Valve Endothelial Cell Exposure to High Levels of Flow Oscillations Exacerbates Valve Interstitial Cell Calcification
Abstract
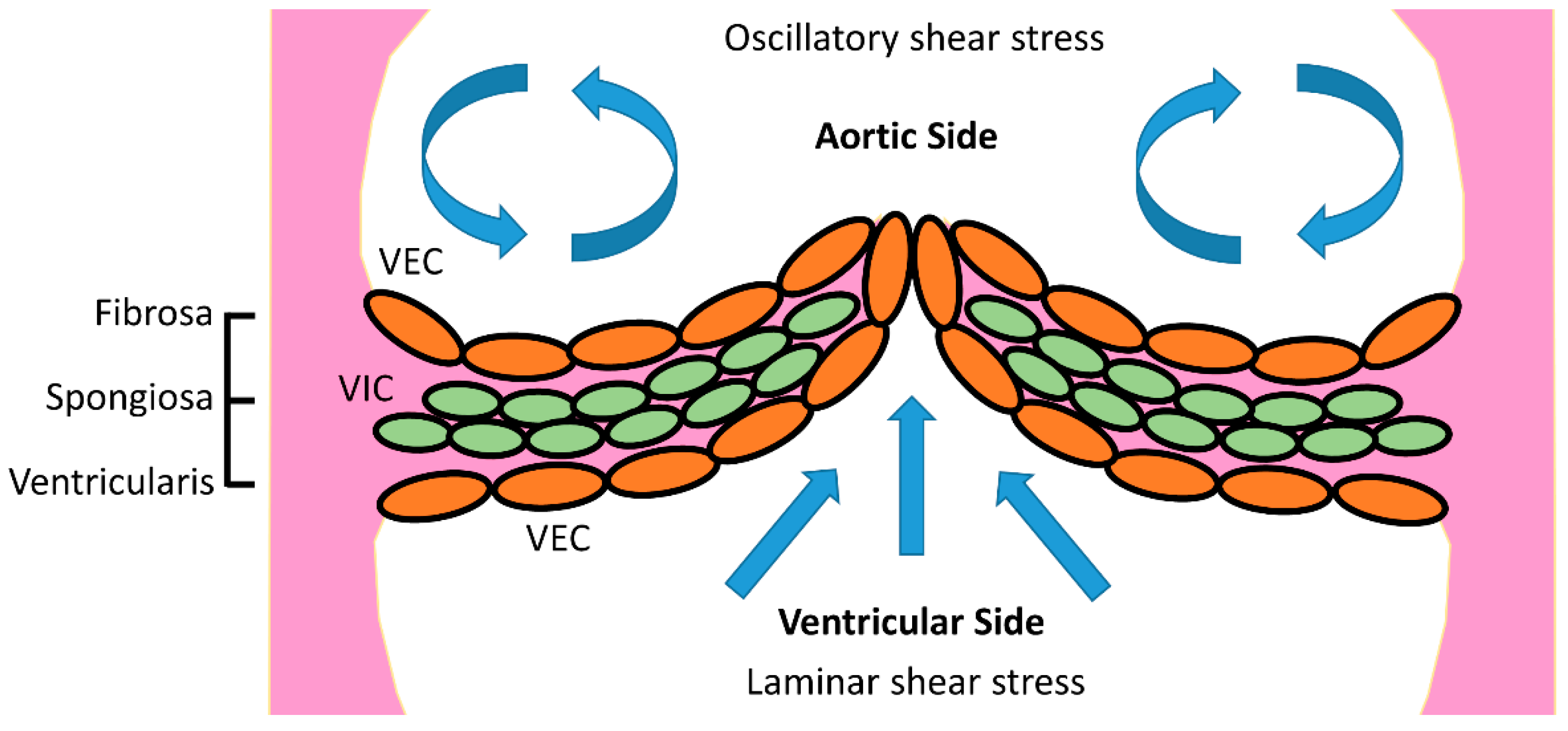
:1. Introduction
2. Materials and Methods
2.1. Computational Fluid Dynamics (CFD)
2.2. Analyses of Excised Aortic Valve Leaflets
2.3. In Vitro Cell Culture Experiments and Subsequent Assessments
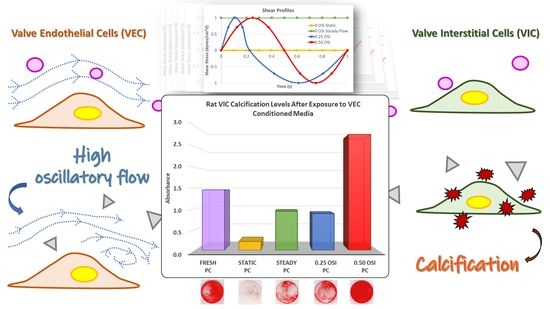
3. Results
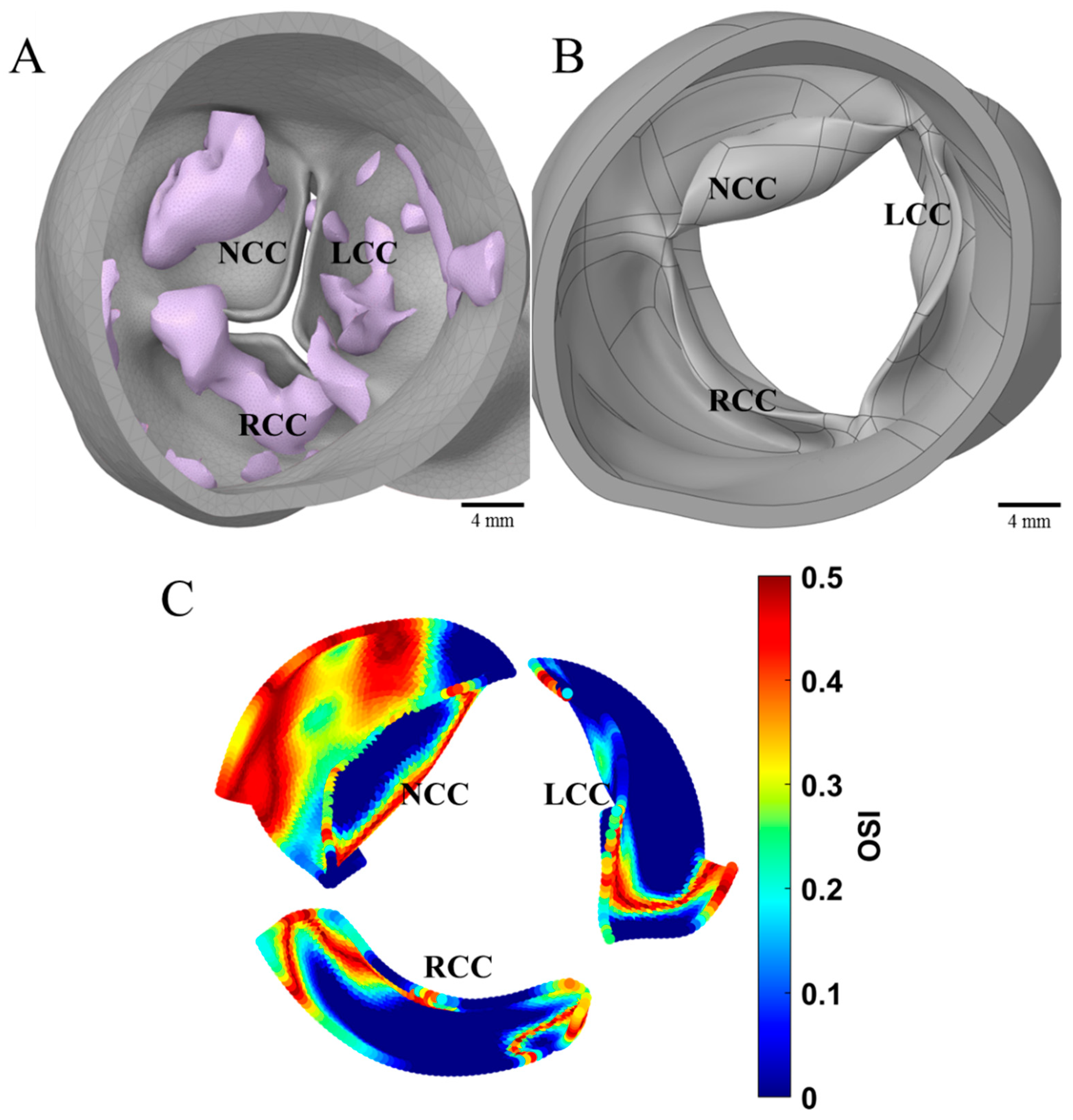
3.1. CFD
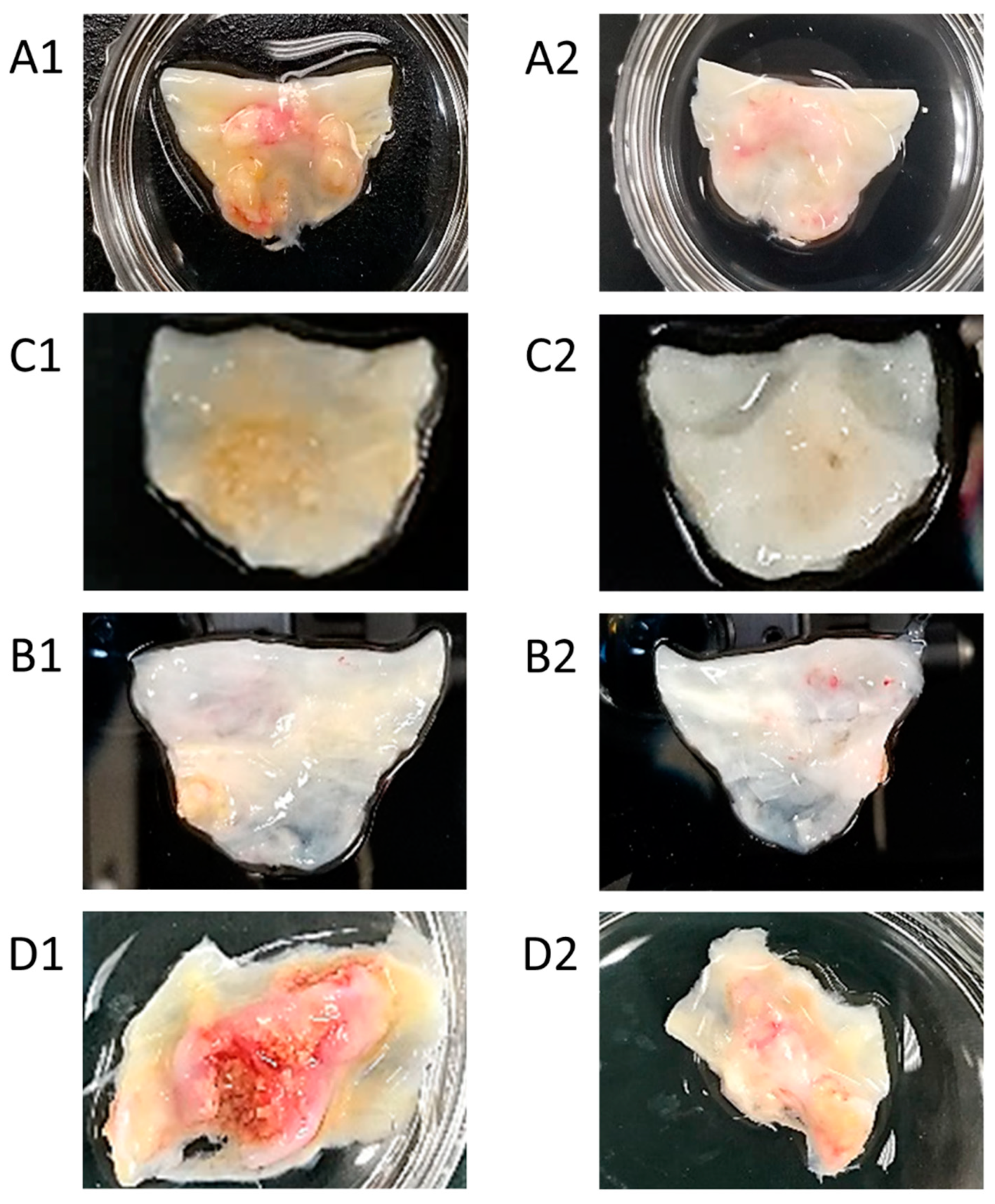
3.2. Analyses of Excised Aortic Valve Leaflets
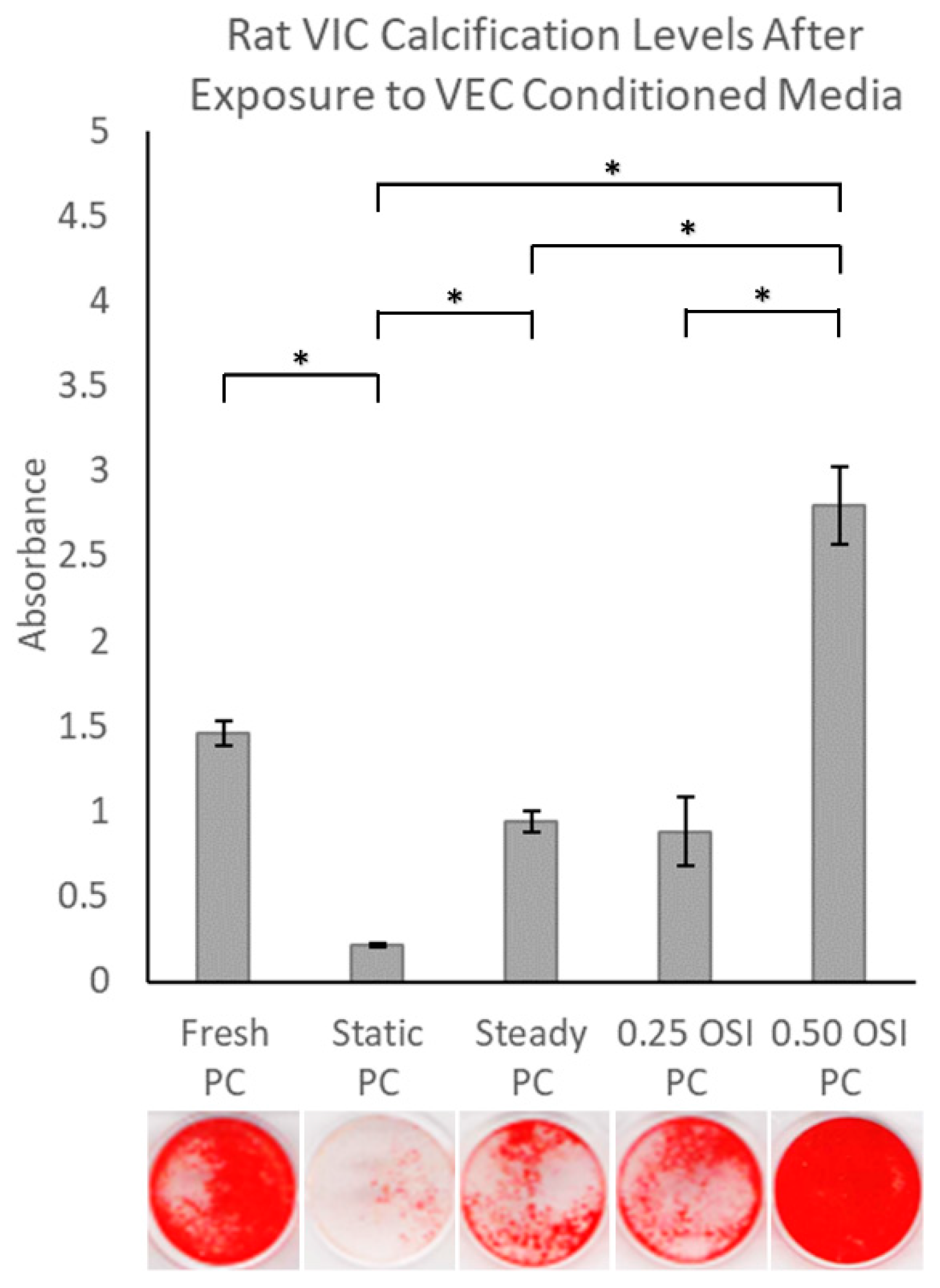
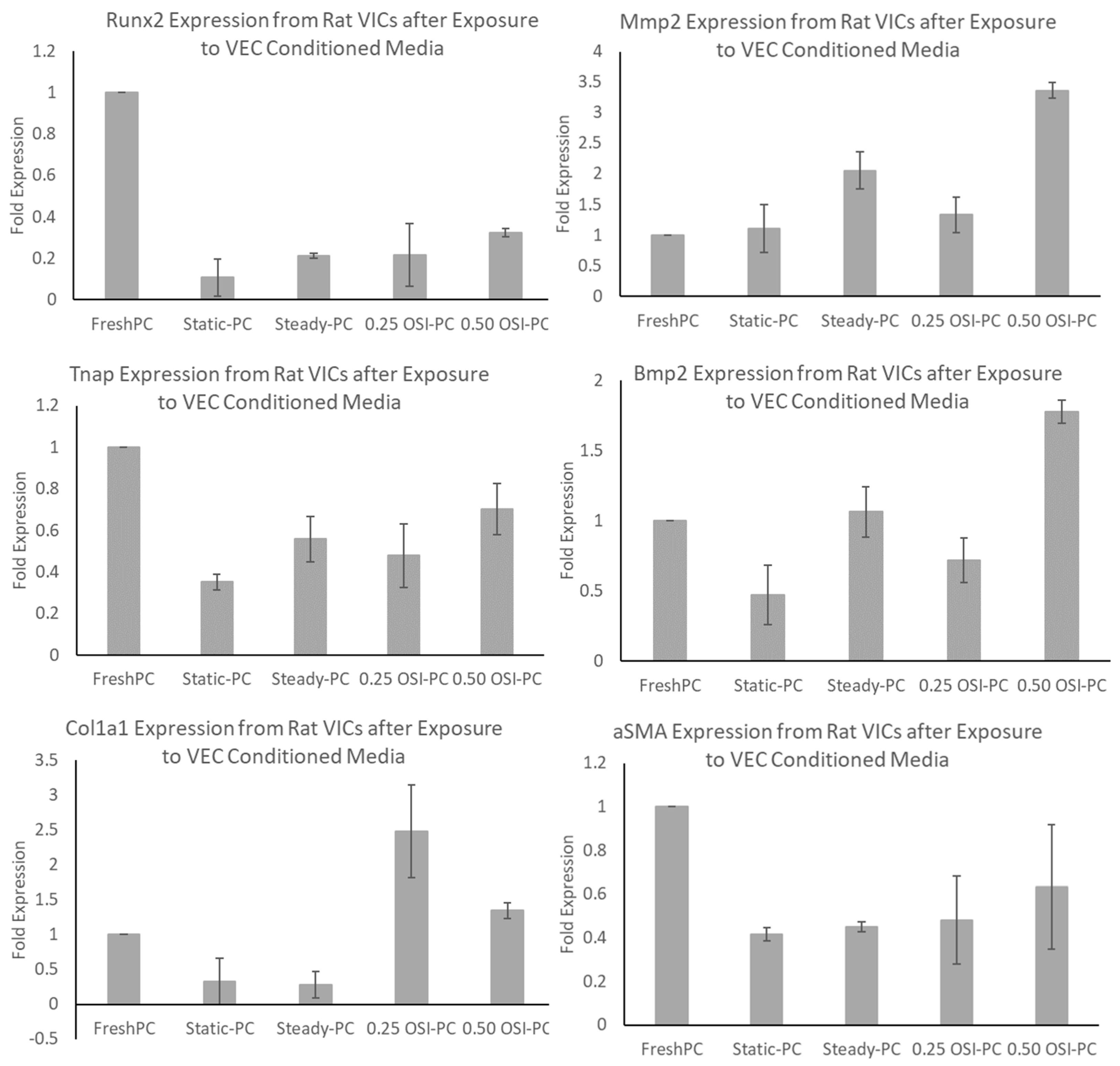
3.3. In Vitro Cell Culture Experiments and Subsequent Assessments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef]
- Hsu, C.-P.D.; Hutcheson, J.D.; Ramaswamy, S. Oscillatory Fluid-Induced Mechanobiology in Heart Valves with Parallels to the Vasculature. Vasc. Biol. 2020, 2, R59–R71. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ali, L.; Foffa, I.; Festa, P.; Andreassi, M.G. Bicuspidia Aortica: Epidemiologia, Genetica e Clinica. Recenti. Prog. Med. 2012, 103, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Cellular Mechanisms of Aortic Valve Calcification. Circ. Cardiovasc. Interv. 