Abstract
The objective of the study was to determine the effect of vintage and cultivar on the total polyphenol content and the antioxidant activity of wines made from the white wine cultivar ‘Solaris’ and the red wine cultivar ‘Zilga’ (both with skin maceration). The second goal was to describe the polyphenolic profile of ‘Solaris’ wine and compare it with that of ‘Zilga’ wine. Spectrophotometric methods were employed to determine the total polyphenol content and the antioxidant activity. High-performance liquid chromatography (HPLC) was used to determine the polyphenol composition. The total polyphenol content and the antioxidant activity of ‘Solaris’ wine differed significantly from that of ‘Zilga’ wine in the experimental years from 2021 to 2023. Significant differences between the wines also occurred on average over the years, with a significantly higher total polyphenol content and antioxidant activity obtained for the red wine of ‘Zilga’. However, both wines were similarly influenced by vintage over the three-year experimental period. The antioxidant activity showed a high correlation with the total polyphenol content. The polyphenolic profile of ‘Solaris’ wine differed notably from that of ‘Zilga’ wine. While ‘Solaris’ wine exhibited lower levels of phenolic acids and flavonols, the content of the flavanols was significantly higher, even double that of ‘Zilga’ wine. Among the flavanols, there was a remarkably high content of catechin and epicatechin.
1. Introduction
The Estonian wine industry has experienced exponential growth in the last decade, and in addition, wine tourism is also increasing in popularity. Estonia lies on the northern edge of the wine industry world. Long and cold winters, combined with summers featuring moderate heat and long, light nights, create unique conditions for growing fruits and berries. This brand-new terroir is currently being explored by local wine producers as well as tourists. Until the year 2022, the wine in Estonia was still mainly produced from berries and fruits, but 8000 L of wine was already being produced from grapes at that time. The most common grape cultivar for white wine production is ‘Solaris’, whereas red wine is mainly produced from ‘Rondo’, ‘Regent’, and the hybrid ‘Zilga’. Estonia and Lithuania were added to the northern coolest viticulture zone, Zone A, of the European Union in December 2021, and, thus, were officially acknowledged as one of the wine-producing countries. However, Estonian and Lithuanian wines remain largely unknown internationally due to the smallness of the countries, and the viticulture for wine is at the very beginning of its development.
Traditional winemaking techniques for white wine typically do not involve prolonged contact between the solid parts of the grapes and the grape must during the fermentation and post-fermentation stages. In order to extract specific aromatic or phenolic compounds, winemakers often employ pre-fermentation maceration, a method commonly applied to aromatic grape cultivars. Orange wines are made from white grape cultivars through prolonged contact of the skin and seeds in the fermentation process, which is actually a technique for producing red wines. The color of orange wines has great variability, ranging from pale to deep orange [1]. Some experiments have shown that maceration treatments strongly affected wine phenolics, and that the concentrations of various phenolic compounds mainly depend on the maceration length [2]. It has been demonstrated that the applied winemaking practices can moderately to strongly affect wine taste, leading to an increase in the overall complexity and quality of the aftertaste, especially when prolonged maceration of late harvest grapes is applied. The increased intensity of yellow wine color is a direct consequence of higher phenolic content, tannin addition, or grape maturity, while prolonged maceration intensifies the wine color the most. It is well known that wine polyphenols have a certain biological activity and, therefore, can contribute to the health benefits of wine. The most abundant polyphenols in orange wine are gallic acid, caffeic acid, and catechin [3]. However, red wine has been shown to be significantly different from orange wine [4]. The total polyphenol content of orange wines is 0.6 times lower than that of red wines and 4.6 times higher than that of white wines [1]. Similarly, the antioxidant activity of orange wine is different. Experiments conducted with the cultivar ‘Solaris’ have shown that cold maceration treatment for 24 h and fermentation on the skin leads to wines with lower acidity and higher glycerol and total polyphenol indexes compared to white wine [5]. Sensory analysis has shown that cold maceration enhances “apricot” and “apple” flavor, while skin fermentation gives rise to increased “rose” and “elderflower” flavors.
