab | 0.18 ± 0.05 a | ** |
| β-Selinene | 0.06 ± 0.02 | <LOD | <LOD | . |
| Isoeugenol methyl ether | 0.05 ± 0.02 | 0.13 ± 0.05 | 0.31 ± 0.23 | n.s. |
| α-Muurolene | 5.86 ± 1.87 | <LOD | <LOD | * |
| Butylated Hydroxytoluene | 0.02 ± 0.01 | 0.79 ± 0.39 | 0.62 ± 0.35 | n.s. |
| Guaiacylacetone | 0.04 ± 0.01 | 0.19 ± 0.07 | 0.24 ± 0.08 | n.s. |
| α-Cadinene | 0.07 ± 0.03 | <LOD | <LOD | . |
| α-Calacorene | 0.17 ± 0.02 | <LOD | <LOD | * |
| Spathulenol | 0.13 ± 0.04 b | 0.26 ± 0.05 a | 0.12 ± 0.03 a | ** |
| Caryophyllene oxide | 0.23 ± 0.03 b | 0.34 ± 0.04 a | 0.09 ± 0.02 a | ** |
| Humulane-1,6-dien-3-ol | 0.17 ± 0.01 b | 0.32 ± 0.07 a | 0.17 ± 0.07 a | * |
| Cubenol | 0.03 ± 0.00 b | 0.04 ± 0.01 a | 0.03 ± 0.01 a | ** |
| epi-α-Cadinol (τ-Cadinol) | 0.26 ± 0.03 b | 0.41 ± 0.10 a | 0.27 ± 0.09 a | ** |
| epi-α-Muurolol | 0.12 ± 0.02 b | 0.21 ± 0.04 a | 0.13 ± 0.04 a | ** |
| α-Cadinol | 0.19 ± 0.06 b | 0.46 ± 0.10 a | 0.31 ± 0.12 a | ** |
| Homovanillic acid | <LOD b | 0.49 ± 0.03 ab | 2.59 ± 1.39 a | * |
| 7-Acetyl-2-hydroxy-2-methyl-5-isopropylbicyclo[4.3.0]nonane | <LOD b | 1.52 ± 0.28 a | 0.75 ± 0.32 a | ** |
| Benzyl Benzoate | 0.01 ± 0.00 | 0.05 ± 0.03 | 0.09 ± 0.04 | n.s. |
| β-Hydroxypropiovanillone | <LOD b | 0.89 ± 0.22 a | 0.95 ± 0.28 a | * |
| p-Coumaric acid ethyl ester | <LOD | <LOD | <LOD | ** |
| Alloaromadendrene oxide-(1) | 0.01 ± 0.01 | <LOD | <LOD | ** |
| Sclarene | 0.02 ± 0.01 | <LOD | <LOD | * |
| Rimuene | 0.04 ± 0.01 | <LOD | <LOD | * |
| Ethyl hexadecanoate | 0.03 ± 0.02 | 0.21 ± 0.07 | 0.24 ± 0.07 | n.s. |
| Isoparvifuran | 0.01 ± 0.00 | <LOD | <LOD | * |
| Thunbergol | <LOD | <LOD | <LOD | * |
| Linoleic acid ethyl ester | 0.01 ± 0.00 b | 0.48 ± 0.09 b | 1.08 ± 0.32 a | ** |
| Ethyl oleate | <LOD b | 0.08 ± 0.02 b | 0.17 ± 0.02 a | ** |
| 1,2,3,4-tetrahydro-5,8-dimethyl-Acridin-9-amine | <LOD b | 42.35 ± 4.91 a | 53.11 ± 6.65 a | ** |
| Levopimaric acid methyl ester | 0.04 ± 0.01 | 0.87 ± 0.29 | 0.54 ± 0.08 | n.s. |
| Dehydroabeityl alcohol | <LOD b | 0.35 ± 0.07 a | 0.19 ± 0.03 a | ** |
| Pinocembrin | <LOD b | 3.58 ± 0.71 a | 4.33 ± 1.42 a | * |
| Methyl levopimarate | <LOD | 0.11 ± 0.05 | 0.04 ± 0.02 | n.s. |
| Total number of compounds identified | 85 | 65 | 65 | |
| Compound | Concentration (%) | ISO 21093:2003 (Range %) |
|---|---|---|
| α-Pinene | 14.53 ± 2.27 | 10–30 |
| β-Pinene | 5.06 ± 0.54 | 3–14 |