2012, 5, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, K.; Sucosky, P.; Yoganathan, A.P. Hemodynamics and Mechanobiology of Aortic Valve Inflammation and Calcification. Int. J. Inflamm. 2011, 2011, 263870. [Google Scholar] [CrossRef] [PubMed]
- Gomel, M.A.; Lee, R.; Grande-Allen, K.J. Comparing the Role of Mechanical Forces in Vascular and Valvular Calcification Progression. Front. Cardiovasc. Med. 2019, 5, 197. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef]
- Rathan, S.; Ankeny, C.J.; Arjunon, S.; Ferdous, Z.; Kumar, S.; Fernandez Esmerats, J.; Heath, J.M.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Identification of Side- and Shear-Dependent microRNAs Regulating Porcine Aortic Valve Pathogenesis. Sci. Rep. 2016, 6, 25397. [Google Scholar] [CrossRef]
- Esmerats, J.F.; Villa-Roel, N.; Kumar, S.; Gu, L.; Salim, T.; Ohh, M.; Taylor, W.R.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1α (Hypoxia-Inducible Factor-1α) Pathway in Endothelial Cells. Arter. Thromb. Vasc. Biol. 2019, 39, 467–481. [Google Scholar] [CrossRef]
- He, X.; Ku, D.N. Pulsatile Flow in the Human Left Coronary Artery Bifurcation: Average Conditions. J. Biomech. Eng. 1996, 118, 74–82. [Google Scholar] [CrossRef]
- Mohammadi, H.; Goode, D.; Fradet, G.; Mequanint, K. Proposed Percutaneous Aortic Valve Prosthesis Made of Cryogel. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2019, 233, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Ramaswamy, S. Importance of Non-Newtonian Computational Fluid Modeling on Severely Calcified Aortic Valve Geometries—Insights from Quasi-Steady State Simulations. J. Biomech. Eng. 2022, 144, 114501. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.M.; Barreto, A.; Boodooram, T.; Ramaswamy, S. Importance of Non-Newtonian Modeling of Blood Flow for Calcified Aortic Valves: Relevance to Sub-Clinical Thrombosis. Struct. Heart 2021, 5, 30. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Rathan, S.; Yoganathan, A.P.; O’Neill, C.W. The Role of Inorganic Pyrophosphate in Aortic Valve Calcification. J. Heart Valve Dis. 2014, 23, 387–394. [Google Scholar] [PubMed]
