Myocyte Culture with Decellularized Skeletal Muscle Sheet with Observable Interaction with the Extracellular Matrix
Abstract
:1. Introduction
2. Materials and Methods
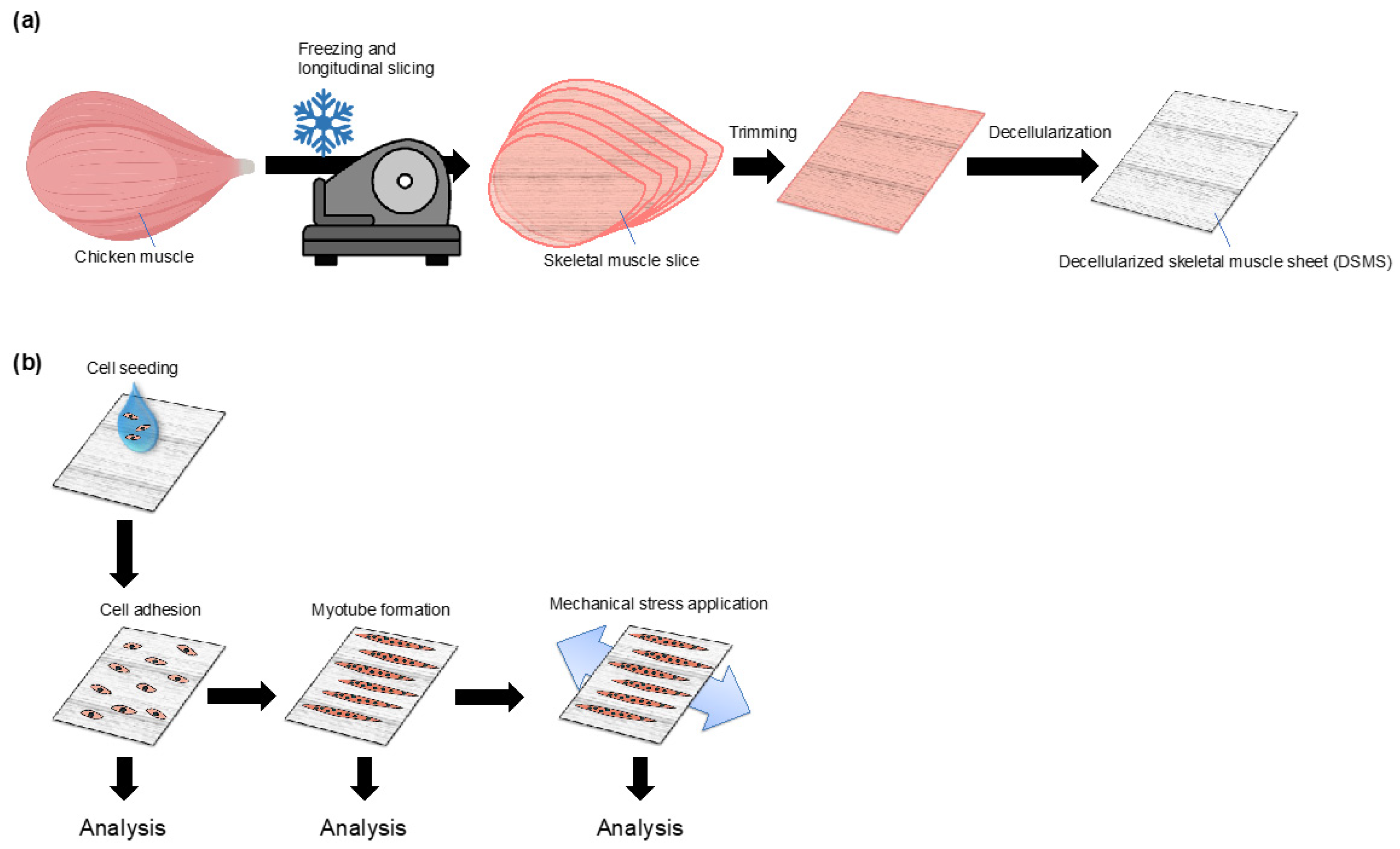
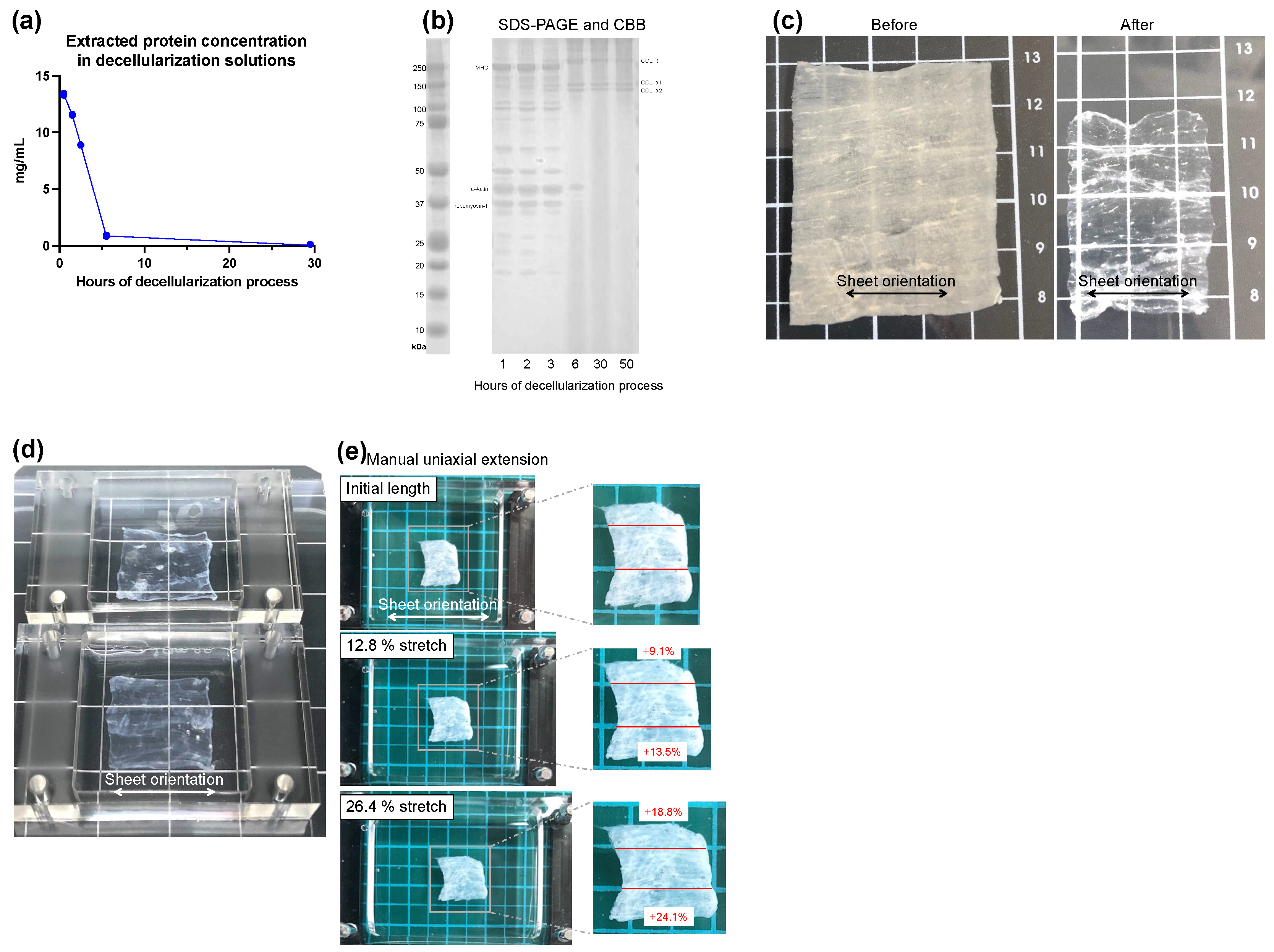
2.1. Fabrication of Decellularized Skeletal Muscle Sheets (DSMS)
2.2. Cell Culture
2.3. Myoblast Adhesion and Myotube Formation on DSMS or Plastic Dish
2.4. Fluorescent Microscopic Observation
2.5. Semi-Quantitative Reverse Transcription Polymerase Chain Reaction
2.6. Statistical Analysis
3. Results
3.1. Preparation and Properties of DSMS
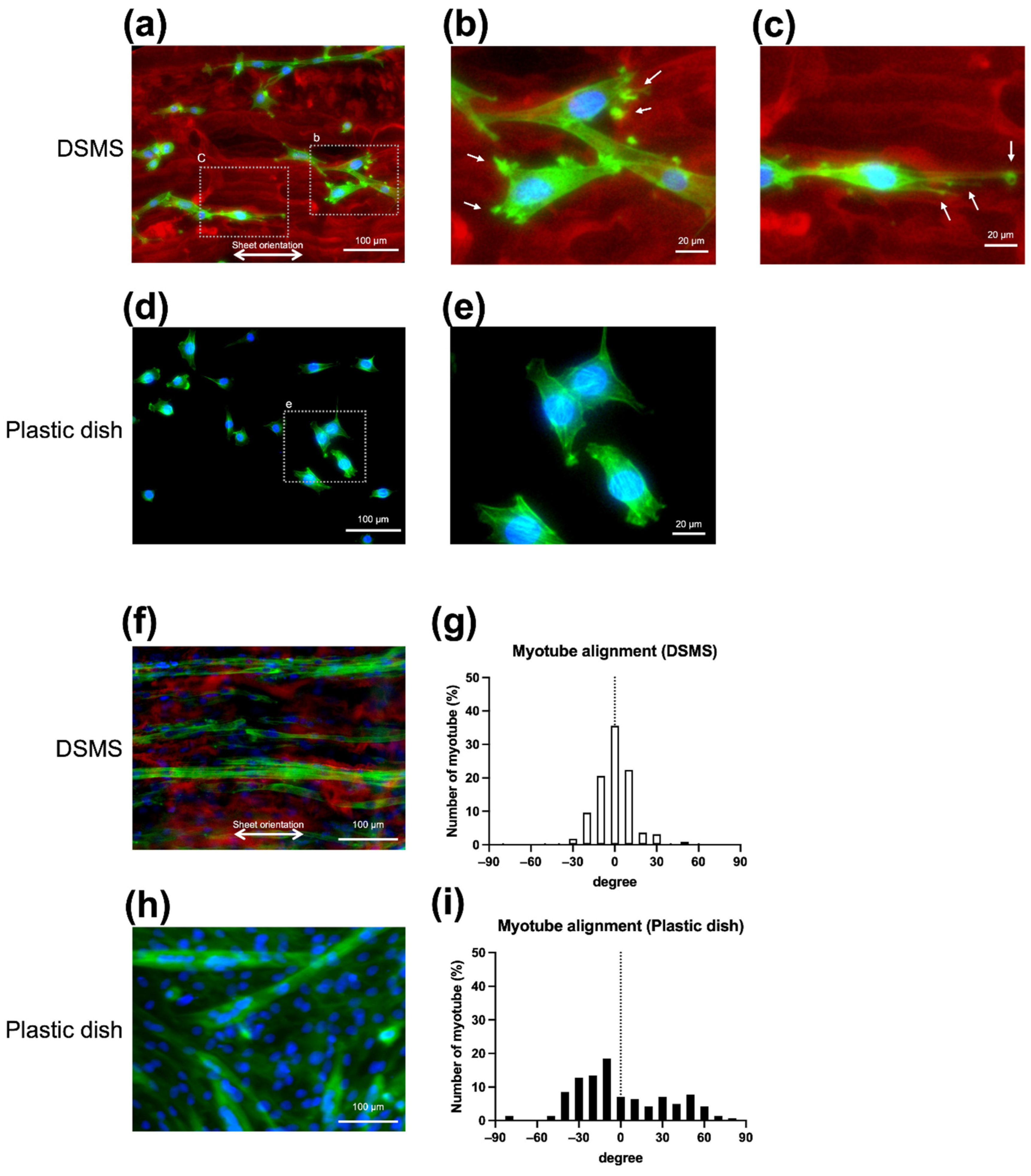
3.2. Morphology of Myoblasts and Myotubes on DSMS
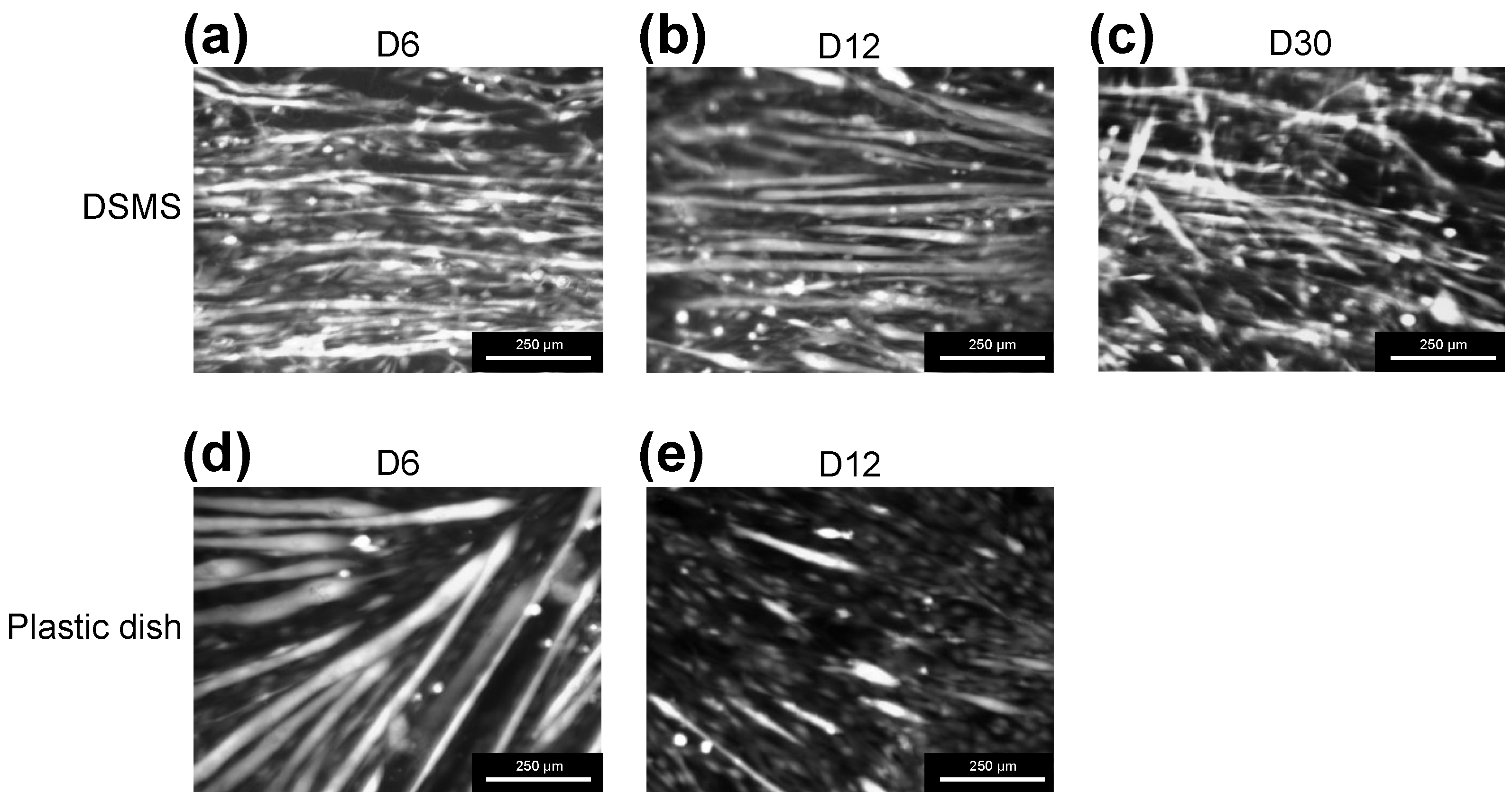
3.3. Myocyte Differentiation on the DSMS
3.4. Myotube Viability on the DSMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, K.D.; Lee, E.J.; Holmes, J.W. Creating alignment and anisotropy in engineered heart tissue: Role of boundary conditions in a model three-dimensional culture system. Tissue Eng. 2003, 9, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Foolen, J.; van Donkelaar, C.; Nowlan, N.; Murphy, P.; Huiskes, R.; Ito, K. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. J. Orthop. Res. 2008, 26, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciech Pawlina, M.H.R. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, 8th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Sanes, J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 2003, 278, 12601–12604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotter, J.A.; Purslow, P.P. Functional morphology of the endomysium in series fibered muscles. J. Morphol. 1992, 212, 109–122. [Google Scholar] [CrossRef]
