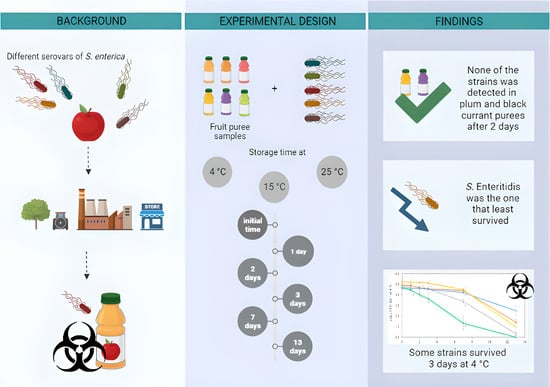
The Survival of Salmonella enterica Strains in Ready-to-Eat Fruit Purees under Different Storage Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Puree Samples and Their Characterization
2.2. Salmonella Strains and Inoculum Preparation
2.3. Salmonella Survival in Fruit Purees
2.4. Data Processing and Statistical Analysis
3. Results
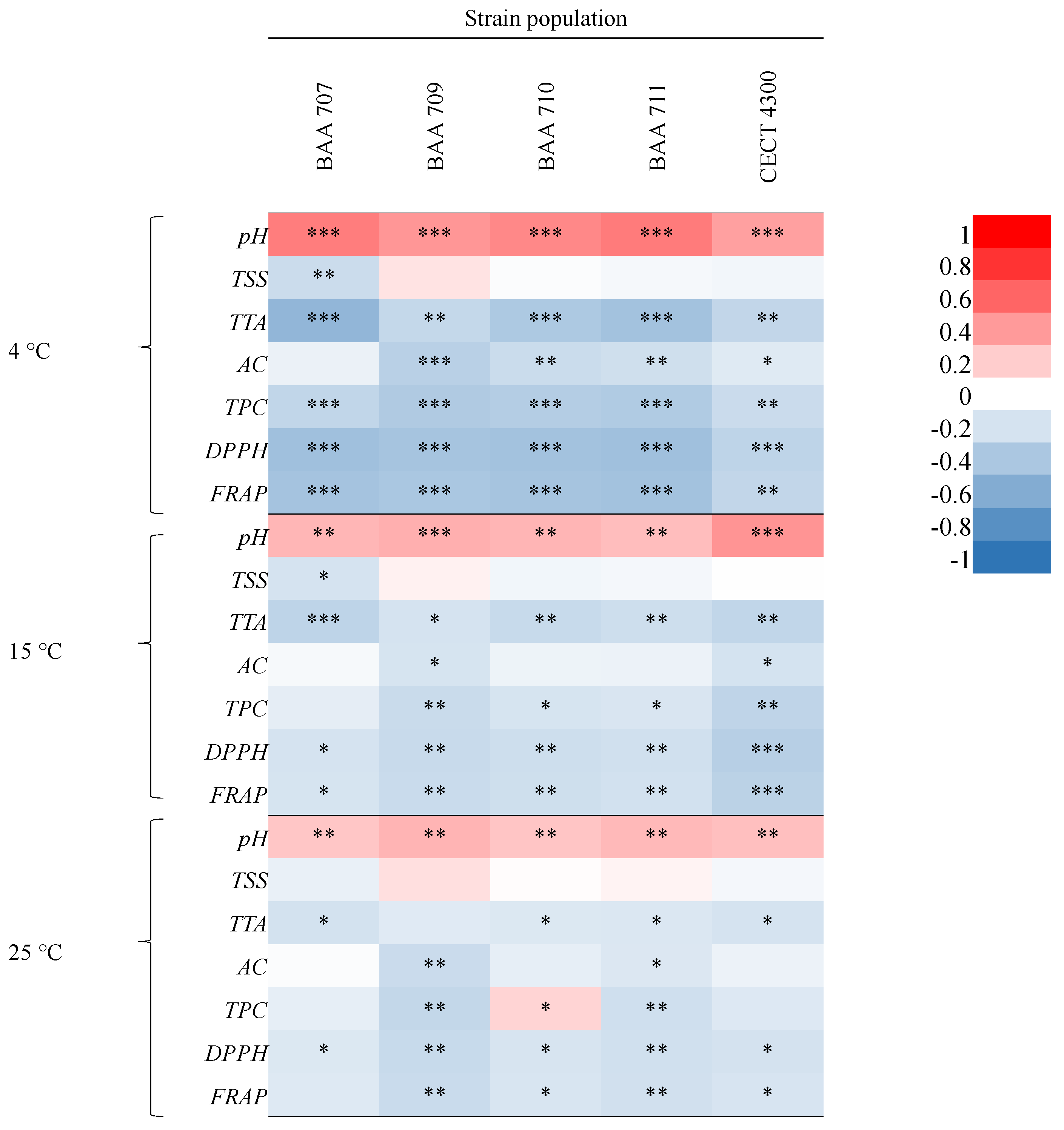
3.1. Physicochemical and Microbiological Characterization
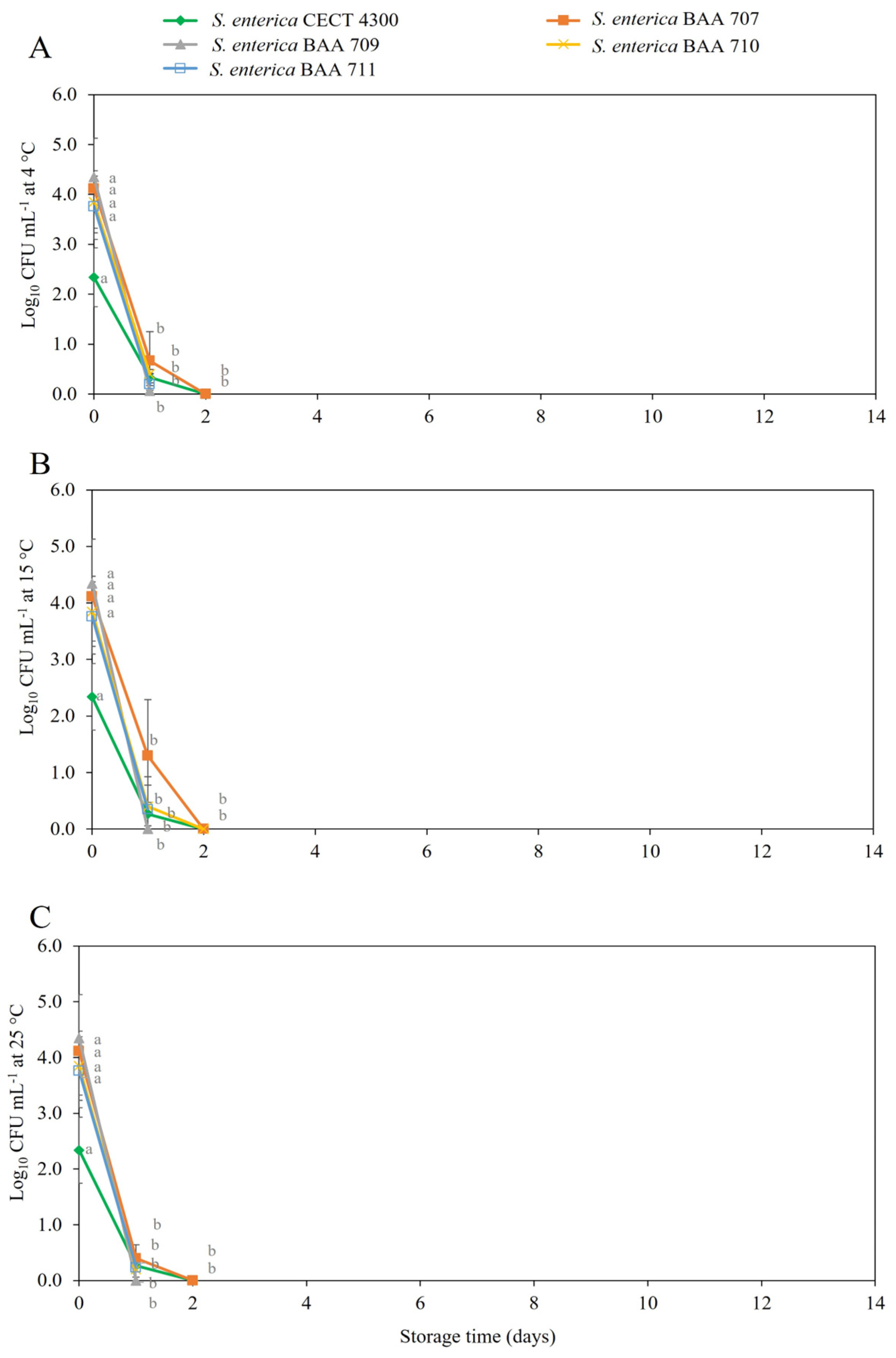
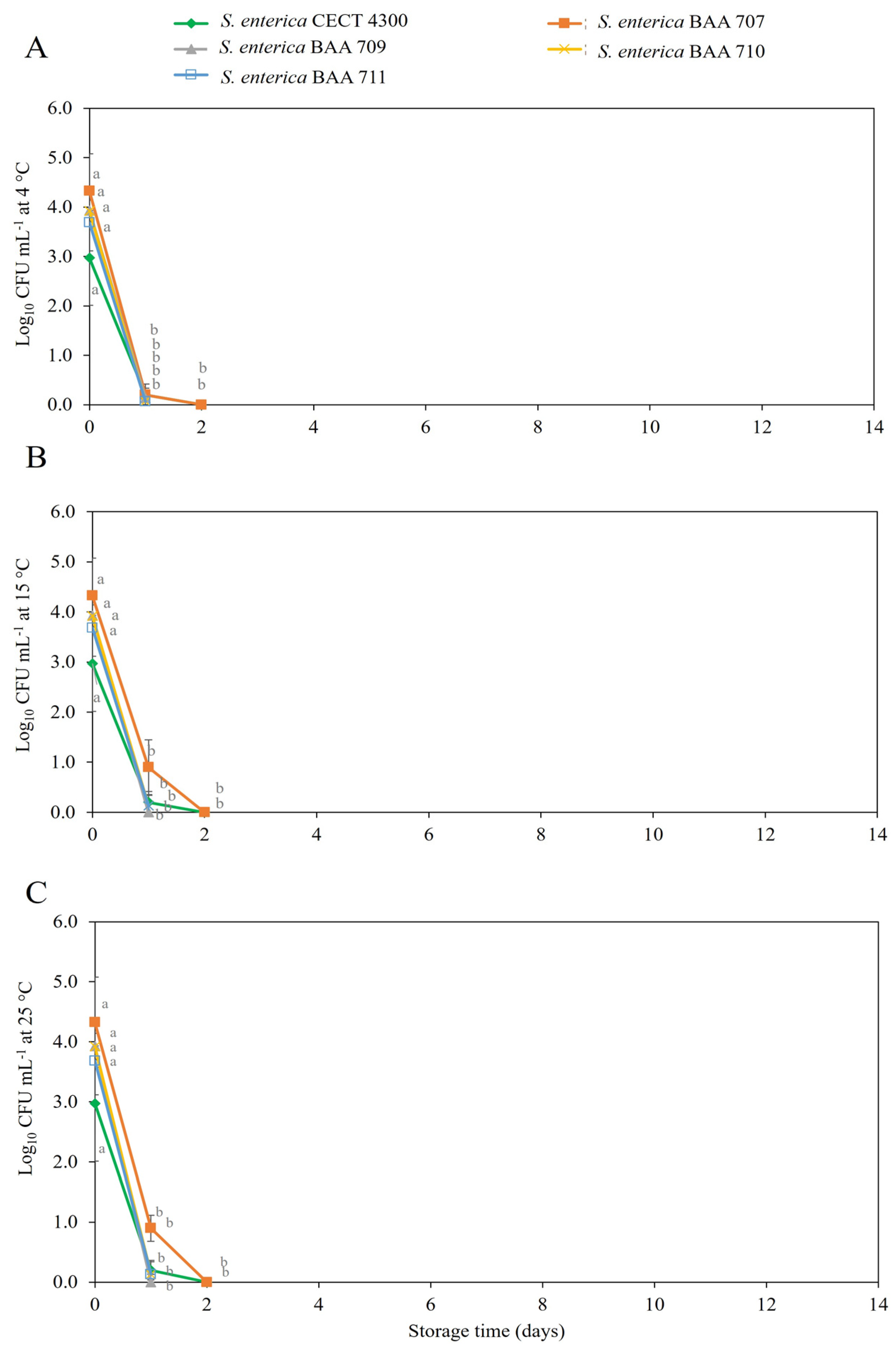
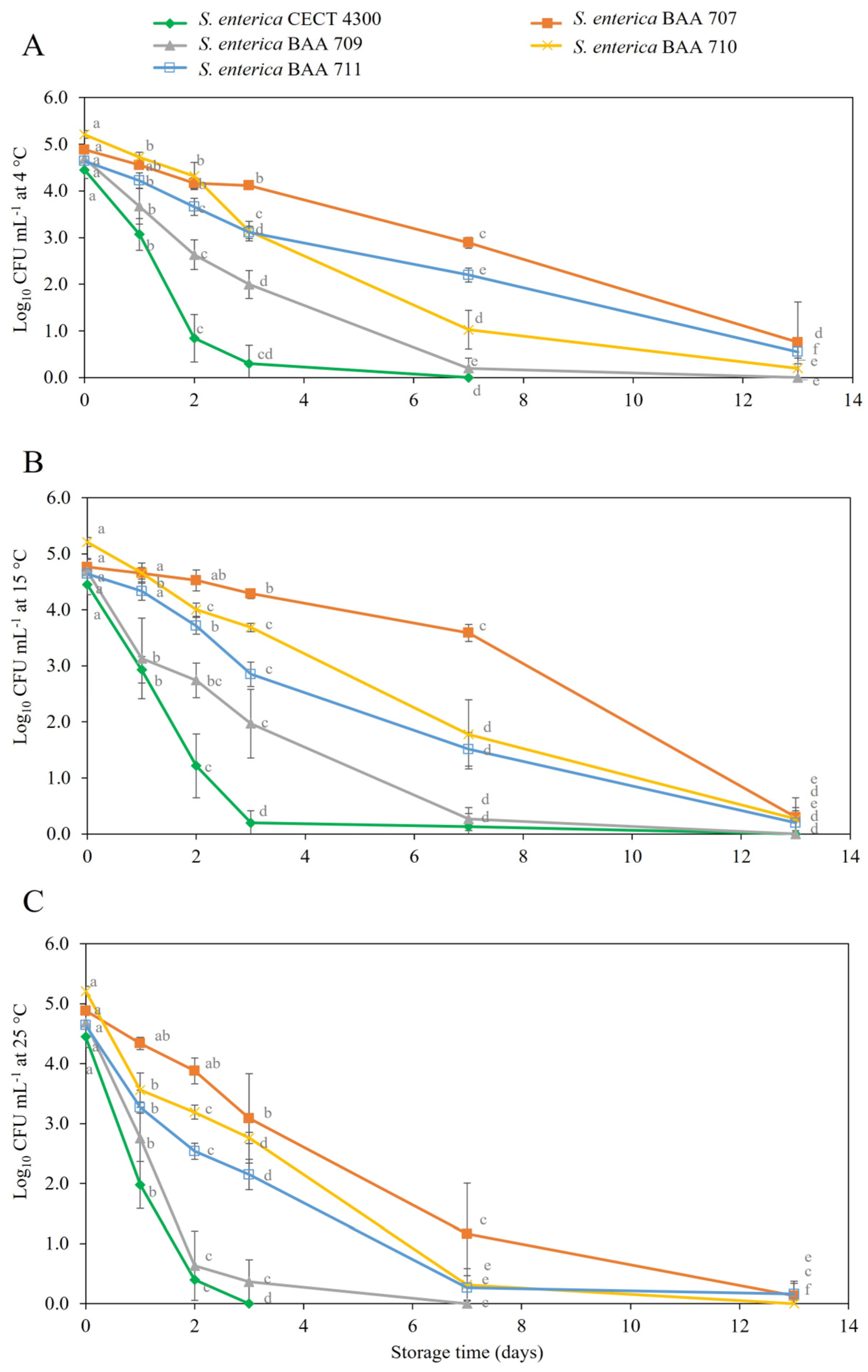
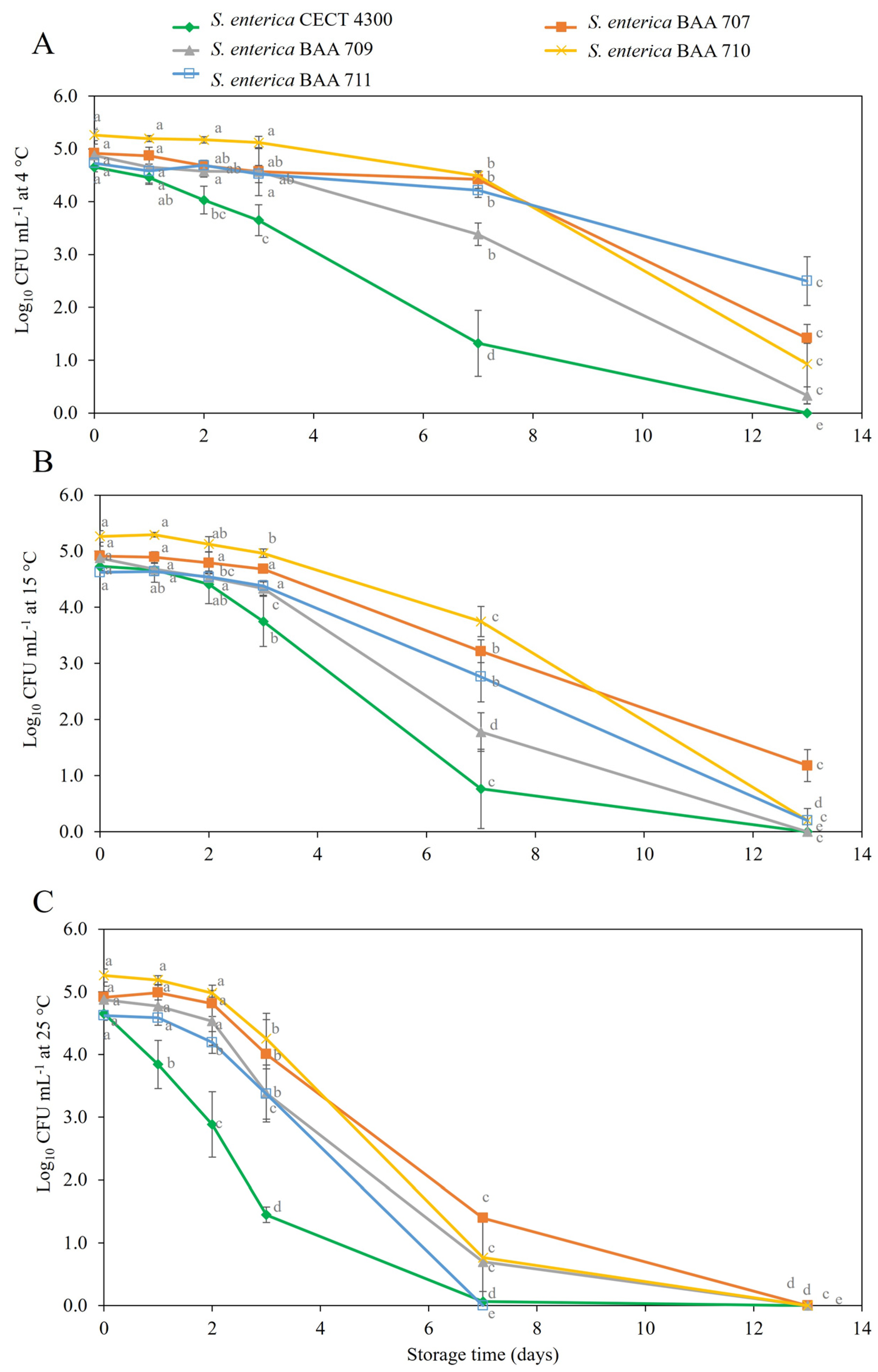
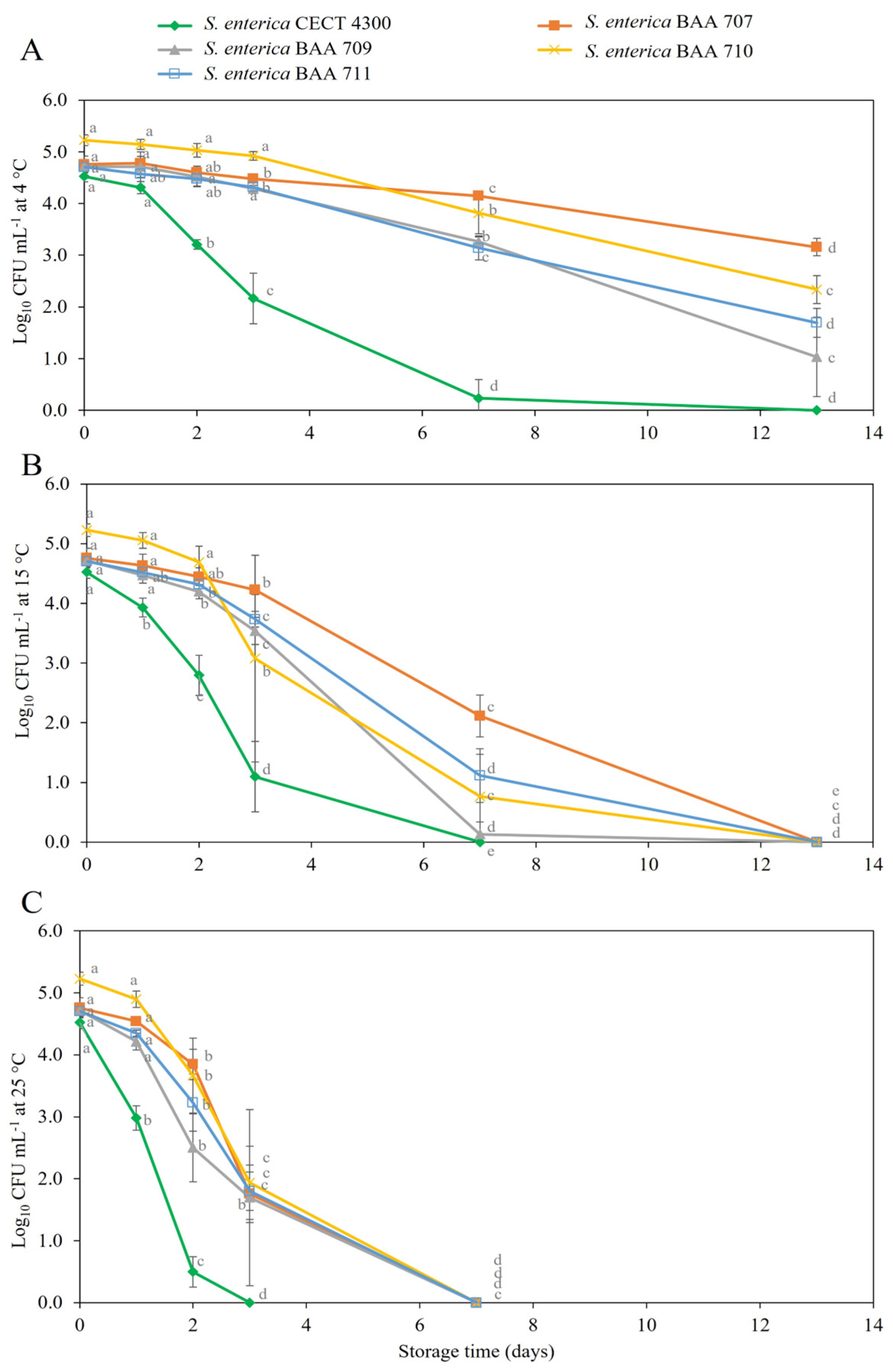
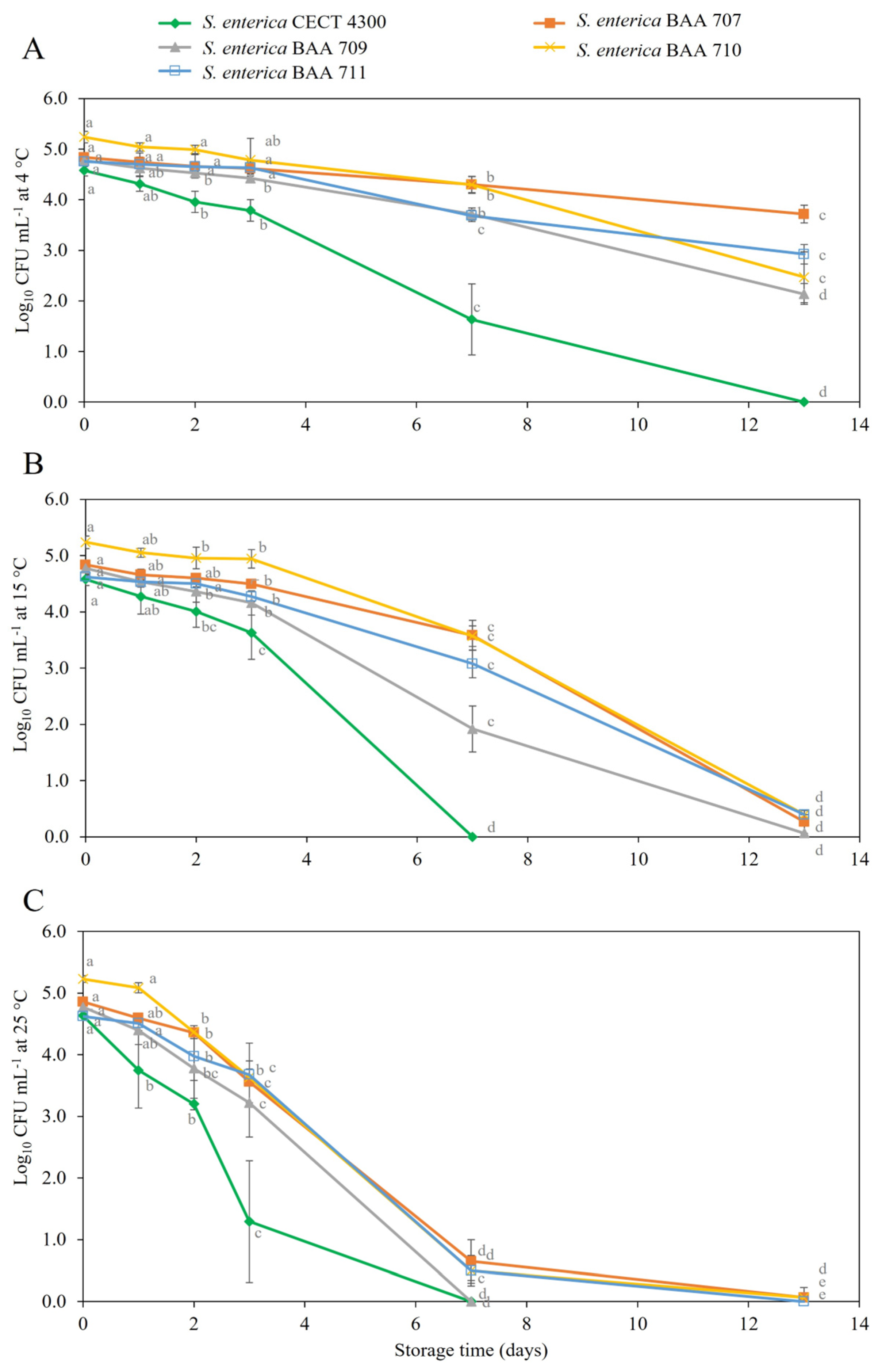
3.2. Survival of S. enterica Strains in Fruit Purees
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wesche, A.M.; Gurtler, J.B.; Marks, B.P.; Ryser, E.T. Stress, sublethal injury; resuscitation, and virulence of bacterial foodborne pathogens. J. Food Prot. 2009, 72, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Krug, M.D.; Chapin, T.; Danyluk, M.; Goodrich-Schneider, R.; Schneider, K.; Harris, L.; Worobo, R. Outbreaks of foodborne disease associated with fruit and vegetable juices, 1922–2019. Food Sci. Hum. Nutr. 2020, 5. [Google Scholar] [CrossRef]
