A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study
Abstract
:1. Introduction
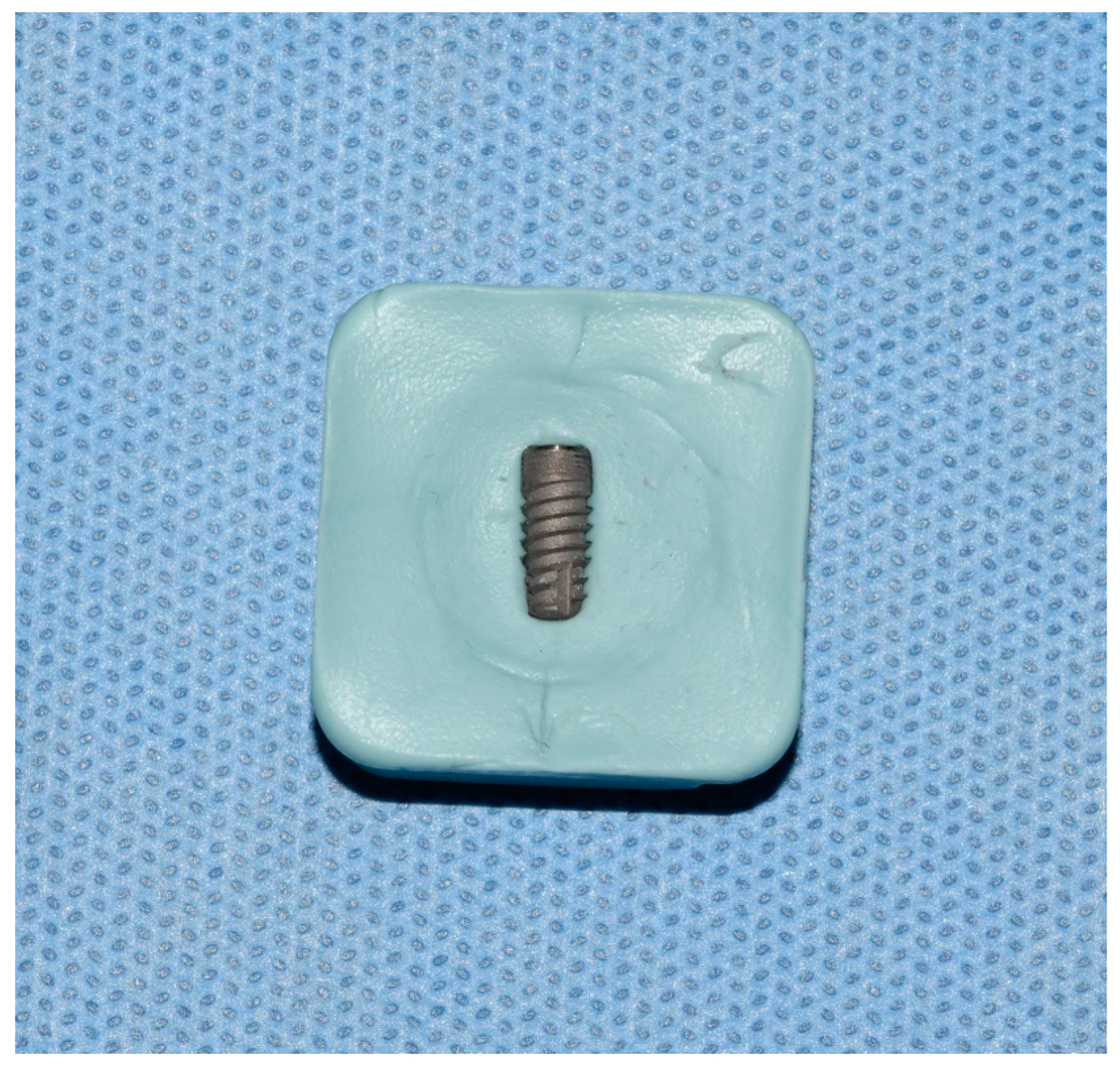
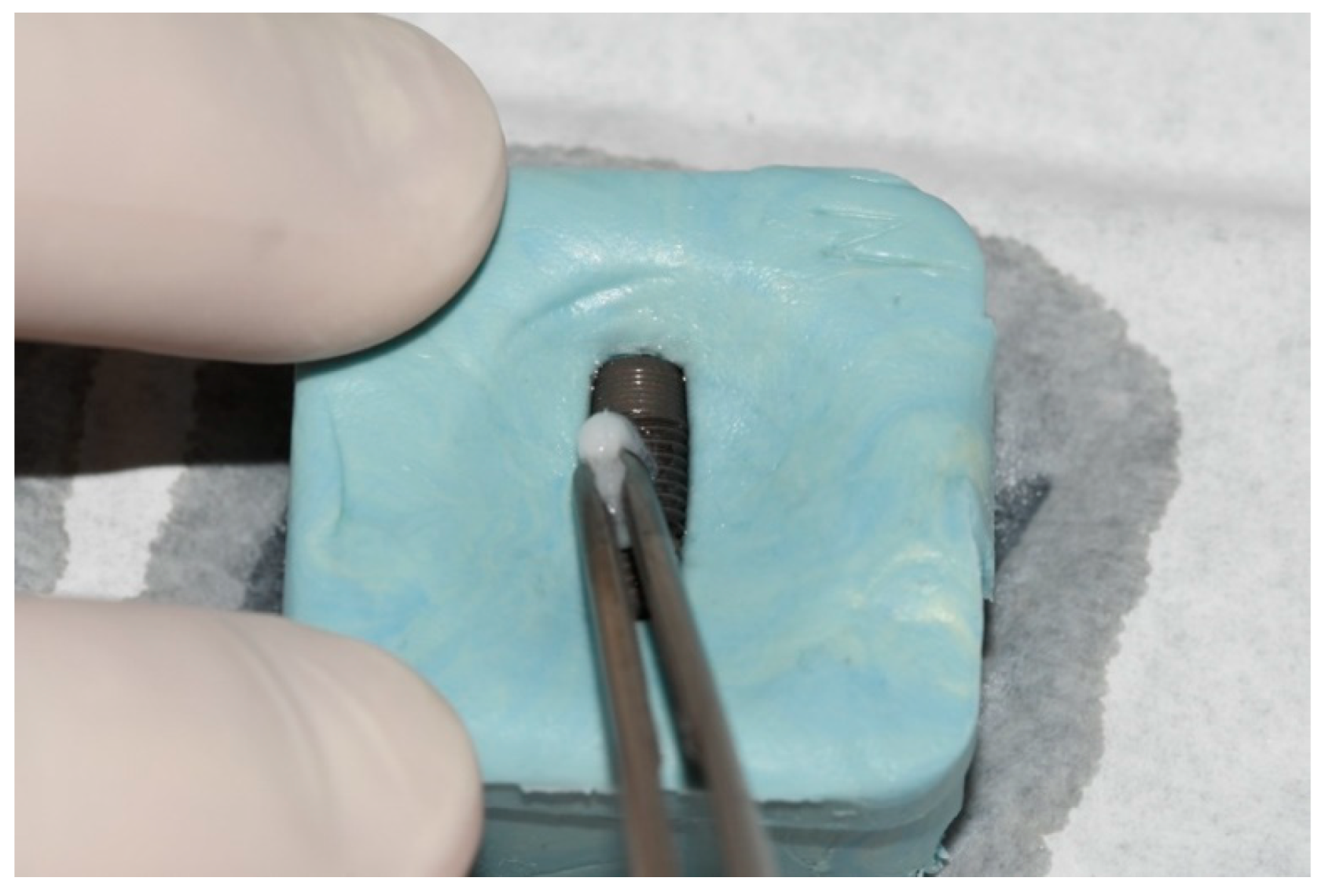
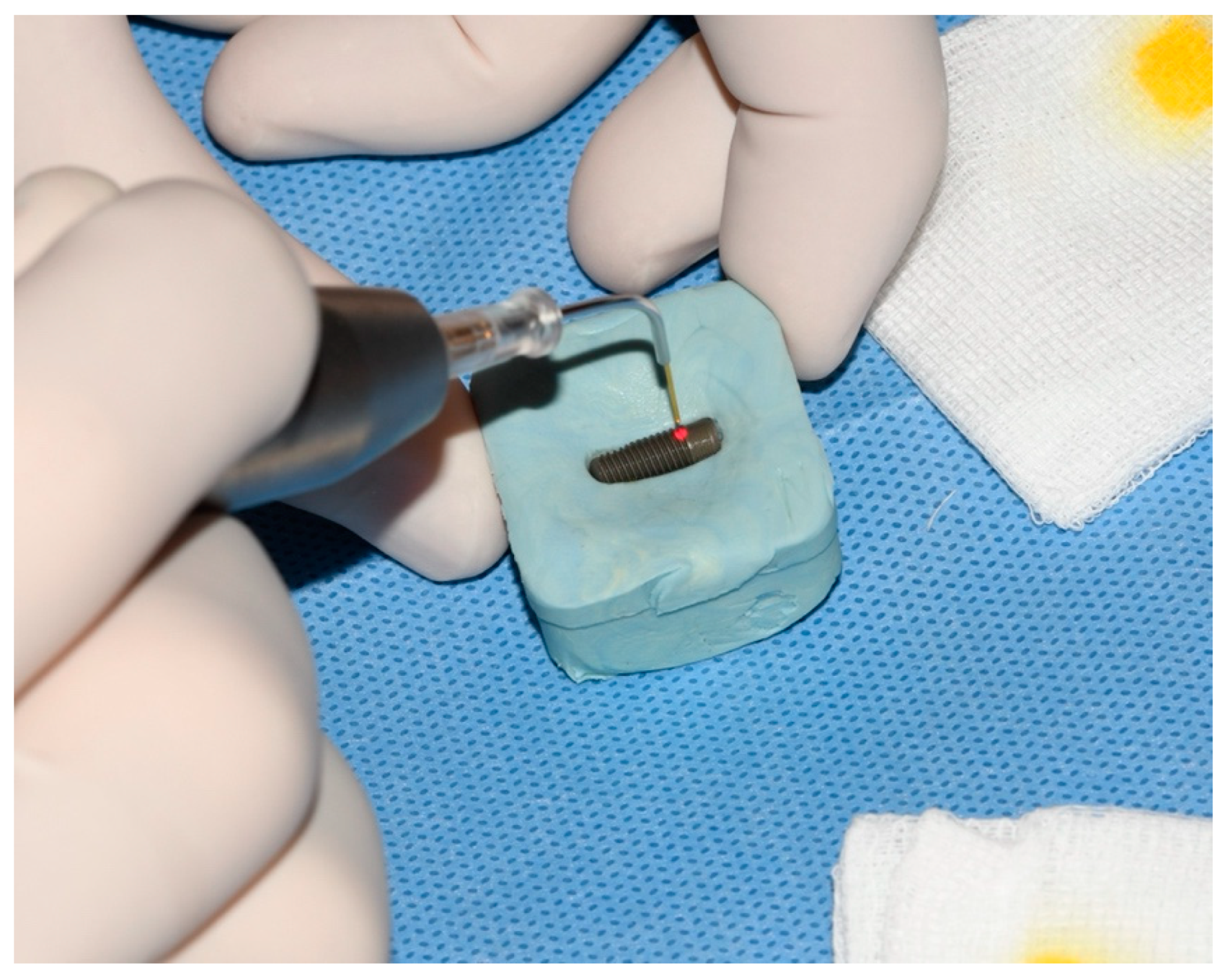
2. Material and Methods
Statistical Analysis
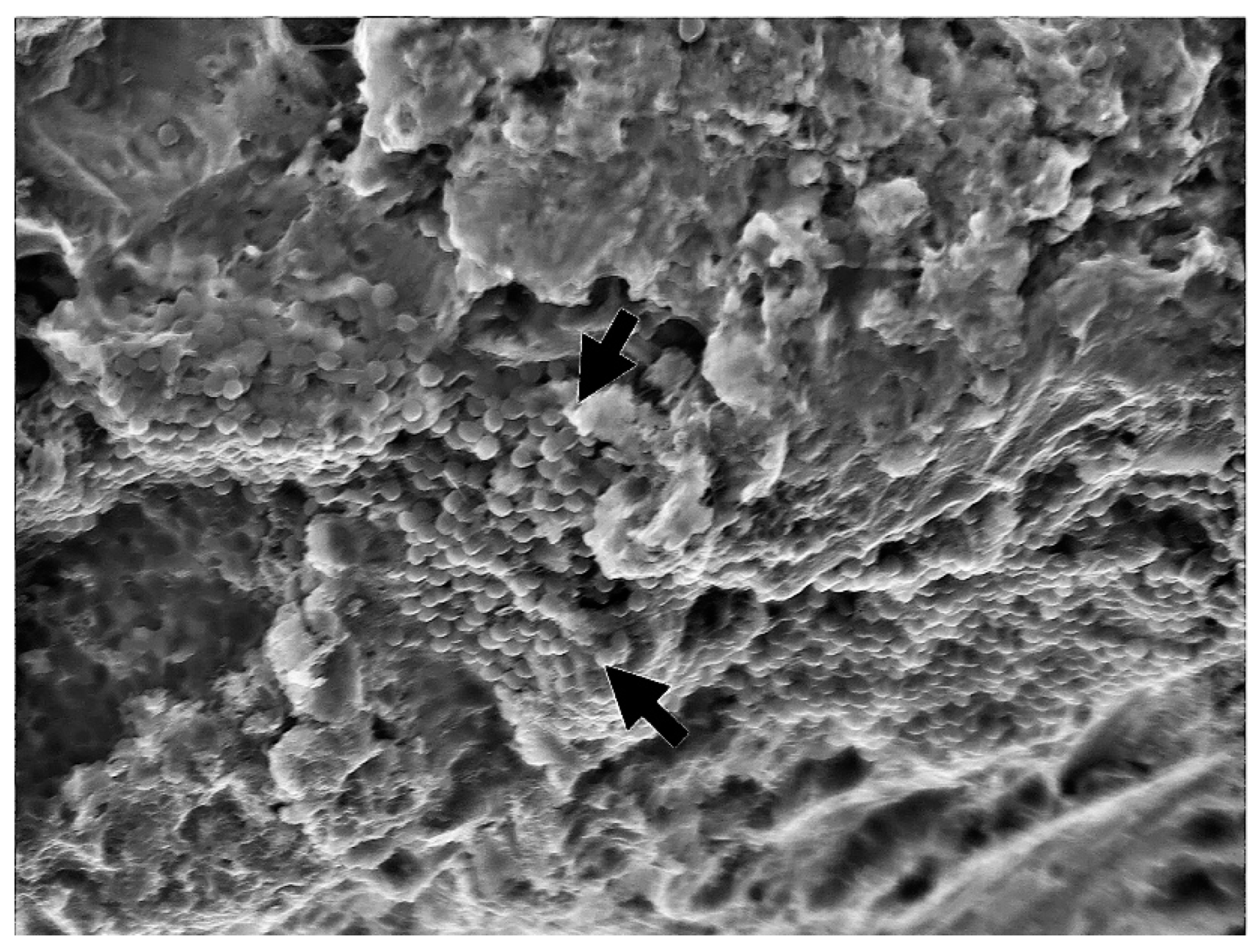
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wada, M.; Mameno, T.; Otsuki, M.; Kani, M.; Tsujioka, Y.; Ikebe, K. Prevalence and risk indicators for peri-implant diseases: A literature review. Jpn. Dent. Sci. Rev. 2021, 57, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Al-Radha, A.S.D.; Pal, A.; Pettemerides, A.P.; Jenkinson, H. Molecular analysis of microbiota associated with peri-implant diseases. J. Dent. 2012, 40, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, C.; Monesi, N.; Ito, I.Y.; Issa, J.P.; de Albuquerque, R.F., Jr. Bacterial diversity of periodontal and implant-related sites detected by the DNA Checkerboard method. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1607–1613. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Fürst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res. 2008, 19, 242–248. [Google Scholar] [CrossRef]
- Tillander, J.; Hagberg, K.; Hagberg, L.; Brånemark, R. Osseointegrated titanium implants for limb prostheses attachments: Infectious complications. Clin. Orthop. Relat. Res. 2010, 468, 2781–2788. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Li, Y.; Wang, Y.; Chen, S.; Jiang, S.; Ge, H.; Lei, L.; Huang, X. Antimicrobial effects of photodynamic therapy with antiseptics on Staphylococcus aureus biofilm on titanium surface. Photodiagnosis Photodyn. Ther. 2019, 25, 382–388. [Google Scholar] [CrossRef]