| 3-Carene | 24.74 ± 1.47 | 5–25 |
| D-Limonene | 17.13 ± 1.86 | 8–14 |
| mg/L | mg/L | mg/L | |||
|---|---|---|---|---|---|
| Ag | <0.01 | Hf | <0.01 | Rh | <0.005 |
| Al | 0.14 | Hg | <0.01 | Ru | <0.001 |
| As | <0.02 | Ho | <0.01 | S | 22.4 |
| Au | <0.01 | I | <5 | Sb | <0.05 |
| B | <0.10 | In | <0.02 | Sc | <0.001 |
| Ba | 0.01 | Ir | <0.01 | Se | <0.05 |
| Be | <0.001 | K | 32.9 | Si | 2.42 |
| Bi | <0.02 | La | <0.01 | Sm | <0.05 |
| Br | <5.00 | Li | 0.00174 | Sn | <0.05 |
| Ca | 26.50 | Lu | <0.001 | Sr | 0.0487 |
| Cd | <0.001 | Mg | 15.7 | Ta | <0.01 |
| Ce | <0.10 | Mn | 0.404 | Tb | <0.02 |
| Cl | <5 | Mo | <0.001 | Te | <0.05 |
| Co | <0.001 | Na | 2.91 | Th | <0.05 |
| Cr | <0.001 | Nb | <0.005 | Ti | 0.00271 |
| Cu | 0.007056 | Nd | <0.01 | Tl | <0.05 |
| Dy | <0.01 | Ni | <0.001 | Tm | <0.005 |
| Er | <0.01 | P | 3.19 | V | 0.001184 |
| Eu | <0.01 | Pb | <0.02 | W | <0.005 |
| Fe | 0.0649 | Pd | <0.01 | Y | <0.001 |
| Ga | <0.01 | Pr | <0.05 | Yb | <0.001 |
| Gd | <0.01 | Pt | <0.01 | Zn | 0.129 |
| Ge | <1 | Re | <0.005 | Zr | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perbellini, A.; Pelloso, F.; Grigolato, S.; Zanchin, A.; Guerrini, L. Evaluation of Fractions Obtained Through Steam Distillation and Hydroalcoholic Maceration of Wood Chips from Pinus mugo for Flavouring Italian Spirit grappa. Beverages 2025, 11, 14. https://doi.org/10.3390/beverages11010014
Perbellini A, Pelloso F, Grigolato S, Zanchin A, Guerrini L. Evaluation of Fractions Obtained Through Steam Distillation and Hydroalcoholic Maceration of Wood Chips from Pinus mugo for Flavouring Italian Spirit grappa. Beverages. 2025; 11(1):14. https://doi.org/10.3390/beverages11010014
Chicago/Turabian StylePerbellini, Anna, Fabio Pelloso, Stefano Grigolato, Alessandro Zanchin, and Lorenzo Guerrini. 2025. "Evaluation of Fractions Obtained Through Steam Distillation and Hydroalcoholic Maceration of Wood Chips from Pinus mugo for Flavouring Italian Spirit grappa" Beverages 11, no. 1: 14. https://doi.org/10.3390/beverages11010014
APA StylePerbellini, A., Pelloso, F., Grigolato, S., Zanchin, A., & Guerrini, L. (2025). Evaluation of Fractions Obtained Through Steam Distillation and Hydroalcoholic Maceration of Wood Chips from Pinus mugo for Flavouring Italian Spirit grappa. Beverages, 11(1), 14. https://doi.org/10.3390/beverages11010014