- Castillo, A.; Villarruel-López, A.; Navarro-Hidalgo, V.; Martínez-González, N.E.; Torres-Vitela, M.R. Salmonella and Shigella in freshly squeezed orange juice, fresh oranges, and wiping cloths collected from public markets and street booths in Guadalajara, Mexico: Incidence and comparison of analytical routes. J. Food Prot. 2006, 69, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, I.; Rubert, J.; Soriano, J.M.; Mañes, J. Incidence of microorganisms from fresh orange juice processed by squeezing machines. Food Control 2012, 23, 282–285. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Viñas, I.; Oliveira, M.; Anguera, M.; Serrano, J.C.E.; Abadias, M. Exposure to minimally processed pear and melon during shelf life could modify the pathogenic potential of Listeria monocytogenes. Food Microbiol. 2017, 62, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, A.; Tokarskyy, O.; Sargent, S.; Schneider, K.R. Survival of Salmonella spp. on surface-inoculated forced-air cooled and hydrocooled intact strawberries, and in strawberry puree. Food Control 2014, 51, 244–250. [Google Scholar] [CrossRef]
- Food and Drugs Administration (FDA). Recommendations of the United States Public Health Service; Food and Drugs Administration (FDA): Silver Spring, MD, USA, 2017. [Google Scholar]