- Purslow, P.P. The structure and role of intramuscular connective tissue in muscle function. Front. Physiol. 2020, 11, 495. [Google Scholar] [CrossRef]
- Olivero, D.K.; Furcht, L.T. Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2825–2834. [Google Scholar]
- Foster, R.F.; Thompson, J.M.; Kaufman, S.J. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: Analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev. Biol. 1987, 122, 11–20. [Google Scholar] [CrossRef]
- Lehka, L.; Rędowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal muscle extracellular matrix—What do we know about its composition, regulation, and physiological roles? A narrative review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Lam, M.T.; Sim, S.; Zhu, X.; Takayama, S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 2006, 27, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Bursac, N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials 2009, 30, 1401–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zeng, H.; Nam, J.; Agarwal, S. Fabrication of skeletal muscle constructs by topographic activation of cell alignment. Biotechnol. Bioeng. 2009, 102, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altomare, L.; Gadegaard, N.; Visai, L.; Tanzi, M.C.; Farè, S. Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 2010, 6, 1948–1957. [Google Scholar] [CrossRef]
- Takahashi, H.; Shimizu, T.; Nakayama, M.; Yamato, M.; Okano, T. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials 2013, 34, 7372–7380. [Google Scholar] [CrossRef] [Green Version]
- Charest, J.L.; García, A.J.; King, W.P. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials 2007, 28, 2202–2210. [Google Scholar] [CrossRef]
- Huang, N.F.; Lee, R.J.; Li, S. Engineering of aligned skeletal muscle by micropatterning. Am. J. Transl. Res. 2010, 2, 43–55. [Google Scholar]
