Decellularised Cartilage ECM Culture Coatings Drive Rapid and Robust Chondrogenic Differentiation of Human Periosteal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Costal Cartilage Harvest
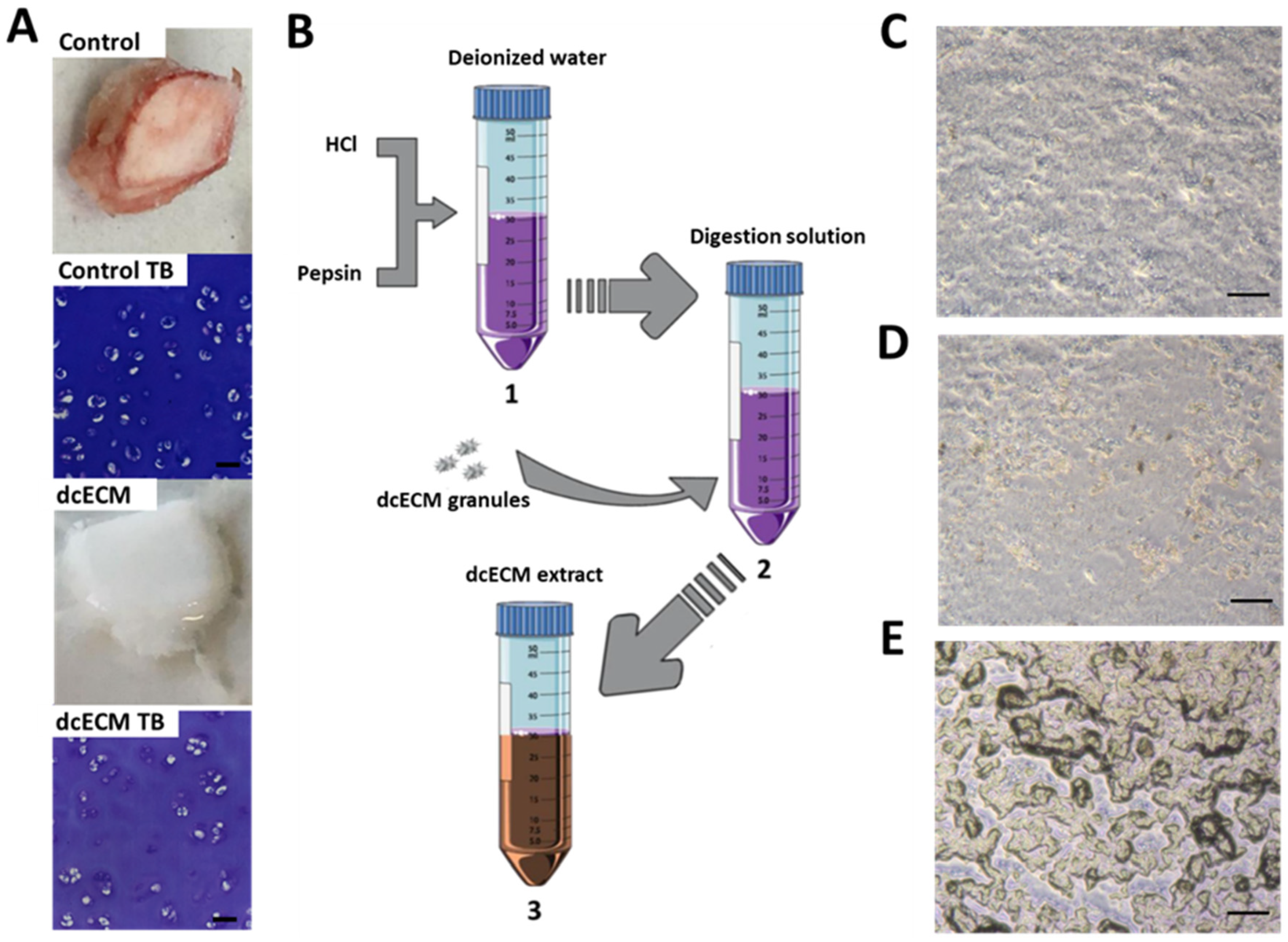
2.2. Decellularisation and Digestion
2.3. Sterilisation and Coating of dcECM-Derived Digests
2.4. Human Periosteum-Derived Cell (hPDC) Culture
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. dcECM Digests Can Coat Tissue Culture Plastic and Alternative Sterilisation Methods Elicit Different Surface Topologies
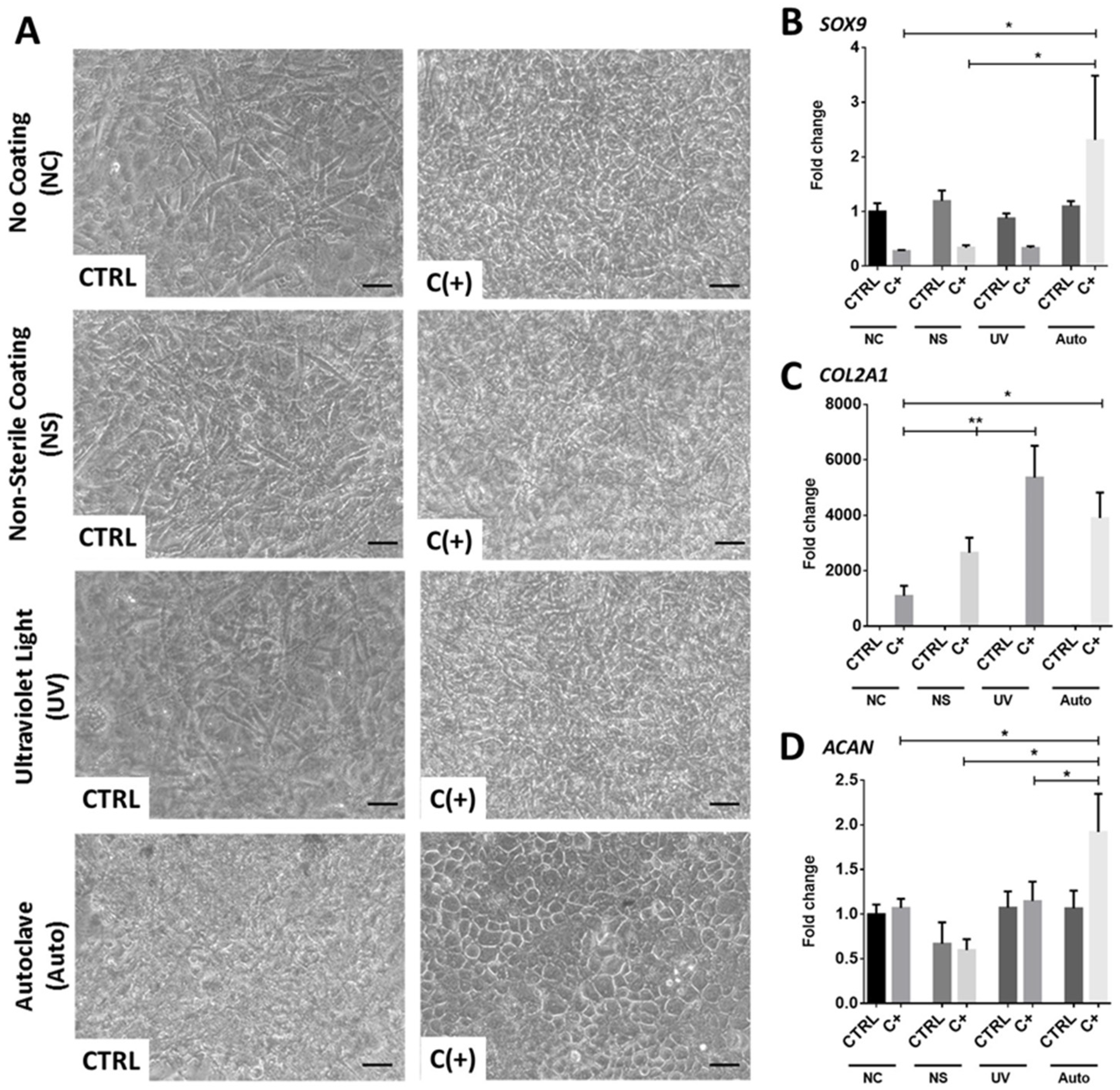
3.2. dcECM Surface Coatings Modify Chondrogenic Gene Expression from hPDCs and Promote Morphological Changes
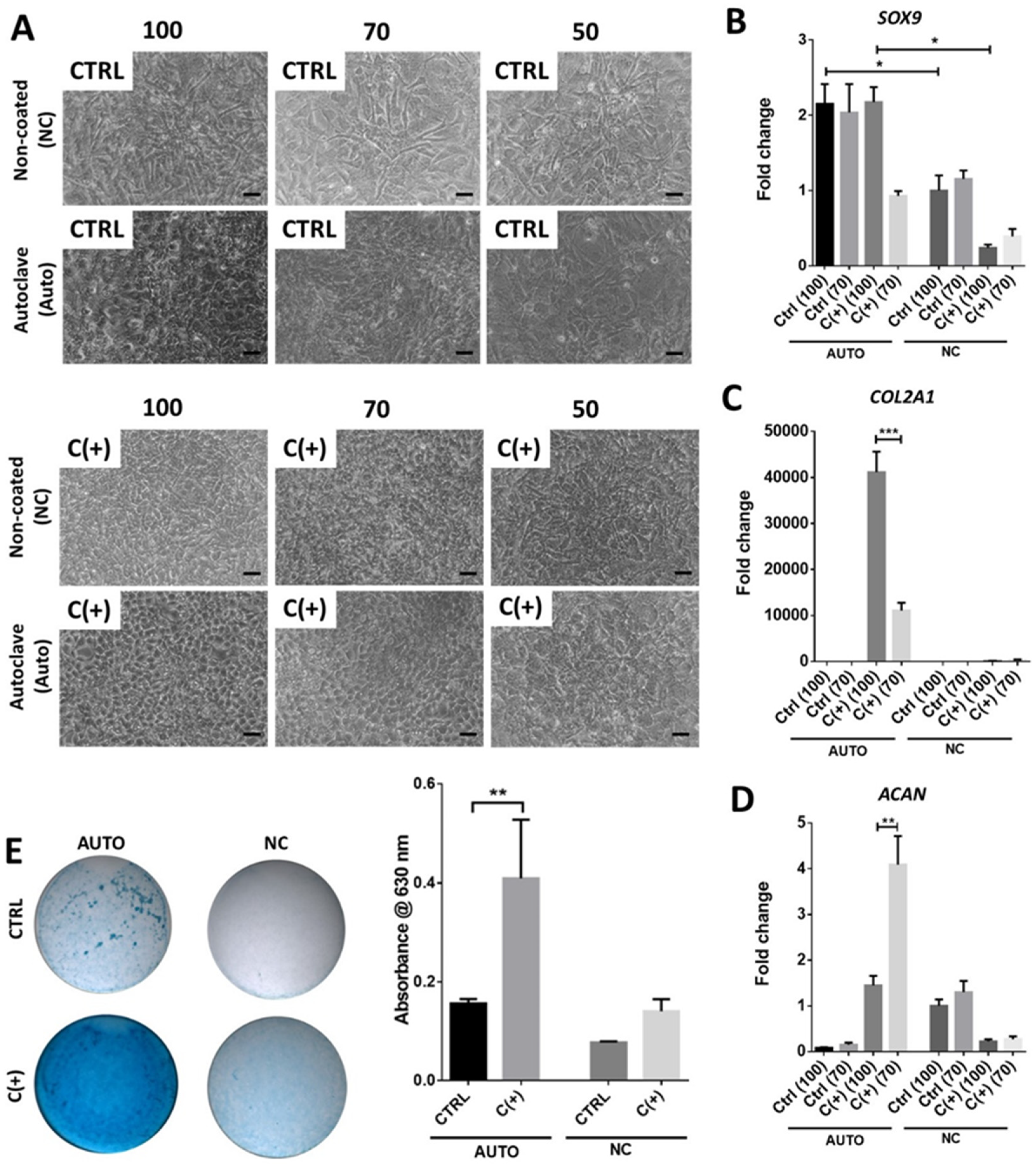
3.3. High Density Culture of hPDCs Is Required for Chondrogenic Efficacy of dcECM (Auto)
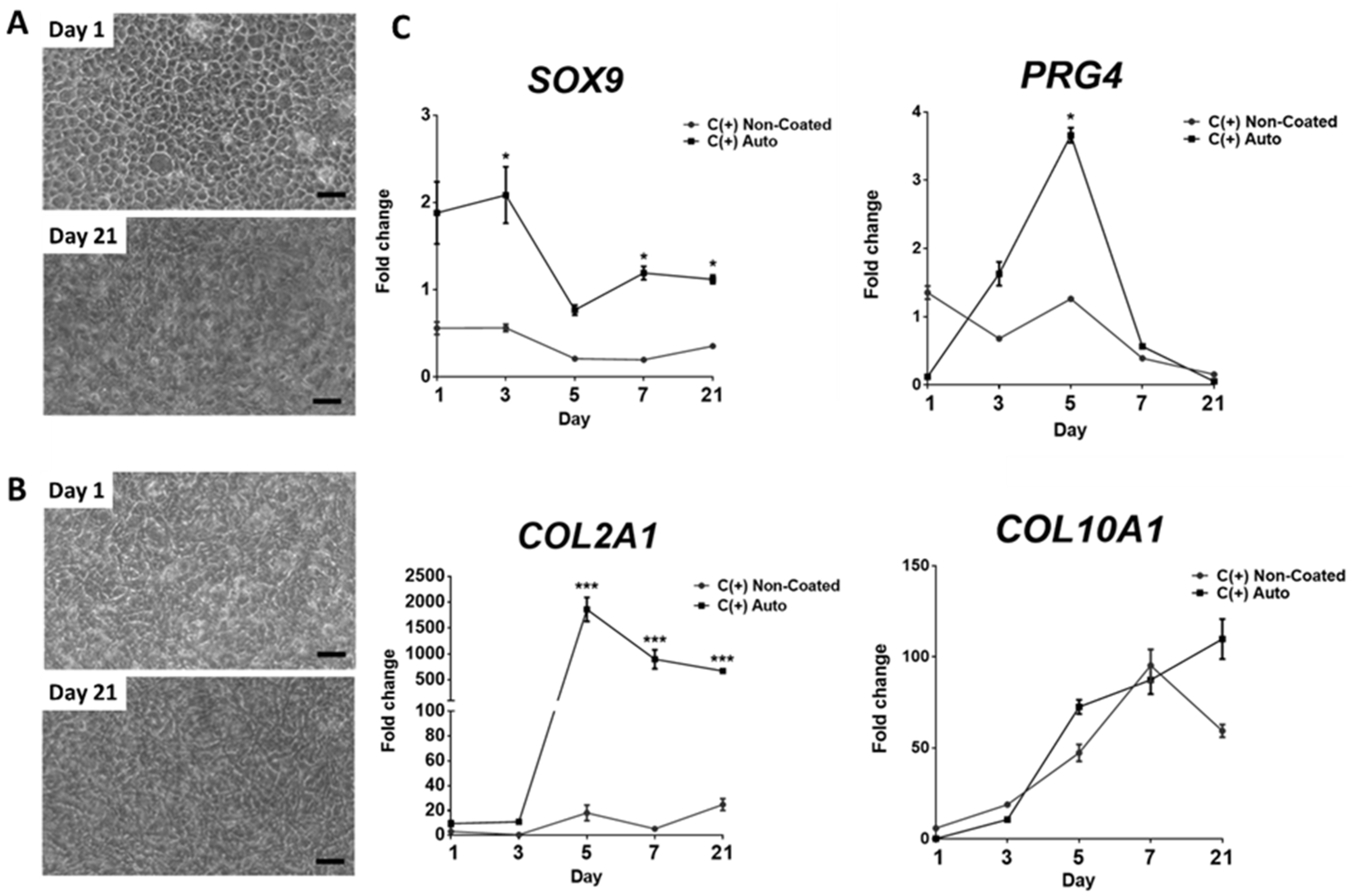
3.4. Chondrogenic Effect Is Maintained over 21 Days, However, May Coincide with Hypertrophic Differentiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2014, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.R.; Ewald, A.J. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Understanding the cancer/tumor biology from 2D to 3D. J. Thorac. Dis. 2016, 8, E1484–E1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [Green Version]