- Huang, J.; Luo, Y.; Zhou, B.; Zheng, J.; Nou, X. Growth and survival of Salmonella enterica and Listeria monocytogenes on fresh-cut produce and their juice extracts: Impacts and interactions of food matrices and temperature abuse conditions. Food Control 2019, 100, 300–304. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Lafarga, T.; Viñas, I.; Abadias, M.; Bobo, G.; Aguiló-Aguayo, I. Ultrasound processing alone or in combination with other chemical or physical treatments as a safety and quality preservation strategy of fresh and processed fruits and vegetables: A Review. Food Bioprocess Technol. 2019, 12, 1452–1471. [Google Scholar] [CrossRef]
- Singh, M.C.; Price, W.E.; Kelso, C.; Arcot, J.; Probst, Y. Measuring the anthocyanin content of the Australian fruit and vegetables for the development of a food composition database. J. Food Compos. Anal. 2022, 112, 104697. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Método Horizontal Para el Recuento de Microorganismos. Parte 1: Recuento de Colonias a 30 °C Mediante la Técnica de Siembra en Profundidad. ISO: Geneva, Switzerland, 2014.
- ISO 21527-1:2008; Método Horizontal Para el Recuento de Levaduras y Mohos. Parte 1: Técnica de Recuento de Colonias en Productos con Actividad de Agua Superior a 0.95. ISO: Geneva, Switzerland, 2008.
- ISO 4832:2006; Método Horizontal de Recuento de Microorganismos Coliformes. ISO: Geneva, Switzerland, 2015.