In traditional wine-producing countries, wine is mainly made from Vitis vinifera cultivars. Wines produced from interspecific V. vinifera × V. labrusca hybrid grape cultivars can exhibit atypical organoleptic characteristics when compared to wines produced only from V. vinifera. Results with wines from hybrid cultivars ‘Rondo’, ‘Zilga’, and ‘Hasansky Sladky’ demonstrate that although the polyphenolic spectra of red wines produced from hybrid grapes are generally similar to those of traditional V. vinifera wines, they show a wider range of anthocyanins [6]. In the hybrid grape wines, peonidin has been found in similar concentrations to malvidin; compared to the traditional wines, the hybrid grape wines also contained less petunidin, and no delphinidin compounds were discovered. The differences found in the anthocyanin profiles, flavonols, and hydroxycinnamic acid derivatives present in ‘Isabel’ (V. vinifera × V. labrusca) wines are the same as those found in V. vinifera red wines [7]. Red wines of ‘Isabel’ show low to medium antioxidant capacity when compared to V. vinifera red wines. However, it turns out that wines with the highest content of total polyphenols do not always show the highest values for antioxidant activity. This shows that the antioxidant activity of wine is more related to the kind of phenolic compounds present in the wines than to their total content. The antioxidant activity of red wines is significantly correlated with polyphenols, but the correlation is different with the flavonoids, especially with flavanol (tannin) and anthocyanin fractions [8]. V. vinifera grapes almost exclusively contain anthocyanin monoglucosides, whereas non-vinifera and hybrid grapes contain a mixture of both anthocyanin monoglucosides and diglucosides [9]. Polymeric pigment forms when anthocyanins react with tannins. Promising results have been obtained from the use of 50% whole clusters as a pre-fermentative technique to manage phenolics and improve the tannin content in red wine from hybrid cultivars [10].
The hypothesis of this work is that the polyphenol content and antioxidant activity of wines varies among different vintages, but that it is not clear how significantly this affects ‘Solaris’ and ‘Zilga’ wines in the conditions of a cool climate. During fermentation with skins and seeds, different polyphenolic compounds are extracted into the wine, and thus, the differences between wines made from white-skinned grapes and those made from black-skinned grapes can be reduced. The first objective is to determine the total polyphenol content and antioxidant activity of wines made from the white wine cultivar ‘Solaris’ and the red wine cultivar ‘Zilga’ (both with skin maceration). The second goal is to describe the polyphenolic profile of ‘Solaris’ wine and compare it with that of ‘Zilga’ wine.
2. Materials and Methods
2.1. Cultivars and Wine Processing
Cultivar ‘Zilga’ is an interspecific hybrid obtained by crossing ‘Smuglyanka’ and ‘Dvietes Zila’ with the ‘Jubileinaja Novgoroda’, and the species used in breeding are V. vinifera and V. labrusca. The country of origin of the cultivar is Latvia, and the selection was performed in 1964. ‘Zilga’ is an early ripening wine grape cultivar, and the vines are vigorous with good disease resistance and winter hardiness; therefore, they can be cultivated in open field conditions [11,12]. The cultivar has small to medium semi-tight clusters with medium-sized black berries with a sky-blue shade.
The German cultivar ‘Solaris’ was obtained by crossing ‘Merzling’ × Gm 6493, with the crossing performed in 1975. The cultivar is bred from the species V. vinifera, but V. amurensis is also present in the pedigree. The clusters are medium-sized with green-blanc berries. The vines are vigorous, with good resistance against downy mildew and low susceptibility to Oidium and Botrytis [13]. In Estonia, ‘Solaris’ has problems with winter damage; therefore, it is mainly cultivated in high plastic tunnels.
The berries for the test wines were harvested from two different vineyards in South Estonia during the years 2021–2023. The berries of ‘Zilga’ were harvested from the experimental open field vineyard at the Estonian University of Life Sciences (58°21′27.0″ N 26°31′16.0″ E). Vines were planted in 2 m × 2 m spaces and trained on low double trunk trellises, and spur pruning was used. Vines of the cultivar ‘Solaris’ were grown in a high plastic tunnel (57°58′58.6″ N 26°34′04.2″ E). The tunnel without foundation was 28 m in length, 7.6 m in width, and 4.6 m in height, covered with 0.18 mm thick UV-stable low-density polyethylene. Vines were planted in 1.6 m × 2 m spaces and trained on low double trunk trellises. At both sites, the vine rows were oriented from north to south, the ground was covered with woven ground cover fabric, and no pesticides, fertilizers, or additional irrigation systems were used. The soil was high-productivity sandy loam Haplic luvisol with sufficient drainage. At both sites, the ground was flat and the soil had uniform fertility. At the beginning of veraison, leaves were removed from the cluster zone. On the day of picking in the experimental years, the berry maturity indicators did not vary significantly and were as follows: ‘Zilga’—soluble solids 17 ± 1 °Brix, total acids 8.7 ± 0.6 g L−1, and pH 3.3 ± 0.1; ‘Solaris’—soluble solids 21.3 ± 1.2 °Brix, total acids 6.5 ± 0.5 g L−1, and pH 3.1 ± 0.2.
The fruits of experimental cultivars were destemmed with a destemmer on the day of harvesting. For ‘Zilga’ and ‘Solaris’ wine, fermentation with skins and seeds took place for 7 days, after which the juice was pressed. Cap punching was performed four times a day. The yeast Bioferm Aromatic (Saccharomyces cerevisiae var. cerevisiae) (Brouwland, Beverlo, Belgium) was used, which has the ability to reduce the malic acid content by 30%. Pressing of all treatments was done with a 20 L stainless water pressure press. In the case of ‘Zilga’, the °Brix value was increased to 20 ± 1 by adding glucose. Further fermentation and maturing were conducted in 100 L size stainless steel tanks equipped with floating lids at a temperature of 18 to 20 °C. All wines were fermented to dryness. After fermentation, 100 mg L−1 SO2 was added. The finished young wine was used 4 months later for instrumental analysis. Wine samples were analyzed in three replications. The chemical parameters of young wines after completing fermentation for ‘Solaris’ were as follows (2021–2023): alcohol, 11 ± 1%; total acid, 7.7 ± 0.6 g L−1; and pH, 3.2 ± 0.1. For ‘Zilga’, alcohol was 9.3 ± 0.6%, total acid was 8.0 ± 0.4 g L−1, and pH was 3.2 ± 0.1.
