Engineering Extracellular Microenvironment for Tissue Regeneration
Abstract
1. Introduction
2. Extracellular Matrix (ECM)
2.1. Native ECM
2.2. Engineering ECM for Tissue Regeneration
2.3. Engineering Artificial ECM-Mimicking Scaffolds for Tissue Regeneration
2.4. Advantages and Disadvantages of Current Engineering Approaches in ECM
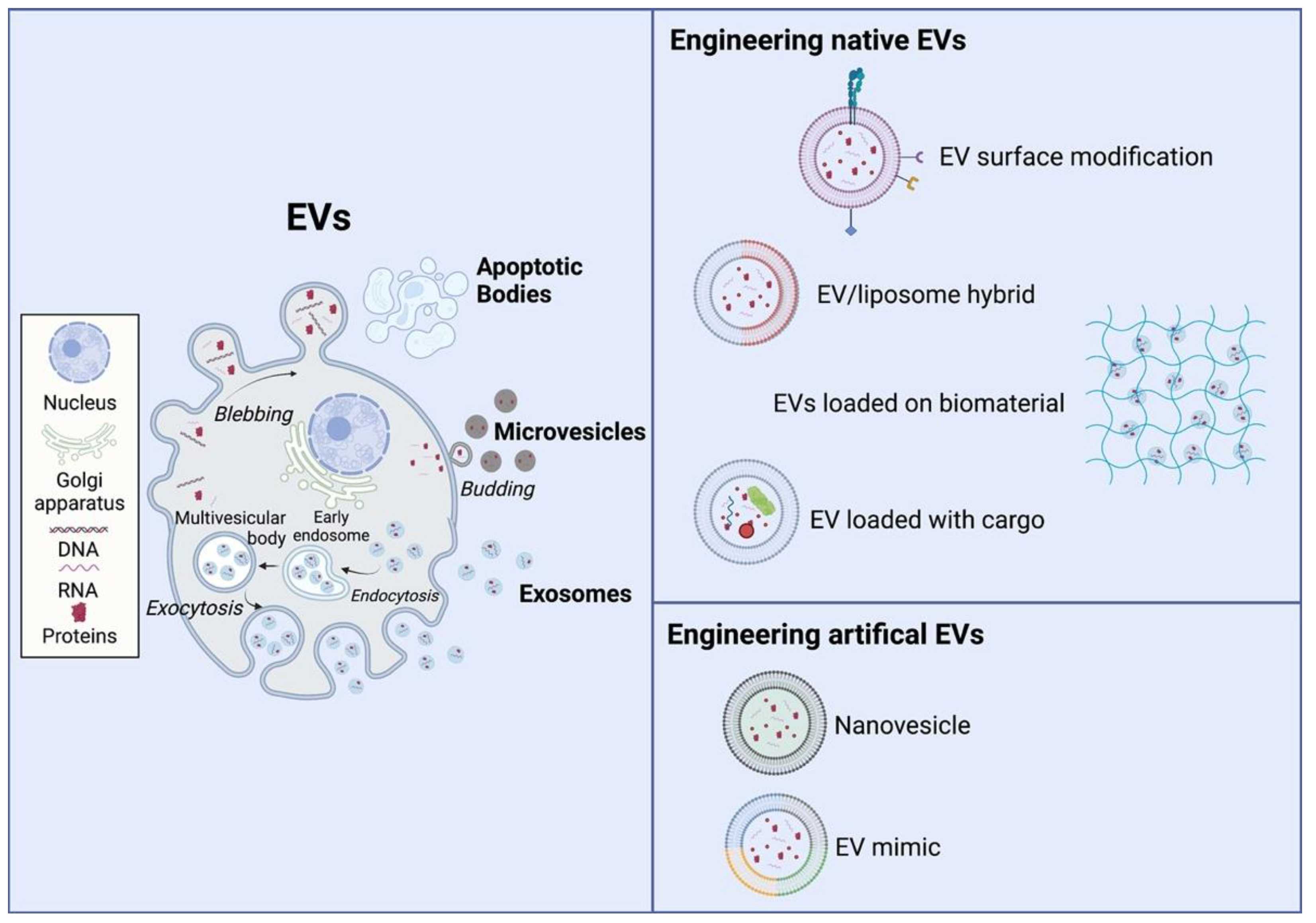
3. Extracellular Vesicles (EVs)
3.1. Native EVs
3.2. Engineering Native EVs for Tissue Regeneration
3.3. Engineering Artificial Evs for Tissue Regeneration
3.4. Advantages and Disadvantages of Current Engineering Approaches in EVs
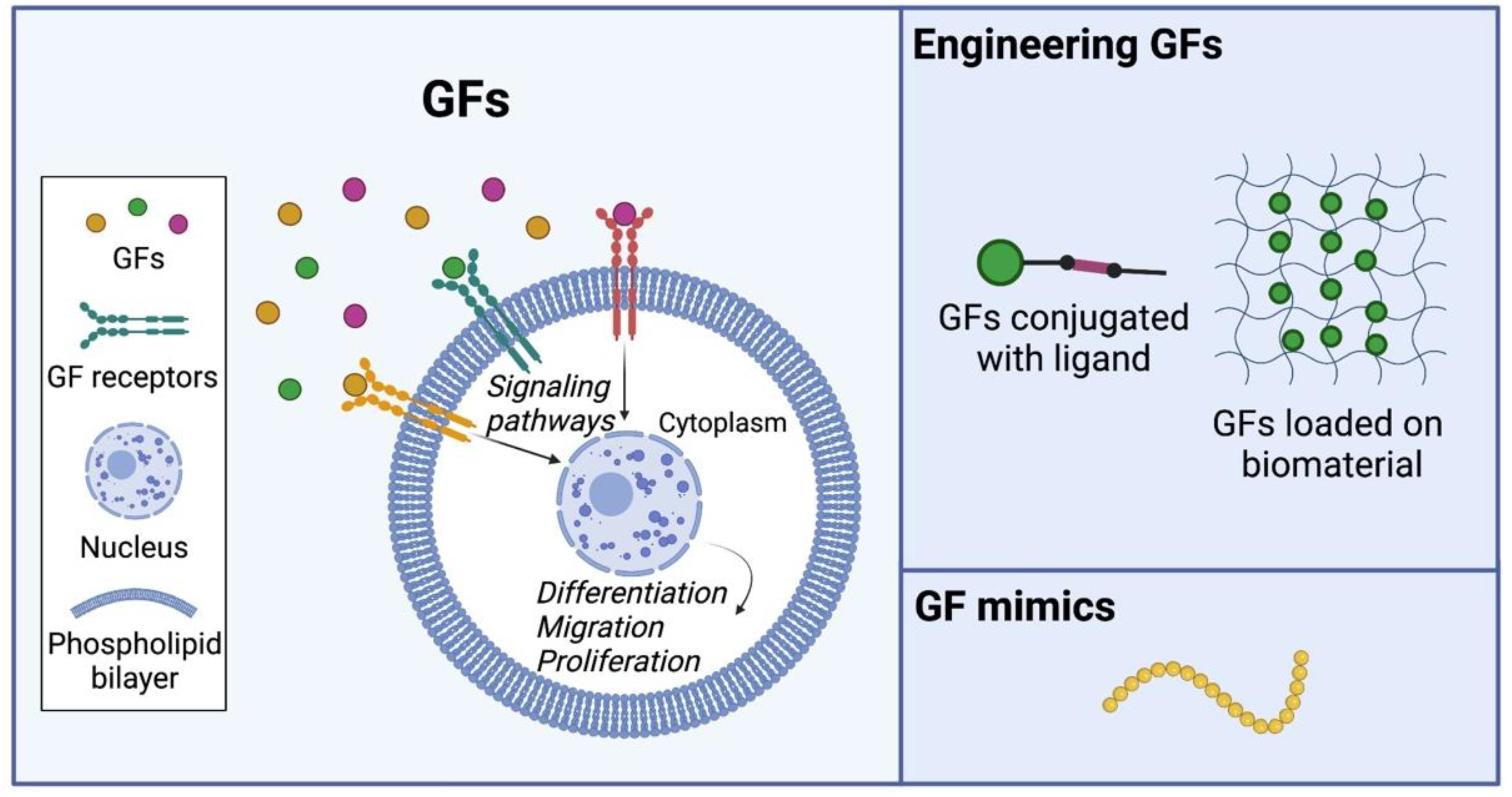
4. Growth Factors (GFs)
4.1. Native GFs
4.2. Engineering GFs for Tissue Regeneration
4.3. Engineering Artificial GF Mimics for Tissue Regeneration
4.4. Advantages and Disadvantages of Current Engineering Approaches in GFs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atala, A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 2004, 7, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.M.; Coakley, D.N.; Walsh, M.T.; McGloughlin, T.M.; Grace, P.A. Regenerative medicine, tissue engineering and vascular surgery: Twenty first century clinical challenges. Ir. J. Med. Sci. 2010, 179, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, M.; Payne, S.L. Biomaterial-based cell delivery strategies to promote liver regeneration. Biomater. Res. 2021, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Jemni-Damer, N.; Guedan-Duran, A.; Fuentes-Andion, M.; Serrano-Bengoechea, N.; Alfageme-Lopez, N.; Armada-Maresca, F.; Guinea, G.V.; Perez-Rigueiro, J.; Rojo, F.; Gonzalez-Nieto, D.; et al. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part II: Cell and Tissue Engineering Therapies. Front. Bioeng. Biotechnol. 2020, 8, 588014. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Cranford, S.W.; de Boer, J.; van Blitterswijk, C.; Buehler, M.J. Materiomics: An -omics approach to biomaterials research. Adv. Mater. 2013, 25, 802–824. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, H.; Wen, H.; Huang, H.; Li, Q.; Liang, M.; Liu, Y.; Dong, H.; Cao, X. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: Insights from a microgel model. Acta Biomater. 2020, 113, 393–406. [Google Scholar] [CrossRef]