- ISO 16649-1:2001; Método Horizontal Para la Enumeración de Escherichia coli Beta-Glucuronidasa Positivo. Parte 1: Técnica de Recuento de Colonias Utilizando Membranas y 5-Bromo-4-Cloro-3-Indolil Beta-D-Glucurónido. ISO: Geneva, Switzerland, 2013.
- ISO 11290-1:2017; Método Horizontal Para la Detección y el Recuento de Listeria monocytogenes y de Listeria spp. Parte 1: Método de Detección. ISO: Geneva, Switzerland, 2018.
- ISO 6579-1:2017; Método Horizontal Para la Detección, Enumeración y Serotipado de Salmonella. Parte 1: Detección de Salmonella spp. ISO: Geneva, Switzerland, 2017.
- Jeffreys, A.G.; Hak, K.M.; Steffan, R.J.; Foster, J.W.; Bej, A.K. Growth, survival and characterization of cspA in Salmonella Enteritidis following cold shock. Curr. Microbiol. 1998, 36, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Beuchat, L.R.; Doyle, M.P.; Chen, J. Survival of Salmonella in pasteurized, refrigerated calcium-fortii ed orange juice. J. Food Prot. 2001, 64, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.C.L.; Oyarzabal, O.A.; Oyarzabal, O.; Gombas, D.E. Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella in cranberry, lemon, and lime juice concentrates. J. Food Prot. 2003, 66, 1637–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savran, D.; Pérez-Rodríguez, F.; Halkman, A.K. Modelling survival of Salmonella Enteritidis during storage of yoghurt at different temperatures. Int. J. Food Microbiol. 