- Jayasuriya, C.T.; Chen, Q. Potential benefits and limitations of utilizing chondroprogenitors in cell-based cartilage therapy. Connect. Tissue Res. 2015, 56, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Kessler, M.W.; Grande, D.A. Tissue engineering and cartilage. Organogenesis 2008, 4, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Law, J.B.K.; He, A.Y.; Low, H.Y.; Hui, J.H.P.; Lim, C.T.; Yang, Z.; Lee, E.H. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1507–1516. [Google Scholar] [CrossRef]
- Gao, L.; McBeath, R.; Chen, C.S. Stem cell shape regulates a chondrogenic versus myogenic fate through rac1 and n-cadherin. Stem Cells 2010, 28, 564–572. [Google Scholar] [CrossRef] [Green Version]
- McBride, S.H.; Knothe Tate, M.L. Modulation of stem cell shape and fate a: The role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng. Part A 2008, 14, 1561–1572. [Google Scholar] [CrossRef]
- Chastain, S.R.; Kundu, A.K.; Dhar, S.; Calvert, J.W.; Putnam, A.J. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ecm ligands and controls their osteogenic differentiation. J. Biomed. Mater. Res. Part A 2006, 78A, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Koochekpour, S.; Merzak, A.; Pilkington, G.J. Extracellular matrix proteins inhibit proliferation, upregulate migration and induce morphological changes in human glioma cell lines. Eur. J. Cancer 1995, 31, 375–380. [Google Scholar] [CrossRef]
- Everitt, E.A.; Malik, A.B.; Hendey, B. Fibronectin enhances the migration rate of human neutrophils in vitro. J. Leukoc. Biol. 1996, 60, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Liberio, M.S.; Sadowski, M.C.; Soekmadji, C.; Davis, R.A.; Nelson, C.C. Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behavior. PLoS ONE 2014, 9, e112122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brafman, D.A.; Shah, K.D.; Fellner, T.; Chien, S.; Willert, K. Defining long-term maintenance conditions of human embryonic stem cells with arrayed cellular microenvironment technology. Stem Cells Dev. 2009, 18, 1141–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaim, C.J.; Teng, D.; Chien, S.; Bhatia, S.N. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008, 17, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Cain, S.A.; Tian, P.; Baldwin, A.K.; Uppanan, P.; Kielty, C.M.; Kimber, S.J. Recombinant extracellular matrix protein fragments support human embryonic stem cell chondrogenesis. Tissue Eng. Part A 2018, 24, 968–978. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Wei, Y.; Hung, C.; Vunjak-Novakovic, G. Chondrogenic properties of collagen type xi, a component of cartilage extracellular matrix. Biomaterials 2018, 173, 47–57. [Google Scholar] [CrossRef]
- Lu, Z.; Doulabi, B.Z.; Huang, C.; Bank, R.A.; Helder, M.N. Collagen type ii enhances chondrogenesis in adipose tissue–derived stem cells by affecting cell shape. Tissue Eng. Part A 2010, 16, 81–90. [Google Scholar] [CrossRef]
- Tiruvannamalai Annamalai, R.; Mertz, D.R.; Daley, E.L.H.; Stegemann, J.P. Collagen type ii enhances chondrogenic differentiation in agarose-based modular microtissues. Cytotherapy 2016, 18, 263–277. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Trevino, E.; Temenoff, J. Cell number and chondrogenesis in human mesenchymal stem cell aggregates is affected by the sulfation level of heparin used as a cell coating. J. Biomed. Mater. Res. A 2016, 104, 1817–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vas, W.J.; Shah, M.; Blacker, T.S.; Duchen, M.R.; Sibbons, P.; Roberts, S.J. Decellularized cartilage directs chondrogenic differentiation: Creation of a fracture callus mimetic. Tissue Eng. Part A 2018, 24, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Sawkins, M.J.; Bowen, W.; Dhadda, P.; Markides, H.; Sidney, L.E.; Taylor, A.J.; Rose, F.R.A.J.; Badylak, S.F.; Shakesheff, K.M.; White, L.J. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013, 9, 7865–7873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: The stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−δδct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Yang, G.; Tuan, R.S. Tissue-specific bioactivity of soluble tendon-derived and cartilage-derived extracellular matrices on adult mesenchymal stem cells. Stem Cell Res. 2017, 8, 133. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rowland, C.R.; Lennon, D.P.; Caplan, A.I.; Guilak, F. The effects of crosslinking of scaffolds engineered from cartilage ecm on the chondrogenic differentiation of mscs. Biomaterials 2013, 34, 5802–5812. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, E.C.; Hernández, J.C.R.; Machado, M.; Mano, J.F.; Ribelles, J.L.G.; Pradas, M.M.; Sánchez, M.S. Human chondrocyte morphology, its dedifferentiation, and fibronectin conformation on different plla microtopographies. Tissue Eng. Part A 2008, 14, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Tgf-beta1, gdf-5, and bmp-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015, 33, 762–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Tao, H.; Jin, C.; Liu, Y.; Lu, X.; Hu, X.; Wang, X. Transforming growth factor-beta1 induces type ii collagen and aggrecan expression via activation of extracellular signal-regulated kinase 1/2 and smad2/3 signaling pathways. Mol. Med. Rep. 2015, 12, 5573–5579. [Google Scholar] [CrossRef]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular interactions and signaling in cartilage development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef] [Green Version]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar]
- Hino, K.; Saito, A.; Kido, M.; Kanemoto, S.; Asada, R.; Takai, T.; Cui, M.; Cui, X.; Imaizumi, K. Master regulator for chondrogenesis, sox9, regulates transcriptional activation of the endoplasmic reticulum stress transducer bbf2h7/creb3l2 in chondrocytes. J. Biol. Chem. 2014, 289, 13810–13820. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Trovato, F.M.; Loreto, C.; Leonardi, R.; Szychlinska, M.A.; Castorina, S.; Mobasheri, A. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: A morphological, immunohistochemical and biochemical study. Acta Histochem. 2014, 116, 965–972. [Google Scholar] [CrossRef]