- Evans, D.J.; Britland, S.; Wigmore, P.M. Differential response of fetal and neonatal myoblasts to topographical guidance cues in vitro. Dev. Genes Evol. 1999, 209, 438–442. [Google Scholar] [CrossRef]
- Warita, K.; Oshima, N.; Takeda-Okuda, N.; Tamura, J.I.; Hosaka, Y.Z. Degree of suppression of mouse myoblast cell line C2C12 differentiation varies according to chondroitin sulfate subtype. Mar. Drugs 2016, 14, 193. [Google Scholar] [CrossRef] [Green Version]
- Mikami, T.; Koyama, S.; Yabuta, Y.; Kitagawa, H. Chondroitin sulfate is a crucial determinant for skeletal muscle development/regeneration and improvement of muscular dystrophies. J. Biol. Chem. 2012, 287, 38531–38542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Uriel, S.; Labay, E.; Francis-Sedlak, M.; Moya, M.L.; Weichselbaum, R.R.; Ervin, N.; Cankova, Z.; Brey, E.M. Extraction and assembly of tissue-derived gels for cell culture and tissue engineering. Tissue Eng. Part C Methods 2009, 15, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Park, S.J.; Yi, H.G.; Lee, H.; Kim, D.S.; Cho, D.W. Muscle-derived extracellular matrix on sinusoidal wavy surfaces synergistically promotes myogenic differentiation and maturation. J. Mater. Chem. B 2018, 6, 5530–5539. [Google Scholar] [CrossRef]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.M.; Myers, R.L.; Hammam, N.; Stern, K.A.; Eberli, D.; Kritchevsky, S.B.; Soker, S.; Van Dyke, M. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 2009, 30, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Carton, F.; Di Francesco, D.; Fusaro, L.; Zanella, E.; Apostolo, C.; Oltolina, F.; Cotella, D.; Prat, M.; Boccafoschi, F. Myogenic potential of extracellular matrix derived from decellularized bovine pericardium. Int. J. Mol. Sci. 2021, 22, 9406. [Google Scholar] [CrossRef]