- Urzúa, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef]
- De La Torre, J.; Quindós, G.; Marcos-Arias, C.; Marichalar-Mendia, X.; Gainza, M.L.; Eraso, E.; Acha-Sagredo, A.; Aguirre-Urizar, J.M. Oral Candida colonization in patients with chronic periodontitis. Is there any relationship? Rev Iberoam Micol. 2018, 35, 134–139. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 89, S286–S291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntrouka, V.; Hoogenkamp, M.; Zaura, E.; van der Weijden, F. The effect of chemotherapeutic agents on titanium-adherent biofilms. Clin. Oral Implant. Res. 2011, 22, 1227–3124. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Iglhaut, G.; Becker, J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: A randomized controlled clinical study. J. Clin. Periodontol. 2011, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Valero, A.; Buitrago-Vera, P.; Solá-Ruiz, M.F.; Ferrer-García, J.C. Decontamination of dental implant surface in peri-implantitis treatment: A literature review. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e869–e876. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Yu, S.H.; Wang, H.L. Non-Surgical Therapy for Peri-Implant Diseases: A Systematic Review. J. Oral Maxillofac Res. 2016, 7, e13. [Google Scholar] [CrossRef] [Green Version]
- Al-Hashedi, A.A.; Laurenti, M.; Benhamou, V.; Tamimi, F. Decontamination of titanium implants using physical methods. Clin. Oral Implant. Res. 2017, 28, 1013–1021. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F.A. Titanium surface alterations following the use of different mechanical instruments: A systematic review. Clin. Oral Implants Res. 2012, 23, 643–658. [Google Scholar] [CrossRef]
- Akram, Z.; Al-Shareef, S.A.; Daood, U.; Asiri, F.Y.; Shah, A.H.; AlQahtani, M.A.; Vohra, F.; Javed, F. Bactericidal Efficacy of Photodynamic Therapy Against Periodontal Pathogens in Periodontal Disease: A Systematic Review. Photomed. Laser Surg. 2016, 34, 137–149. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Hayek, R.R.; Araújo, N.S.; Gioso, M.A.; Ferreira, J.; Baptista-Sobrinho, C.A.; Yamada, A.M.; Ribeiro, M.S. Comparative Study Between the Effects of Photodynamic Therapy and Conventional Therapy on Microbial Reduction in Ligature-Induced Peri-Implantitis in Dogs. J. Periodontol. 2005, 76, 1275–1281. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy —What we know and what we don′t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kömerik, N.; Nakanishi, H.; MacRobert, A.J.; Henderson, B.; Speight, P.; Wilson, M. In Vivo Killing of Porphyromonas gingivalis by Toluidine Blue-Mediated Photosensitization in an Animal Model. Antimicrob. Agents Chemother. 2003, 47, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.-Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontology 2000 2009, 51, 109–140. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of perio-dontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J Periodontol. 2018, 89, 783–803. [Google Scholar] [PubMed]