2.2. Chemical Analyses
The total polyphenol content (TPC) of the wine samples (years 2021–2023) was determined in three replications by the Folin–Ciocalteu phenol reagent method [14]. Results were expressed as mg of gallic acid equivalent (GAE) per 100 mL of wine (mg 100 mL−1 GAE). The absorbance was measured after 2 h at 765 nm. The antioxidant activity was determined by applying the DPPH (2.2-diphenyl-1-picrylhydrazyl) radical scavenging method [15] and expressed as percentage (%) DPPH radical scavenging activity. The absorbance was measured after 60 min at 515 nm. Spectrophotometric measurements were made with a Cary 60 UV-Vic spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA).
The determination of the most abundant polyphenols and the polyphenol profiling of the wine samples (the year 2021) were performed using a Shimadzu Nexera X2 UHPLC coupled with a diode array detector SPD-M20A and a mass spectrometer LCMS 8040 (Shimadzu Scientific Instruments, Kyoto, Japan), as described in Ben-Othman et al. [16]. Individual phenolic compounds were identified by comparing the retention times, UV spectra, and parent and daughter ion masses with those of the standard compounds (Table 1). The anthocyanidins were determined using UV spectrum at 520 nm and the concentrations were calculated using the malvidin-3-gluoside for calibration. The standard compounds of caffeic acid, protocatechuic acid, syringic acid, malvidin-3-glucoside, catechin, epicatechin, procyanidin B1, procyanidin B2, rutin, kaempferol, kaempferol-3-glucoside, phloretin, quercetin, quercetin-3-galactoside, quercetin-3-glucoside, quercetin-3-glucuronide, and quercitrin were of laboratory grade. The results were expressed in μg per L (μg L−1). All chemicals used were of laboratory grade and purchased from Sigma (Steinheim am Albuch, Germany) or Cayman (Cayman Chemical, Ann Arbor, MI, USA).

Table 1.
The names of detected compounds, their parent and daughter ion masses (m/z), and their retention times.
2.3. Weather Conditions
The coolest months of the vegetation period in the experimental years were May and September, during which night frosts also occurred (Figure 1). In 2021, June and July experienced high temperatures; however, August and September were cooler than they were, on average, in previous years. In this year, May and August were rainy months (Figure 2). In June, the precipitation was below average, followed by drought in July. The year 2022 had a warmer August compared to other years, but a very cool September followed. Precipitation was more evenly distributed, with September being the driest month. In 2023, August was warmer than it had been, on average, for many years, and September was exceptionally warm. The drought occurred in spring in May, while rainfall in July was higher than it was in the other experimental years.

Figure 1.
Monthly average, minimum, and maximum air temperature (°C) compared to the average of many years (1991–2020) from May to September in 2021–2023.
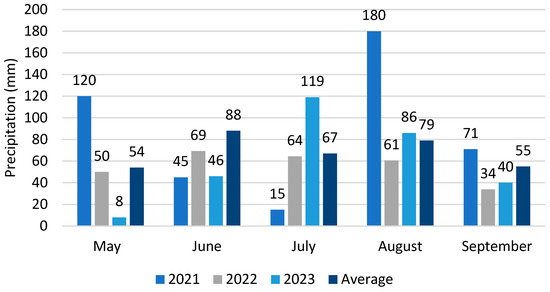
Figure 2.
Monthly total precipitation (mm) and average of many years (1991–2020) from May to September in 2021–2023.
2.4. Statistical Analysis
The results in figures and Table 2 are represented as means of three replicates, and in Table 2, the means ± standard deviations are presented. One-way analysis of variance (ANOVA) was performed to evaluate the existence of differences between experimental wines′ polyphenolic compounds and their antioxidant activity. Two-way analysis of variance was performed to evaluate the effect of cultivars (‘Zilga’, ‘Solaris’) and vintage (2021–2023). Comparison of means was done by using Fisher’s Least Significant Difference (LSD) test to confirm the statistically significant differences. Different letters in the figures and table indicate statistically significant differences (p < 0.05). Regression analysis was used to determine the relationship between the polyphenols content and the antioxidant activity. The coefficient of determination (R2) was calculated (n = 18).

Table 2.
The most abundant polyphenols (µg L−1) in skin-contact fermented wines of ‘Solaris’ and ‘Zilga’. Different letters in the same row between means (±STDV) of polyphenol groups present statistically significant differences at p ≤ 0.05.