- Brazile, B.; Lin, S.; Copeland, K.M.; Butler, J.R.; Cooley, J.; Brinkman-Ferguson, E.; Guan, J.; Liao, J. Ultrastructure and Biomechanics of Skeletal Muscle ECM. In Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–160. [Google Scholar]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V.; De Caro, R. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci. 2015, 16, 14808–14831. [Google Scholar] [CrossRef] [Green Version]
- Jank, B.J.; Xiong, L.; Moser, P.T.; Guyette, J.P.; Ren, X.; Cetrulo, C.L.; Leonard, D.A.; Fernandez, L.; Fagan, S.P.; Ott, H.C. Engineered composite tissue as a bioartificial limb graft. Biomaterials 2015, 61, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Urciuolo, A.; Urbani, L.; Perin, S.; Maghsoudlou, P.; Scottoni, F.; Gjinovci, A.; Collins-Hooper, H.; Loukogeorgakis, S.; Tyraskis, A.; Torelli, S.; et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci. Rep. 2018, 8, 8398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, H.; Hioki, H.; Namiki, K.; Hoshida, T.; Kurokawa, H.; Ishidate, F.; Kaneko, T.; Akagi, T.; Saito, T.; Saido, T.; et al. ScaleS: An optical clearing palette for biological imaging. Nat. Neurosci. 2015, 18, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Lee, J.S.; Kim, J.G.; Kang, K.T.; Jang, J.W.; Shim, Y.B.; Moon, S.H. Mechanical properties of decellularized tendon cultured by cyclic straining bioreactor. J. Biomed. Mater. Res. A 2013, 101, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; Dye, D.E.; Kinnear, B.F.; Van Kuppevelt, T.H.; Grounds, M.D.; Coombe, D.R. Interactions between skeletal muscle myoblasts and their extracellular matrix revealed by a serum free culture system. PLoS ONE 2015, 10, e0127675. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Caswell, P.T.; Zech, T. Actin-based cell protrusion in a 3D matrix. Trends Cell Biol. 2018, 28, 823–834. [Google Scholar] [CrossRef]