- Katalinić, I.; Budimir, A.; Bošnjak, Z.; Jakovljević, S.; Anić, I. The photo-activated and photo-thermal effect of the 445/970 nm diode laser on the mixed biofilm inside root canals of human teeth in vitro: A pilot study. Photodiagnosis Photodyn. Ther. 2019, 26, 277–283. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Gustumhaugen, E.; Lönn-Stensrud, J.; Scheie, A.A.; Lyngstadaas, S.P.; Ekfeldt, A.; Taxt-Lamolle, S. Effect of chemical and mechanical debridement techniques on bacterial re-growth on rough titanium surfaces: An in vitro study. Clin. Oral Implants Res. 2014, 25, 707–713. [Google Scholar] [CrossRef]
- Deppe, H.; Ahrens, M.; Behr, A.V.; Marr, C.; Sculean, A.; Mela, P.; Ritschl, L.M. Thermal effect of a 445 nm diode laser on five dental implant systems: An in vitro study. Sci. Rep. 2021, 11, 20174. [Google Scholar] [CrossRef]
- Malmqvist, S.; Liljeborg, A.; Qadri, T.; Johannsen, G.; Johannsen, A. Using 445 nm and 970 nm Lasers on Dental Im-plants-An In Vitro Study on Change in Temperature and Surface Alterations. Materials 2019, 12, 3934. [Google Scholar] [CrossRef] [Green Version]
- Widodo, A.; Spratt, D.; Sousa, V.; Petrie, A.; Donos, N. An in vitro study on disinfection of titanium surfaces. Clin. Oral Implants Res. 2016, 27, 1227–1232. [Google Scholar] [CrossRef]