- Goto, S.; Rogers, M.A.; Blaser, M.C.; Higashi, H.; Lee, L.H.; Schlotter, F.; Body, S.C.; Aikawa, M.; Singh, S.A.; Aikawa, E. Standardization of Human Calcific Aortic Valve Disease in vitro Modeling Reveals Passage-Dependent Calcification. Front. Cardiovasc. Med. 2019, 6, 49. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Valente, V.; Teixeira, S.A.; Neder, L.; Okamoto, O.K.; Oba-Shinjo, S.M.; Marie, S.K.; Scrideli, C.A.; Paçó-Larson, M.L.; Carlotti, C.G. Selection of Suitable Housekeeping Genes for Expression Analysis in Glioblastoma Using Quantitative RT-PCR. BMC Mol. Biol. 2009, 10, 17. [Google Scholar] [CrossRef]
- Yi, B.; Zeng, W.; Lv, L.; Hua, P. Changing Epidemiology of Calcific Aortic Valve Disease: 30-year Trends of Incidence, Prevalence, and Deaths Across 204 Countries and Territories. Aging 2021, 13, 12710–12732. [Google Scholar] [CrossRef]
- Ladich, E.; Nakano, M.; Carter-Monroe, N.; Virmani, R. Pathology of Calcific Aortic Stenosis. Futur. Cardiol. 2011, 7, 629–642. [Google Scholar] [CrossRef]
- Yip, C.Y.Y.; Simmons, C.A. The Aortic Valve Microenvironment and Its Role in Calcific Aortic Valve Disease. Cardiovasc. Pathol. 2011, 20, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zebhi, B.; Lazkani, M.; Bark, D.J. Calcific Aortic Stenosis—A Review on Acquired Mechanisms of the Disease and Treatments. Front. Cardiovasc. Med. 2021, 8, 734175. [Google Scholar] [CrossRef] [PubMed]