2018, 271, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Valdés, L.; Bernardo, A.; Prieto, M.; López, M. Survival of acid adapted and non-acid adapted Salmonella Typhimurium in pasteurized orange juice and yogurt under different storage temperatures. Food Sci. Technol. Int. 2013, 19, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Fernández, A.; López, M.; Bernardo, A. Relationship between membrane fatty acid composition and heat resistance of acid and cold stressed Salmonella Senftenberg CECT 4384. Food Microbiol. 2009, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Fernández, A.; López, M.; Arenas, R.; Bernardo, A. Modifications in membrane fatty acid composition of Salmonella Typhimurium in response to growth conditions and their effect on heat resistance. Int. J. Food Microbiol. 2008, 123, 212–219. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Parish, M.E.; Narciso, J.A.; Friedrich, L.M. Survival of Salmonella in orange juice. J. Food Saf. 1997, 17, 273–281. [Google Scholar] [CrossRef]
- Han, Y.; Linton, R.H. Fate of Escherichia coli O157:H7 and Listeria monocytogenes in strawberry juice and acidified media at different ph values and temperatures. J. Food Prot. 2004, 67, 2443–2449. [Google Scholar] [CrossRef]
- Nielsen, L.; Knøchel, S. Inactivation of Salmonella strains in acidified broth and raw egg yolk as a function of pH and acid type. Food Microbiol. 2020, 92, 103574. [Google Scholar] [CrossRef]
- Nutt, J.D.; Li, X.; Woodward, C.L.; Zabala-Díaz, I.B.; Ricke, S.C. Growth kinetics response of a Salmonella Typhimurium poultry marker strain to fresh produce extracts. Bioresour. Technol. 2003, 89, 313–316. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]
- Yin, X.; Gyles, C.L.; Gong, J. Grapefruit juice and its constituents augment the effect of low pH on inhibition of survival and adherence to intestinal epithelial cells of Salmonella enterica serovar Typhimurium PT193. Int. J. Food Microbiol. 2012, 158, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Paunović, S.M.; Mašković, P.; Nikolić, M.; Miletić, R. Bioactive compounds and antimicrobial activity of black currant (Ribes nigrum L.) berries and leaves extract obtained by different soil management system. Sci. Hortic. 2017, 222, 69–75. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Cavestri, C.; Savard, P.; Fliss, I.; Emond-Rhéault, J.-G.; Hamel, J.; Kukavica-Ibrulj, I.; Boyle, B.; Daigle, F.; Malo, D.; Bekal, S.; et al. Salmonella enterica subsp. enterica virulence potential can be linked to higher survival within a dynamic in vitro human gastrointestinal model. Food Microbiol. 2022, 101, 103877. [Google Scholar] [CrossRef]