3. Results
3.1. Total Polyphenols and Antioxidant Activity
The TPC differed considerably between ‘Solaris’ and ‘Zilga’ wines; it was more than double in the ‘Zilga’ wine in 2022 and 2023 (Figure 3). The highest TPC was found in the red wine of ‘Zilga’, where it ranged from 39 to 168 mg 100 mL−1 GAE. A lower TPC was found in the ‘Solaris’ wine, with the content ranging from 25 to 75 mg 100 mL−1 GAE. Significant differences between the wines also occurred on average over the years, with a significantly higher TPC obtained for the red wine of ‘Zilga’ compared to the wine of ‘Solaris’. The average effect of the vintage was also significant, with the TPC ranging from 32 to 121 mg 100 mL−1 GAE.

Figure 3.
Total polyphenol content (mg 100 mL–1 GAE) of the skin-contact fermented wines of ‘Solaris’ and ‘Zilga’. Different letters on the bars indicate statistically significant differences (p < 0.05).
The determined values of antioxidant activity in experimental wines were within the range from 11 to 83% inhibition of DPPH (Figure 4). Every year, there was a significant variation between the cultivars—the antioxidant activity of the ‘Solaris’ wine ranged from 11 to 28% DPPH, while for ‘Zilga’, the results were significantly higher in each year, with a variability from 36 to 83% DPPH. There were also significant differences between the wines of ‘Solaris’ and ‘Zilga’ on average over the years, with the vintage having a significant effect on the results. The antioxidant activity exhibits a high correlation with the total polyphenol content (Figure 5).
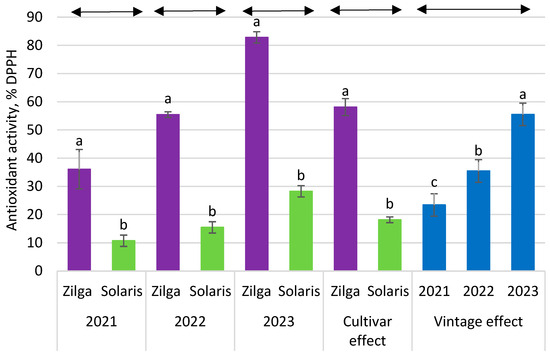
Figure 4.
Antioxidant activity of DPPH radical scavenging (%) of the skin-contact fermented wines of ‘Solaris’ and ‘Zilga’. Different letters on the bars indicate statistically significant differences (p < 0.05).

Figure 5.
Linear regression between total polyphenol content (mg 100 mL–1 GAE) and antioxidant activity (% DPPH) values in wines.
3.2. Individual Phenolic Compounds
Within the wines of ‘Solaris’ and ‘Zilga’, the represented groups of polyphenols were phenolic acids, anthocyanins, flavanols, and flavonols (Table 2). However, differences were evident in their profiles and contents. The amount of phenolic acids in the wine of ‘Solaris’ was 52.0 µg L−1, while in the wine of ‘Zilga’ it was 129.0 µg L−1. Considering the singularity of each phenolic acid constituent, the most abundant compounds in the studied wines were caffeic acid and protocatechuic acid. They presented significant differences between wines, with the ‘Zilga’ wine containing more of both.
The totality of anthocyanins in ‘Zilga’ wine was 4812.0 µg L−1 (Table 2). The anthocyanins’ profile showed that the predominant anthocyanidin in the red wine of ‘Zilga’ was malvidin-3-diglucoside, followed by delphinidin-3-glucoside and malvidin-3-glucoside. In addition, petunidin-3-glucoside, malvidin, and peonidin-3-glucoside were also represented.
Catechin was present as the dominant flavanol in both wines, with its content in the wine of ‘Solaris’ being 640.7 µg L−1, and less in ‘Zilga’ wine, with a content of 268.0 µg L−1 (Table 2). In addition to the mentioned compounds, both wines contained epicatechin, procyanidin B1, and procyanidin B2. The total content of flavanols in ‘Solaris’ wine was 1062.7 µg L−1, which was twice as high as that in ‘Zilga’ wine, where the content was 558.4 µg L−1.
In both experimental wines, among the flavonols, isorhamnetin-3-glucoside+isorhamnetin-3-galactoside, phloretin, quercetin-3-glucoside, and quercetin-3-glucuronide were represented (Table 2). Rutin was present in lower amounts. The wine of ‘Zilga’ also contained quercetin, but it was not detected in the ‘Solaris’ wine. The total flavonols amount was significantly higher in ‘Zilga’ wine and lower in ‘Solaris’, at 604.0 and 272.7 µg L−1, respectively.