- Kazik, H.B.; Kandail, H.S.; LaDisa, J.F.J.; Lincoln, J. Molecular and Mechanical Mechanisms of Calcification Pathology Induced by Bicuspid Aortic Valve Abnormalities. Front. Cardiovasc. Med. 2021, 8, 677977. [Google Scholar] [CrossRef]
- Wirrig, E.E.; Yutzey, K.E. Developmental Pathways in CAVD. In Calcific Aortic Valve Disease; Aikawa, E., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Faure, E.; Bertrand, E.; Gasté, A.; Plaindoux, E.; Deplano, V.; Zaffran, S. Side-Dependent Effect in the Response of Valve Endothelial Cells to Bidirectional Shear Stress. Int. J. Cardiol. 2020, 323, 220–228. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis as an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.R.; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone Formation and Inflammation in Cardiac Valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Cao, K.; Bukač, M.; Sucosky, P. Three-Dimensional Macro-Scale Assessment of Regional and Temporal Wall Shear Stress Characteristics on Aortic Valve Leaflets. Comput. Methods Biomech. Biomed. Eng. 2015, 19, 603–613. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Deng, C.; Li, F.; Wang, Y.; Hu, X.; Shi, F.; Dong, N. MicroRNA-204 Targets Runx2 to Attenuate BMP-2-induced Osteoblast Differentiation of Human Aortic Valve Interstitial Cells. J. Cardiovasc. Pharmacol. 2015, 66, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmouliere, A. Fibroblasts and Myofibroblasts in Wound Healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef]
- Bosse, K.; Hans, C.P.; Zhao, N.; Koenig, S.N.; Huang, N.; Guggilam, A.; LaHaye, S.; Tao, G.; Lucchesi, P.A.; Lincoln, J.; et al. Endothelial Nitric Oxide Signaling Regulates Notch1 in Aortic Valve Disease. J. Mol. Cell. Cardiol. 2013, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Nasim, S.; Salinas, M.; Moshkforoush, A.; Tsoukias, N.; Ramaswamy, S. A “Sweet-Spot” for Fluid-Induced Oscillations in the Conditioning of Stem Cell-Based Engineered Heart Valve Tissues. J. Biomech. 2017, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Prendergast, B. Aortic-Valve Stenosis—From Patients at Risk to Severe Valve Obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-P.D.; Tchir, A.; Mirza, A.; Chaparro, D.; Herrera, R.E.; Hutcheson, J.D.; Ramaswamy, S. Valve