- Ryu, H.S.; Kim, Y.I.; Lim, B.S.; Lim, Y.J.; Ahn, S.J. Chlorhexidine Uptake and Release from Modified Titanium Surfaces and Its Antimicrobial Activity. J. Periodontol. 2015, 86, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.K.; Garcia, J.; Væth, M.; Schlafer, S. Comparison of Riboflavin and Toluidine Blue O as Photosensitizers for Photoactivated Disinfection on Endodontic and Periodontal Pathogens In Vitro. PLoS ONE 2015, 10, e0140720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bärenfaller, V.; Clausen, C.; Sculean, A.; Eick, S. Effect of photoactivated disinfection using light in the blue spectrum. J. Photochem. Photobiol. B Biol. 2016, 158, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Silver, J.; Marsh, P.J.; Birss, A.J. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: An oxidative buffer and possible pathogenic mechanism. Biochem. J. 1998, 331, 681–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Durantini, E.N. New insights into the antimicrobial blue light inactivation of Candida albicans. Virulence 2016, 7, 493–494. [Google Scholar] [CrossRef] [Green Version]
- Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains-An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Owen, B.; Wataha, J.C.; Campo, M.A.; Lange, N.; Schrenzel, J. Intracellular reactive oxygen species in monocytes generated by photosensitive chromophores activated with blue light. Dent. Mater. 2008, 24, 1070–1076. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Wataha, J.C.; Zapata, O.; Campo, M.; Lange, N.; Schrenzel, J. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomed Laser Surg. 2010, 28, 519–525. [Google Scholar] [CrossRef]