4. Discussion
4.1. Total Polyphenols and Antioxidant Activity
For both wines produced from the experimental cultivars, a significant variation was found in the TPC and the antioxidant activity across the test years, and the tendency of the effect of vintage and that of cultivar on both parameters was similar. Regression analysis showed that the variations in antioxidant activity were related to the content of polyphenols in the wine, which has also been revealed in previous experiments [15,17,18]. Polyphenols are important constituents in red wines, contributing to the sensory properties, but in addition, they contribute also to the antioxidant activity of wines [19]. The most important factors causing differences between vintages are temperature and precipitation, especially during the period of grape veraison [20,21]. Soil moisture also has a significant impact [22]. Soils with less water produce grapes with better winemaking quality, affecting their berry characteristics and anthocyanin profiles [23]. The experimental cultivars grew under different conditions, and therefore, the effect of precipitation varies. The cultivar ‘Solaris’ was grown in a tunnel, making the impact of precipitation more significant for the cultivar ‘Zilga’. In 2021, the amount of precipitation was significantly higher in August and September compared to the average precipitation across previous years, which influenced the results of that year’s experiment.
Long-term studies showed that Estonian weather varies from year to year, and warmer and longer vegetation periods enhanced grapes’ content of polyphenols, while more precipitation and higher humidity decreased it [21]. The weather was different during the experimental years. In 2021, it was warm, and the conditions were favorable for flower fertilization during the blooming time of vines, but at veraison in August, the weather was cool. The highest TPC was determined in the wines produced in 2023. That year, the weather conditions were unsuitable, due to the late spring night frosts damaging the vines, causing the growth of new shoots from dormant buds that emerged after the first shoots had been damaged. At the same time, it was very warm in August and September, which probably contributed to the high TPC. Previous studies with V. vinifera cultivars in warmer climates have also confirmed that the polyphenols content of wines is strongly dependent on weather conditions [24]. Higher temperatures during maturation have been shown to result in a higher content of polyphenolic compounds related to grape quality [25]. The effect of temperatures during ripening is different, and excessively high temperatures have a negative effect. For instance, when the maximum temperature exceeds 35 °C during berry ripening, it inhibits color formation [26]. In addition, regarding flavonols, previous studies [27] seem to agree that 25 °C is related to an increase in their concentration. This was not an issue for outdoor cultivation because the maximum temperatures during the experimental years were below 35 °C. Such a problem in this experiment could have arisen in the tunnel for the cultivar ‘Solaris’. In our experience, in tunnel conditions, the temperature can rise above 35 °C [12]. However, our previous studies revealed that warmer temperatures in the tunnel did not negatively affect the formation of polyphenols compared to open vineyards [21]. The tunnels used in viticulture in Estonia are high and open at the ends, and, therefore, the temperatures do not rise to extremely high levels. Among the weather factors, the duration of sunshine also has an effect. In Nordic countries, the days are longer, and, therefore, the effect of temperature and light is greater. Additionally, due to the angle of inclination of the sun, the berries are not shaded by the canopy, so they are affected by the sun’s radiation throughout the long day. In both experimental vineyards, the rows of vines were located in a north-south direction, allowing the berries to receive local radiation from both sides. Previous studies have shown that the accumulation of flavonols is significantly influenced by light quality, which is known to be the main abiotic driver of flavonol biosynthesis regulation [28]. Thus, the three-year experimental results show that the antioxidant activity and the TPC of the wines are strongly influenced by the vintage. In addition to studying the weather impact on berries, it is essential to understand how the attributes of wine quality will be affected and to develop new strategies to mitigate climate effects on wine grapes.
4.2. Individual Phenolic Compounds
Limited data are available on the composition of on skin-fermented wines, which are produced from hybrid cultivars or from white berries, and are often referred to as orange wine. The qvevri wine is well known, but the essence of the qvevri method is that both the alcoholic fermentation and the further aging occur in the clay vessel [29]. Maceration treatments strongly affect wine phenolics, and the concentrations of various phenolic compounds mainly depend on the maceration length [2]. In wine produced via the qvevri method, the content of polyphenols is much higher than it is in white wine and close to the amount in red wines [30]. In our experiment, the skins were fermented for 7 days, and, therefore, the experimental wines were still different. The obtained results confirm previous findings with other cultivars, where significant differences were also observed between orange and red wines [1]. It can be concluded that further experiments are necessary in Estonia to investigate the effect of longer skin-fermentation times on the polyphenol content of wine, particularly with ‘Solaris’. In the case of ‘Zilga’, longer fermentation with the skins is problematic because it can intensify the specific aroma and taste of the hybrids. Therefore, the sensory properties of hybrid grape wines might benefit from shorter on-skin maceration times. White wine pomace can be used in red winemaking as a natural additive that could improve the concentrations of polyphenols in hybrid red wines [31].