- Dy, P.; Wang, W.; Bhattaram, P.; Wang, Q.; Wang, L.; Ballock, R.T.; Lefebvre, V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 2012, 22, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Xu, J.; Guo, X. Maintaining the phenotype stability of chondrocytes derived from mscs by c-type natriuretic peptide. Front. Physiol. 2017, 8, 143. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vas, W.J.; Shah, M.; Roberts, H.C.; Roberts, S.J. Decellularised Cartilage ECM Culture Coatings Drive Rapid and Robust Chondrogenic Differentiation of Human Periosteal Cells. Bioengineering 2022, 9, 203. https://doi.org/10.3390/bioengineering9050203
Vas WJ, Shah M, Roberts HC, Roberts SJ. Decellularised Cartilage ECM Culture Coatings Drive Rapid and Robust Chondrogenic Differentiation of Human Periosteal Cells. Bioengineering. 2022; 9(5):203. https://doi.org/10.3390/bioengineering9050203
Chicago/Turabian StyleVas, Wollis J., Mittal Shah, Helen C. Roberts, and Scott J. Roberts. 2022. "Decellularised Cartilage ECM Culture Coatings Drive Rapid and Robust Chondrogenic Differentiation of Human Periosteal Cells" Bioengineering 9, no. 5: 203. https://doi.org/10.3390/bioengineering9050203
APA StyleVas, W. J., Shah, M., Roberts, H. C., & Roberts, S. J. (2022). Decellularised Cartilage ECM Culture Coatings Drive Rapid and Robust Chondrogenic Differentiation of Human Periosteal Cells. Bioengineering, 9(5), 203. https://doi.org/10.3390/bioengineering9050203