- Guillén, S.; Marcén, M.; Mañas, P.; Cebrián, G. Differences in resistance to different environmental stresses and non-thermal food preservation technologies among Salmonella enterica subsp. enterica strains. Food Res. Int. 2020, 132, 109042. [Google Scholar] [CrossRef] [PubMed]
- Norberto, A.P.; Alvarenga, V.O.; Hungaro, H.M.; Sant’Ana, A.S. Desiccation resistance of a large set of Salmonella enterica strains and survival on dry- and wet-inoculated soybean meal through storage. LWT 2022, 158, 113153. [Google Scholar] [CrossRef]
| pH | TSS | TTA | AC | TPC | Antioxidant Capacity | ||
|---|---|---|---|---|---|---|---|
| DPPH | FRAP | ||||||
| Plum | 3.40 ± 0.06 cd | 12.5 ± 0.0 b | 8.80 ± 0.49 b | nd | 103.21 ± 0.20 d | 2909.7 ± 17.3 c | 3110.6 ± 6.8 c |
| Black currant | 3.23 ± 0.09 d | 15.1 ± 0.0 a | 21.18 ± 0.19 a | 25.14 ± 9.51 b | 486.79 ± 0.23 a | 7347.7 ± 31.7 a | 12,665.3 ± 7.8 a |
| Blueberry | 3.57± 0.16 c | 9.5 ± 0.1 e | 3.93 ± 0.11 c | 110.88 ± 6.03 a | 446.99 ± 0.47 b | 4964.2 ± 165.2 b | 7490.1 ± 7.8 b |
| Peach | 4.42 ± 0.07 a | 12.5 ± 0.0 b | 3.11 ± 0.18 d | nd | 42.01 ± 0.12 e | 1746.1 ± 23.4 d | 1656.4 ± 3.9 d |