- Arnold, H.H.; Braun, T. 4 Genetics of muscle determination and development. Curr. Top. Dev. Biol. 1999, 48, 129–164. [Google Scholar]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef] [Green Version]
- Megeney, L.A.; Rudnicki, M.A. Determination versus differentiation and the MyoD family of transcription factors. Biochem. Cell Biol. 1995, 73, 723–732. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Ortuste Quiroga, H.P.; Zammit, P.S.; Hinits, Y.; Hughes, S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, W.E.; Sassoon, D.A.; Lin, V.K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 1989, 56, 607–617. [Google Scholar] [CrossRef]
- Agarwal, M.; Sharma, A.; Kumar, P.; Kumar, A.; Bharadwaj, A.; Saini, M.; Kardon, G.; Mathew, S.J. Myosin heavy chain-embryonic regulates skeletal muscle differentiation during mammalian development. Development 2020, 147, dev184507. [Google Scholar] [CrossRef] [PubMed]
- Biressi, S.; Molinaro, M.; Cossu, G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 2007, 308, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Schiaffino, S.; Reggiani, C. Myosin isoforms in mammalian skeletal muscle. J. Appl. Physiol. 1994, 77, 493–501. [Google Scholar] [CrossRef]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Vaz, R.; Martins, G.G.; Thorsteinsdóttir, S.; Rodrigues, G. Fibronectin promotes migration, alignment and fusion in an in vitro myoblast cell model. Cell Tissue Res. 2012, 348, 569–578. [Google Scholar] [CrossRef]
- Vandenburgh, H.H.; Karlisch, P.; Farr, L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitr Cell. Dev. Biol. 1988, 24, 166–174. [Google Scholar] [CrossRef]