Endothelial Cell Exposure to High Levels of Flow Oscillations Exacerbates Valve Interstitial Cell Calcification. Bioengineering 2022, 9, 393. https://doi.org/10.3390/bioengineering9080393
Hsu C-PD, Tchir A, Mirza A, Chaparro D, Herrera RE, Hutcheson JD, Ramaswamy S. Valve Endothelial Cell Exposure to High Levels of Flow Oscillations Exacerbates Valve Interstitial Cell Calcification. Bioengineering. 2022; 9(8):393. https://doi.org/10.3390/bioengineering9080393
Chicago/Turabian StyleHsu, Chia-Pei Denise, Alexandra Tchir, Asad Mirza, Daniel Chaparro, Raul E. Herrera, Joshua D. Hutcheson, and Sharan Ramaswamy. 2022. "Valve Endothelial Cell Exposure to High Levels of Flow Oscillations Exacerbates Valve Interstitial Cell Calcification" Bioengineering 9, no. 8: 393. https://doi.org/10.3390/bioengineering9080393
APA StyleHsu, C.-P. D., Tchir, A., Mirza, A., Chaparro, D., Herrera, R. E., Hutcheson, J. D., & Ramaswamy, S. (2022). Valve Endothelial Cell Exposure to High Levels of Flow Oscillations Exacerbates Valve Interstitial Cell Calcification. Bioengineering, 9(8), 393. https://doi.org/10.3390/bioengineering9080393