| Apple | 4.11 ± 0.03 b | 11.8 ± 0.1 d | 2.57 ± 0.10 d | nd | 137.64 ± 7.59 c | 1283.5 ± 97.2 e | 1229.7 ± 3.9 e |
| Pear | 4.06 ± 0.01 b | 12.1 ± 0.1 c | 2.55 ± 0.12 d | nd | 98.42 ± 4.25 d | 1081.4 ± 12.3 e | 1023.5 ± 3.4 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bainotti, M.B.; Colás-Medà, P.; Viñas, I.; Garza, S.; Alegre, I. The Survival of Salmonella enterica Strains in Ready-to-Eat Fruit Purees under Different Storage Temperatures. Beverages 2024, 10, 17. https://doi.org/10.3390/beverages10010017
Bainotti MB, Colás-Medà P, Viñas I, Garza S, Alegre I. The Survival of Salmonella enterica Strains in Ready-to-Eat Fruit Purees under Different Storage Temperatures. Beverages. 2024; 10(1):17. https://doi.org/10.3390/beverages10010017
Chicago/Turabian StyleBainotti, Maria Belén, Pilar Colás-Medà, Inmaculada Viñas, Salvador Garza, and Isabel Alegre. 2024. "The Survival of Salmonella enterica Strains in Ready-to-Eat Fruit Purees under Different Storage Temperatures" Beverages 10, no. 1: 17. https://doi.org/10.3390/beverages10010017
APA StyleBainotti, M. B., Colás-Medà, P., Viñas, I., Garza, S., & Alegre, I. (2024). The Survival of Salmonella enterica Strains in Ready-to-Eat Fruit Purees under Different Storage Temperatures. Beverages, 10(1), 17. https://doi.org/10.3390/beverages10010017