- Bettadapur, A.; Suh, G.C.; Geisse, N.A.; Wang, E.R.; Hua, C.; Huber, H.A.; Viscio, A.A.; Kim, J.Y.; Strickland, J.B.; McCain, M.L. Prolonged culture of aligned skeletal myotubes on micromolded gelatin hydrogels. Sci. Rep. 2016, 6, 28855. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell. Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Duffy, R.; Lee, A.; Feinberg, A.W. Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater. 2013, 9, 7885–7894. [Google Scholar] [CrossRef] [PubMed]
- Duffy, R.M.; Sun, Y.; Feinberg, A.W. Understanding the role of ECM protein composition and geometric micropatterning for engineering human skeletal muscle. Ann. Biomed. Eng. 2016, 44, 2076–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.Y.; Thissen, H.; Tsai, W.B. The roles of RGD and grooved topography in the adhesion, morphology, and differentiation of C2C12 skeletal myoblasts. Biotechnol. Bioeng. 2012, 109, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Toral-Ojeda, I.; Aldanondo, G.; Lasa-Elgarresta, J.; Lasa-Fernandez, H.; Vesga-Castro, C.; Mouly, V.; de Munain, A.L.; Vallejo-Illarramendi, A. A Novel Functional In Vitro Model That Recapitulates Human Muscle Disorders. In Muscle Cell and Tissue—Current Status of Research Field; IntechOpen: London, UK, 2018; p. 13. [Google Scholar]
- Guo, X.; Greene, K.; Akanda, N.; Smith, A.S.T.; Stancescu, M.; Lambert, S.; Vandenburgh, H.; Hickman, J.J. In vitro differentiation of functional human skeletal myotubes in a defined system. Biomater. Sci. 2014, 2, 131–138. [Google Scholar] [CrossRef]
- Gillies, A.R.; Smith, L.R.; Lieber, R.L.; Varghese, S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng. Part C Methods 2011, 17, 383–389. [Google Scholar] [CrossRef] [Green Version]