- Leelanarathiwat, K.; Katsuta, Y.; Katsuragi, H.; Watanabe, F. Antibacterial activity of blue high-power light-emitting diode-activated flavin mononucleotide against Staphylococcus aureus biofilm on a sandblasted and etched surface. Photodiagnosis Photodyn. Ther. 2020, 31, 101855. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, G.; Plotino, G.; Grande, N.M.; Nocca, G.; Lupi, A.; Giardina, B.; De Luca, M.; Testarelli, L. In vitro evaluation of the cy-totoxicity of FotoSan™ light-activated disinfection on human fibroblasts. Med. Sci. Monit. 2011, 17, MT21–MT25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The photodynamic effect of methylene blue and toluidine blue on Candida albicans is dependent on medium conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Amirzadeh, Z.; Rezai, M.; Lawaf, S.; Rahimi, A. Effect of photodynamic therapy with two photosensitizers on Candida albicans. J. Photochem. Photobiol. B Biol. 2016, 158, 267–273. [Google Scholar] [CrossRef]
- Pereira, C.A.; Romeiro, R.L.; Costa, A.C.; Machado, A.K.; Junqueira, J.C.; Jorge, A.O. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med. Sci. 2011, 26, 341–348. [Google Scholar] [CrossRef]
- Rosa, L.P.; da Silva, F.C.; Nader, S.A.; Meira, G.A.; Viana, M.S. Antimicrobial photodynamic inactivation of Staphylococcus aureus biofilms in bone specimens using methylene blue, toluidine blue ortho and malachite green: An in vitro study. Arch. Oral Biol. 2015, 60, 675–680. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: In vitro study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Alasqah, M.N. Antimicrobial efficacy of photodynamic therapy on dental implant surfaces: A systematic review of in vitro studies. Photodiagnosis Photodyn. Ther. 2019, 25, 349–353. [Google Scholar] [CrossRef]