The TPC was lower in ‘Solaris’ wine, but differences appeared in the profile of polyphenols. While the content of phenolic acids, and flavonols was lower, the content of flavanols was double that of ‘Zilga’ wine. Similar results have also been obtained in other experiments, where the concentration of flavanols in ‘Bianchetta Trevigiana’ wine was similar or even higher than that of red wines [32]. Additionally, the release of various polyphenolic compounds depends on the maceration length. Additionally, the grape cultivar and the phenolic ripeness appear to be the key factors affecting the flavanols or tannin concentration in wine [33]. Among the flavanols in ‘Solaris’ wine, the content of catechin and epicatechin was significantly higher compared to that of ‘Zilga’ wine. A high catechin content has also been reported in orange wines from other V. vinifera cultivars, but this varies depending on the cultivar [34].
In the current experiment, the antioxidant activity of ‘Solaris’ wine was lower than that of ‘Zilga’, which could be attributed to the presence of anthocyanins in ‘Zilga’ wine. Anthocyanins are considered important antioxidants, especially in red wines [35,36]. It is also important to consider the influence of cultivar properties. For example, the berries of ‘Zilga’ have had a consistently lower anthocyanin content than those of ‘Rondo’ [21]. However, it is not always possible to associate the biochemical composition of berries with the wine. For example, some authors have shown that the anthocyanin profile of finished wines is different from those observed in grapes [37,38]. The anthocyanins found in experimental red wines of ‘Zilga’ are mainly based on malvidin, and they were in the form of glucosides and the non-vinifera characteristic diglucosides, as expected. As previously pointed out in the analysis of wines made from V. vinifera cultivars, the malvidin-3-glucoside is the most abundant [39,40]. It has also been pointed out earlier that in hybrid grape wines, the majority of malvidin compounds was in the form of malvidin-3.5-O-diglucoside, whereas in common grape wines, malvidin-3-O-glucoside was found to be the most abundant form [6]. Similar results have been obtained with red wine produced from ‘Isabel’ (V. vinifera × V. labrusca) [7].
Anthocyanins were not found in ‘Solaris’ wine. Anthocyanins are known to impart the red color of wines, and white wines typically do not contain anthocyanins. However, there may also be differences. The production of anthocyanin involves a multi-step biosynthetic pathway regulated by several genes. When these genes are activated or expressed, enzymes are produced, which then facilitate the synthesis of anthocyanin compounds [41]. Despite being white-skinned, many white grape cultivars may retain the genes necessary for anthocyanin production due to their black-skinned ancestors. In the case of the experimental cultivar ‘Solaris’, its pedigree includes a species with black-skinned fruits of V. amurensis, which may also affect the polyphenol composition of ‘Solaris’. A study conducted by Arapitsas et al. [42] demonstrated trace amounts of delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, peonidin-3-O-glucoside, petunidin-3-O-glucoside, and malvidin-3-O-glucoside in the white grape cultivars ‘Chardonnay’, ‘Riesling’, ‘Gewurztraminer’, ‘Moscateller’, and ‘Sauvignon Blanc’. The anthocyanin contents in white grapes were several- to hundreds-fold lower than in colored grapes [43]. Rosé coloration of the skin was observed to sometimes develop late during ripening on normally white grapes [44]. Small concentrations of anthocyanins have also been discovered in various orange wines produced in Serbia [34]. Wines produced from white grape cultivars also show low amounts of anthocyanins, mainly malvidin-3-O-glucoside [45]. It was also revealed that the effect on color depends on the content of anthocyanins; for example, the minimum anthocyanins content required for achieving the pink color of wine was 0.3 mg L−1.
5. Conclusions
The experimental results showed that the polyphenol content and the antioxidant activity of ‘Zilga’ and ‘Solaris’ wines vary significantly in different vintages in the conditions of a cool climate. The content of polyphenols and the antioxidant activity of ‘Solaris’ wine exhibited significant variations compared to ‘Zilga’ wine across the experimental years. However, both wines were similarly influenced by vintage, and the antioxidant activity showed a high correlation with the total polyphenol content. The polyphenolic profile of ‘Solaris’ wine differed notably from that of ‘Zilga’ wine. While ‘Solaris’ wine exhibited lower levels of phenolic acids and flavonols, the content of the flavanols was significantly higher, even double that of ‘Zilga’ wine. Particularly noteworthy was the elevated presence of catechin and epicatechin among the flavanols.