| Gene | Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| Myf5 | NM_008656 | 5′-TGTATCCCCTCACCAGAGGAT-3′ | 5′-GGCTGTAATAGTTCTCCACCTGTT-3′ |
| MyoD | NM_010866 | 5′-AGTGAATGAGGCCTTCGAGA-3′ | 5′-CTGGGTTCCCTGTTCTGTGT-3′ |
| Myogenin | NM_031189 | 5′-ACCAGGAGCCCCACTTCTAT-3′ | 5′-ACGATGGACGTAAGGGAGTG-3′ |
| MHC embryoic (MYH3) | NM_001099635 | 5′-TCCGACAACGCCTACCAGTT-3′ | 5′-CCCGGATTCTCCGGTGAT-3′ |
| MHC neonatal (MYH8) | NM_177369 | 5′-CAGGAGCAGGAATGATGCTCTGAG-3′ | 5′-AGTTCCTCAAACTTTCAGCAGCCAA-3′ |
| GAPDH | NM_008084 | 5′-ACTCCACTCACGGCAAATTC-3′ | 5′-CCTTCCACAATGCCAAAGTT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, S.; Yamashita, Y.; Akiba, S.; Shima, T.; Arikawa-Hirasawa, E. Myocyte Culture with Decellularized Skeletal Muscle Sheet with Observable Interaction with the Extracellular Matrix. Bioengineering 2022, 9, 309. https://doi.org/10.3390/bioengineering9070309
Nakada S, Yamashita Y, Akiba S, Shima T, Arikawa-Hirasawa E. Myocyte Culture with Decellularized Skeletal Muscle Sheet with Observable Interaction with the Extracellular Matrix. Bioengineering. 2022; 9(7):309. https://doi.org/10.3390/bioengineering9070309
Chicago/Turabian StyleNakada, Satoshi, Yuri Yamashita, Seiya Akiba, Takeru Shima, and Eri Arikawa-Hirasawa. 2022. "Myocyte Culture with Decellularized Skeletal Muscle Sheet with Observable Interaction with the Extracellular Matrix" Bioengineering 9, no. 7: 309. https://doi.org/10.3390/bioengineering9070309
APA StyleNakada, S., Yamashita, Y., Akiba, S., Shima, T., & Arikawa-Hirasawa, E. (2022). Myocyte Culture with Decellularized Skeletal Muscle Sheet with Observable Interaction with the Extracellular Matrix. Bioengineering, 9(7), 309. https://doi.org/10.3390/bioengineering9070309