- Vohra, F.; Al-Rifaiy, M.Q.; Lillywhite, G.; Abu Hassan, M.I.; Javed, F. Efficacy of mechanical debridement with adjunct antimicrobial photodynamic therapy for the management of peri-implant diseases: A systematic review. Photochem. Photobiol. Sci. 2014, 13, 1160–1168. [Google Scholar] [CrossRef]


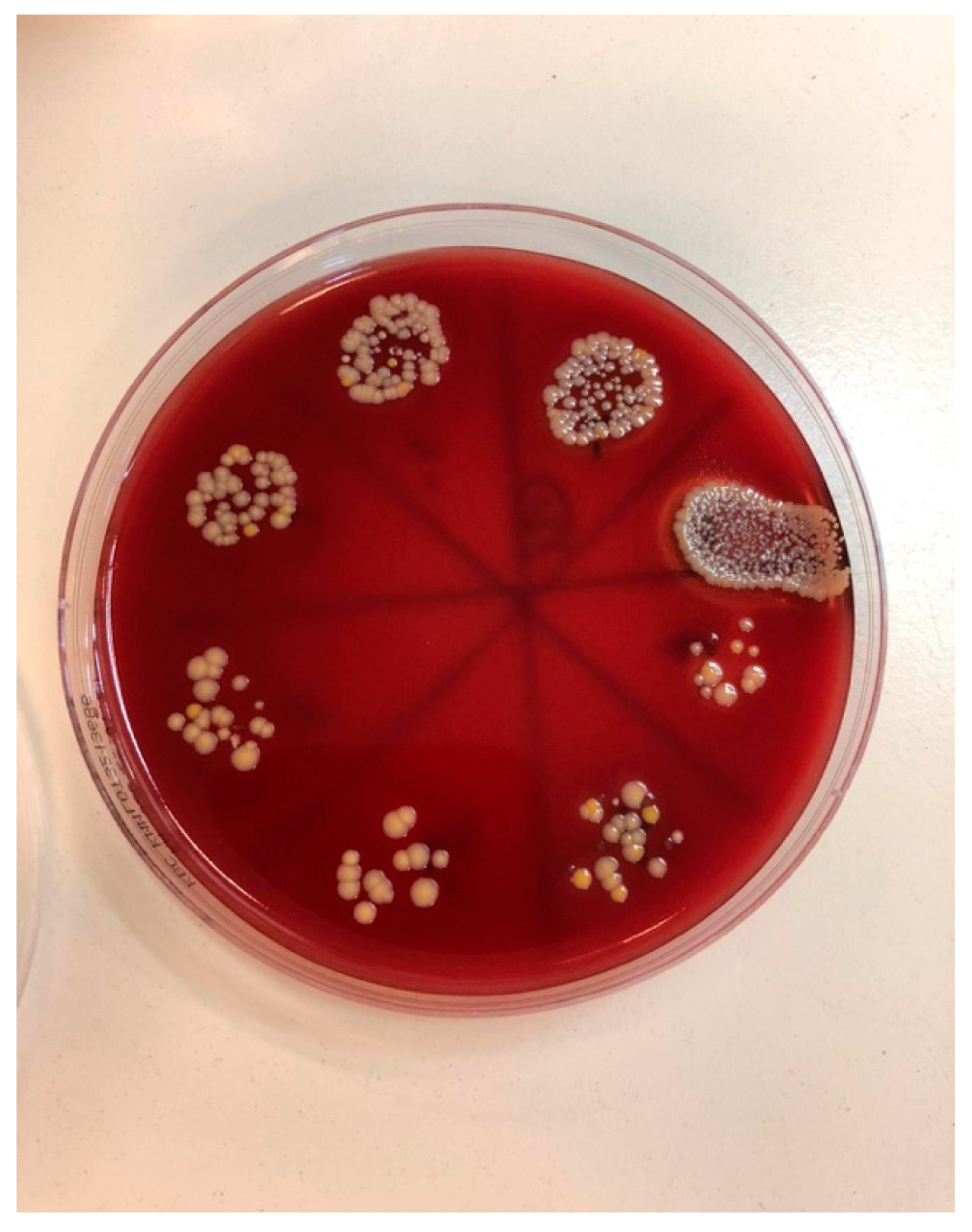




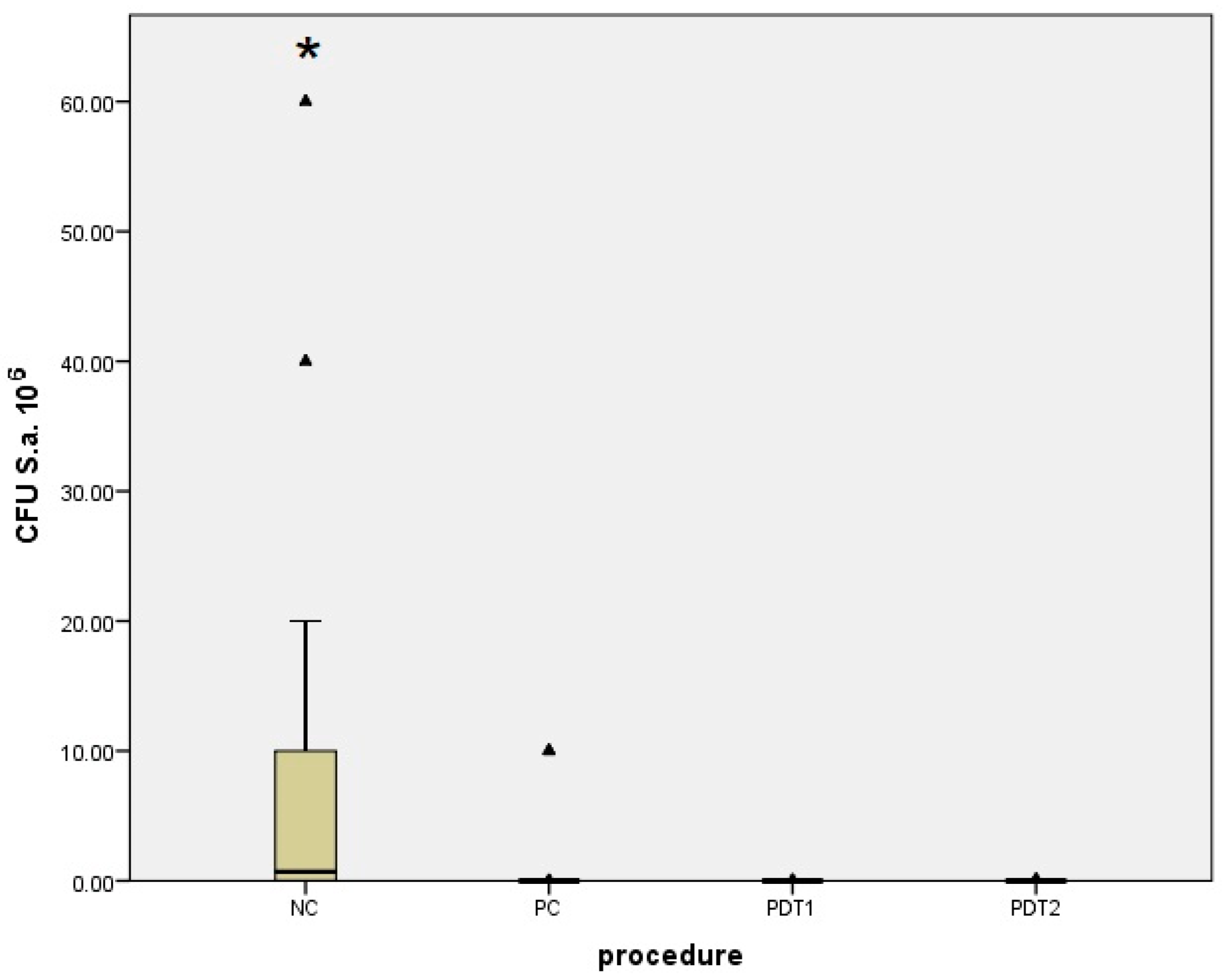
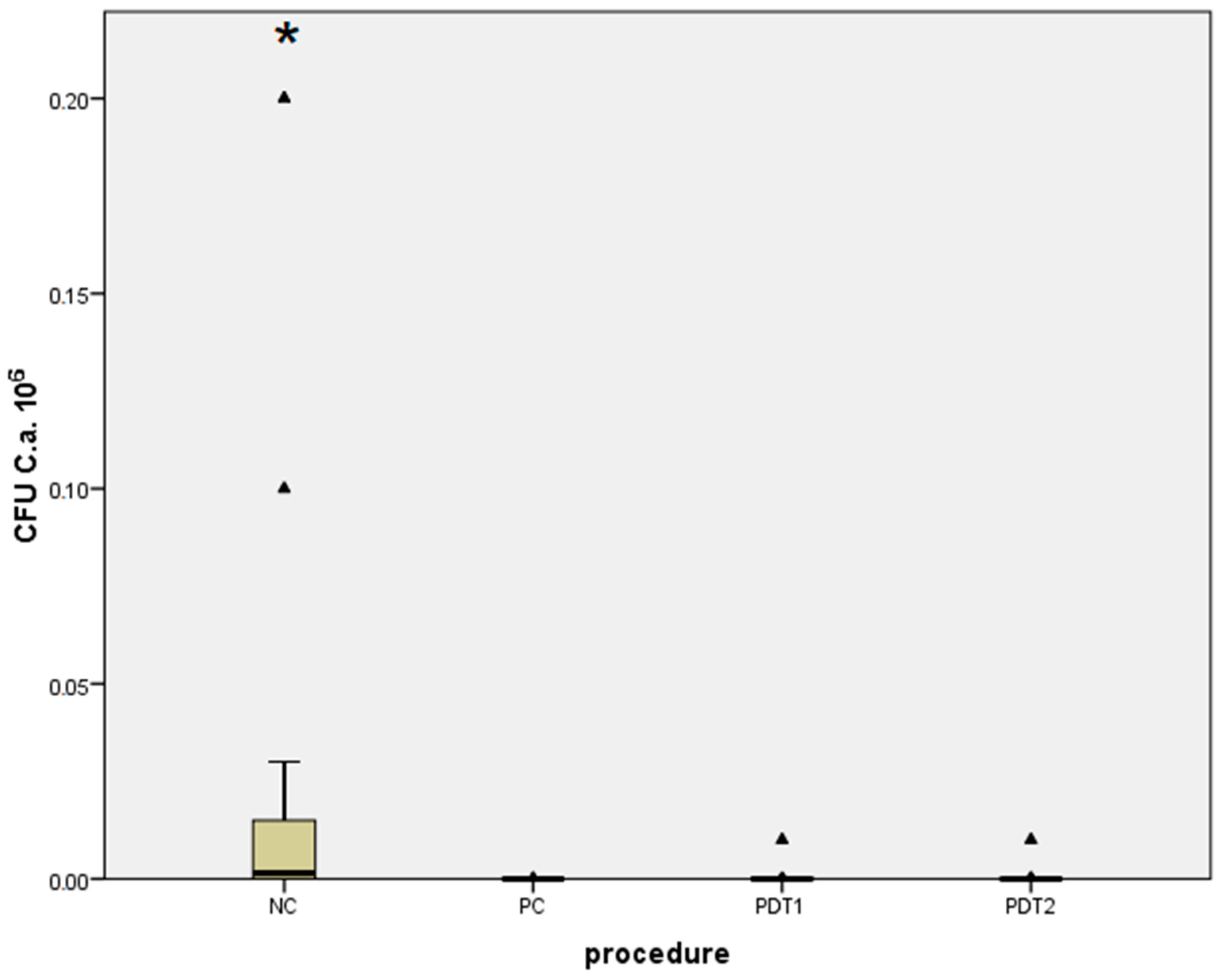
| Staphylococcus aureus | Median (Interquartile Range) | Minimum–Maximum | Difference † | 95% CI | p * |
|---|---|---|---|---|---|
| PDT1 | 0 0 (0–5.5) | 0–3 × 104 | 3.187 × 106 | 104 to 107 | <0.001 |
| NC | 3.2 × 106 (104–1.5 × 107) | 10–108 | |||
| PDT1 | 0 0 (0–0.51) | 0–1 | 0 | 0 to 0 | 0.34 |
| PC | 0 (0–0) | 0–107 | |||
| PDT2 | 0.5 (0–1) | 0–105 | 3.15 × 106 | 104 to 107 | <0.001 |
| NC | 3.2 × 106 (104–1.5 × 107) | 10–108 | |||
| PDT2 | 0.5 (0–1) | 0–105 | 0 | −1 to 0 | 0.09 |
| PC | 0 (0–0) | 0–107 | |||
| NC | 3.2 × 106 (104–1.5 × 107) | 10–108 | −4 × 105 | −1 × 107 to −1 × 104 | <0.001 |
| PC | 0 (0–0) | 0–107 |
| Candida albicans | Median (Interquartile Range) | Minimum–Maximum | Difference † | 95% CI | p * |
|---|---|---|---|---|---|
| PDT1 | 0 (0–1) | 0–104 | 103 | 20 to 104 | <0.001 |
| NC | 1.5 × 103 (20–1.5 × 104) | 0–2 × 105 | |||
| PDT1 | 0 (0–1) | 0–104 | 0 | 0 to 0 | 0.15 |