Author Contributions
Conceptualization, K.K. and M.M.-K.; methodology, R.R., H.K. and M.M.-K.; software, A.K. and L.M.; validation, K.K. and M.M.-K.; formal analysis, R.R., H.K. and M.M.-K.; investigation, K.K. and M.M.-K.; resources, R.R., K.K. and U.M.; data curation, K.K., R.R., H.K. and P.P.; writing—original draft preparation, M.M.-K., K.K. and R.R.; writing—review and editing, U.M. and P.P.; visualization, K.K., R.R. and M.M.-K.; project administration, K.K. and R.R.; funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Estonian Ministry of Rural Affairs. Project: “Mapping and quality analysis of grape wine cultivars grown in Estonia for domestic grape breeding”. In addition, this research was supported by the European Union’s Horizon 2020 research and innovation program project VALORTECH under grant agreement No. 810630. The research was conducted using the “Plant Biology Infrastructure—TAIM” funded by the Estonian Research Council (TT5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the datasets. The datasets presented in this article are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Salemnia, S.; Garcia-Torres, R.; Herman, D.; Fajardo-Lira, C. Red, White, And…Orange? A New Look into an Old Wine (P20-007-19). Curr. Dev. Nutr. 2019, 3, 1765. [Google Scholar] [CrossRef]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Peršurić, A.S.I.; Jeromel, A.; Radeka, S. Comparison of different maceration and non-maceration treatments for enhancement of phenolic composition, colour intensity, and taste attributes of Malvazija istarska (Vitis vinifera L.) white wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Beara, I.; Majkić, T.; Milovanović, L.; Svirčev, E.; Torović, L. Polyphenolic profile and in vitro biological activity of Serbian orange (skin fermented white) wines. Food Chem. 2024, 447, 138933. [Google Scholar] [CrossRef]
- Garcia-Torres, R.; Ramírez-Rodrigues, M.M.; Pérez-Alva, A. Polyphenolic Profile of range wines. Curr. Deve. Nutr. 2021, 5, 1158. [Google Scholar] [CrossRef]
- Zhang, S.; Petersen, M.A.; Liu, J.; Toldam-Andersen, T.B. Influence of Pre-Fermentation Treatments on Wine Volatile and Sensory Profile of the New Disease Tolerant Cultivar Solaris. Molecules 2015, 20, 21609–21625. [Google Scholar] [CrossRef]
- Pedastsaar, P.; Vaher, M.; Helmja, K.; Kulp, M.; Kaljurand, M.; Karp, K.; Raal, A.; Karathanos, V.; Puessa, T. Chemical composition of red wines made from hybrid grape and common grape (Vitis vinifera L.) cultivars. Proc. Est. Acad. Sci. Chem. 2014, 63, 444–453. [Google Scholar] [CrossRef]
- Nixdorf, S.L.; Hermosín-Gutiérrez, I. Brazilian red wines made from the hybrid grape cultivar Isabel: Phenolic composition and antioxidant capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines. I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571. [Google Scholar] [CrossRef]
- Gapinski, A.D.; Horton, A.C.; Watrelot, A.A. Effect of Whole Cluster Fermentation on Phenolics in Cold-Hardy Hybrid Wines. Food Bioprocess. Technol. 2023, 16, 1595–1608. [Google Scholar] [CrossRef]
- Karvonen, J. Vitis cv. Zilga is a vine for the northern temperate climate—Short communication. Hortic. Sci. 2014, 41, 147–151. [Google Scholar] [CrossRef]
- Maante-Kuljus, M.; Rätsep, R.; Mainla, L.; Moor, U.; Starast, M.; Põldma, P.; Karp, K. Technological maturity of hybrid vine (Vitis) fruits under Estonian climate conditions. Acta Agric. Scand. Sect. B Soil. Plant Sci. 2019, 69, 706–714. [Google Scholar] [CrossRef]
- Basler, P.; Pfenninger, H.; Bill, R. Evaluation of German grape varieties Johanniter, Solaris, Bronner and Fr.242-73. Obst-und Weinbau 2002, 138, 442–446. [Google Scholar]
- Waterhouse, A. Folin-Ciocalteau Micro Method for Total Phenol in Wine; Department of Viticulture & Enology University of California: Davis, CA, USA, 2019; Available online: https://waterhouse.ucdavis.edu/folin-ciocalteau-micro-method-total-phenol-wine (accessed on 18 March 2024).