| PC | 0 (0–0) | 0–102 | |||
| PDT2 | 0(0–0) | 0–104 | 103 | 20 to 104 | 0.001 |
| NC | 1.5 × 103 (20–1.5 × 104) | 0–2 × 105 | |||
| PDT2 | 0 (0–0) | 0–104 | 0 | 0 to 0 | 0.38 |
| PC | 0 (0–0) | 0–102 | |||
| NC | 1.5 × 103 (20–1.5 × 104) | 0–2 × 105 | −1450 | −1 × 104 to −1 × 102 | <0.001 |
| PC | 0 (0–0) | 0–102 |
| Microorganism | Median (Interquartile Range) | Difference † | 95% CI | p * | |
|---|---|---|---|---|---|
| PDT1 | PDT2 | ||||
| Staphylococcus aureus | 0 (0–5.5) | 0.5 (0–1) | 0 | −1 to 0 | 0.55 |
| Candida albicans | 0 (0–1) | 0 (0–0) | 0 | 0 to 0 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelato, L.; Budimir, A.; Smojver, I.; Katalinić, I.; Vuletić, M.; Ajanović, M.; Gabrić, D. A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering 2022, 9, 308. https://doi.org/10.3390/bioengineering9070308
Morelato L, Budimir A, Smojver I, Katalinić I, Vuletić M, Ajanović M, Gabrić D. A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering. 2022; 9(7):308. https://doi.org/10.3390/bioengineering9070308
Chicago/Turabian StyleMorelato, Luka, Ana Budimir, Igor Smojver, Ivan Katalinić, Marko Vuletić, Muhamed Ajanović, and Dragana Gabrić. 2022. "A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study" Bioengineering 9, no. 7: 308. https://doi.org/10.3390/bioengineering9070308
APA StyleMorelato, L., Budimir, A., Smojver, I., Katalinić, I., Vuletić, M., Ajanović, M., & Gabrić, D. (2022). A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering, 9(7), 308. https://doi.org/10.3390/bioengineering9070308