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A.C. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of ultrasound-assisted extraction of phloretin and other phenolic compounds from apple tree leaves (Malus domestica Borkh.) and comparison of different cultivars from Estonia. Antioxidants 2021, 10, 189. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Villaño, D.; Garcia-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Tekos, F.; Gkasdrogka, M.; Vardakas, P.; Skaperda, Z.; Kouretas, D. Determination of the polyphenolic content and the antioxidant activities of four indigenous Greek red and white wine varieties. Int. J. Funct. Nutr. 2023, 4, 3. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Nikfardjam, M.S.P.; Márk, L.; Avar, P.; Figler, M.; Ohmacht, R. Polyphenols, anthocyanins, and trans-resveratrol in red wines from the Hungarian Villány region. Food Chem. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Maante-Kuljus, M.; Rätsep, R.; Moor, U.; Mainla, L.; Põldma, P.; Koort, A.; Karp, K. Effect of vintage and viticultural practices on the phenolic content of hybrid winegrapes in very cool climate. Agriculture 2020, 10, 169. [Google Scholar] [CrossRef]
- Aru, V.; Nittnaus, A.P.; Sørensen, K.M.; Toldam-Andersen, T.B.; Engelsen, S.B. Effects of Water Stress, Defoliation and Crop Thinning on Vitis vinifera L. cv. Solaris Must and Wine Part II: 1H NMR Metabolomics. Metabolites 2022, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Rouxinol, M.I.; Martins, M.R.; Salgueiro, V.; Costa, M.J.; Barroso, J.M.; Rato, A.E. Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera. Beverages 2023, 9, 8. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, A.; Mercenaro, L.; Del Caro, A.; Pretti, L.; Nieddu, G. Distinctive anthocyanin accumulation responses to temperature and natural UV radiation of two field-grown (Vitis vinifera L.) cultivars. Molecules 2015, 20, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.P.; Mochioka, R.; Beppu, K.; Kataoka, I. Influence of Temperature on Berry Composition of Interspecific Hybrid Wine Grape ‘Kadainou R-1’ (Vitis ficifolia var. ganebu × V. vinifera ‘Muscat of Alexandria’). J. Jpn. Soc. Hort. Sci. 2009, 78, 169–174. [Google Scholar] [CrossRef][Green Version]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition Under Altered Light and Temperature Conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Buican, B.C.; Colibaba, L.C.; Luchian, C.E.; Kallithraka, S.; Cotea, V.V. “Orange” Wine—The Resurgence of an Ancient Winemaking Technique: A Review. Agriculture 2023, 13, 1750. [Google Scholar] [CrossRef]
- Bene, Z.; Kállay, M. Polyphenol contents of skin-contact fermented white wines. Acta Aliment. 2019, 48, 515–524. [Google Scholar] [CrossRef]
- Nicolle, P.; Marcotte, C.; Angers, P.; Pedneault, K. Co-fermentation of red grapes and white pomace: A natural and economical process to modulate hybrid wine composition. Food Chem. 2018, 242, 481–490. [Google Scholar] [CrossRef]
- Lomolino, G.; Zocca, F.; Spettoli, P.; Zanin, G.; Lante, A. A preliminary study on changes in phenolic content during Bianchetta Trevigiana winemaking. J. Food Compos. Anal. 2010, 23, 575–579. [Google Scholar] [CrossRef]
- Schneider, V.; Chichua, D. Orange wines: Tannin extraction kinetics during maceration of white grapes. J. Vitic. Enol. 2021, 7, 1–9. [Google Scholar]
- Beara, I.; Majkić, T.; Milovanović, L.; Torović, L. In Search of Biological Activity of Orange Wines: Polyphenolic Profile and In Vitro Inhibition of Digestive Enzymes. Proceedings 2023, 91, 351. [Google Scholar] [CrossRef]
- Lapidot, T.; Harel, S.; Akiri, B.; Granit, R.; Kanner, J. PH-dependent forms of red wine anthocyanins as antioxidants. J. Agric. Food Chem. 1999, 47, 67–70. [Google Scholar] [CrossRef]
- Radovanović, B.; Radovanović, A. Free Radical Scavenging Activity and Anthocyanin Profile of Cabernet Sauvignon Wines from the Balkan Region. Molecules 2010, 15, 4213–4226. [Google Scholar] [CrossRef] [PubMed]
- Revilla, E.; García-Beneytez, E.; Cabello, F.; Martín-Ortega, G.; Ryan, J.M. Value of high-performance liquid chromatographic analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. J. Chromatogr. A 2001, 915, 53–60. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Revilla, E.; Cabell, F. Anthocyanin pattern of several red grape cultivars and wines made from them. Eur. Food Res. Technol. 2002, 215, 32–37. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Vercauteren, J.; de Freitas, V. Occurrence of Anthocyanin-Derived Pigments in Red Wines. Agric. Food Chem. 2001, 49, 4836–4840. [Google Scholar] [CrossRef] [PubMed]
- Casavecchia, C.; Magnisi, R.; Pera, L.L.; Maisano, R.; Dugo, G. Classification of Sicilian Red Wines from Autochthonous and Allochthonous Cultivars According to Anthocyanin Pattern. Am. J. Enol. Vitic. 2007, 58, 286–290. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Arapitsas, P.; Oliveira, J.; Mattivi, F. Do white grapes really exist? Food Res. Int. 2015, 69, 21–25. [Google Scholar] [CrossRef]
- Niu, S.; Hao, F.; Mo, H.; Jiang, J.; Wang, H.; Liu, C.; Fan, X.; Zhang, Y. Phenol profiles and antioxidant properties of white skinned grapes and their coloured genotypes during growth. Biotechnol. Biotechnol. Equip. 2016, 31, 58–67. [Google Scholar] [CrossRef]
- Gholami, M.; Coombe, B.G. Occurrence of anthocyanin pigments in berries of the white cultivar Muscat Gordo Blanco (Vitis vinifera L.). Aust. J. Grape Wine Res. 1995, 1, 67–70. [Google Scholar] [CrossRef]
- Cosme, F.; Andrea-Silva, J.; Filipe-Ribeiro, L.; Moreira, A.S.P.; Malheiro, A.C.; Coimbra, M.A.; Domingues, M.R.M.; Nunes, F.M. The origin of pinking phenomena in white wines: An Update. BIO Web Conf. 2019, 12, 02013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).