- Huang, N.F.; Li, S. Regulation of the matrix microenvironment for stem cell engineering and regenerative medicine. Ann. Biomed. Eng. 2011, 39, 1201–1214. [Google Scholar] [CrossRef]
- Metallo, C.M.; Mohr, J.C.; Detzel, C.J.; de Pablo, J.J.; Van Wie, B.J.; Palecek, S.P. Engineering the stem cell microenvironment. Biotechnol. Prog. 2007, 23, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. 1), S20–S23. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef]
- Mitrovic, S.; Mitrovic, D.; Todorovic, V. Fibronectin—A multifunctional glycoprotein. Srp. Arh. Za Celok. Lek. 1995, 123, 198–201. [Google Scholar]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2020, 56, 50–55. [Google Scholar] [CrossRef]
- Miettinen, M.; Foidart, J.M.; Ekblom, P. Immunohistochemical demonstration of laminin, the major glycoprotein of basement membranes, as an aid in the diagnosis of soft tissue tumors. Am. J. Clin. Pathol. 1983, 79, 306–311. [Google Scholar] [CrossRef]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin—A glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar] [CrossRef]
- Abaskharoun, M.; Bellemare, M.; Lau, E.; Margolis, R.U. Expression of hyaluronan and the hyaluronan-binding proteoglycans neurocan, aggrecan, and versican by neural stem cells and neural cells derived from embryonic stem cells. Brain Res. 2010, 1327, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Das, G.; Khan, J.; Mukherjee, N.; Gupta, V.; Mallesh, R.; Ghosh, S. Extracellular Matrix (ECM)-Mimicking Neuroprotective Injectable Sulfo-Functionalized Peptide Hydrogel for Repairing Brain Injury. ACS Biomater. Sci. Eng. 2020, 6, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Papa, M. Matrix Metalloproteinases, Neural Extracellular Matrix, and Central Nervous System Pathology. Prog Mol. Biol. Transl. Sci. 2017, 148, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.D.; San Antonio, J.D.; Jacenko, O. Heparan sulfate proteoglycans: A GAGgle of skeletal-hematopoietic regulators. Dev. Dyn. 2008, 237, 2622–2642. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Drescher, K.; Wingreen, N.S.; Bassler, B.L. Extracellular matrix structure governs invasion resistance in bacterial biofilms. ISME J. 2015, 9, 1700–1709. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Walboomers, X.F.; Jansen, J.A. Cell and tissue behavior on micro-grooved surfaces. Odontology 2001, 89, 2–11. [Google Scholar] [CrossRef]
- Han, Y.L.; Wang, S.; Zhang, X.; Li, Y.; Huang, G.; Qi, H.; Pingguan-Murphy, B.; Li, Y.; Lu, T.J.; Xu, F. Engineering physical microenvironment for stem cell based regenerative medicine. Drug Discov. Today 2014, 19, 763–773. [Google Scholar] [CrossRef]
- Sun, G.; Gerecht, S. Vascular regeneration: Engineering the stem cell microenvironment. Regen. Med. 2009, 4, 435–447. [Google Scholar] [CrossRef]
- Vadala, G.; Ambrosio, L.; Russo, F.; Papalia, R.; Denaro, V. Interaction between Mesenchymal Stem Cells and Intervertebral Disc Microenvironment: From Cell Therapy to Tissue Engineering. Stem Cells Int. 2019, 2019, 2376172. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Yokoyama, U. Extracellular matrix-mediated remodeling and mechanotransduction in large vessels during development and disease. Cell Signal. 2021, 86, 110104. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K. Extracellular Matrix Remodeling in Vascular Development and Disease. In Etiology and Morphogenesis of Congenital Heart Disease; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Janpan, 2016; pp. 221–226. [Google Scholar] [CrossRef]
- Allen, S.C.; Widman, J.A.; Datta, A.; Suggs, L.J. Dynamic extracellular matrix stiffening induces a phenotypic transformation and a migratory shift in epithelial cells. Integr. Biol. 2020, 12, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Chen, R.; Ma, Z.; Shi, Q.; Ataullakhanov, F.I.; Panteleev, M.; Yin, J. A dynamic remodeling bio-mimic extracellular matrix to reduce thrombotic and inflammatory complications of vascular implants. Biomater. Sci. 2020, 8, 6025–6036. [Google Scholar] [CrossRef]
- Qi, C.; Yan, X.; Huang, C.; Melerzanov, A.; Du, Y. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell 2015, 6, 638–653. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Mulyasasmita, W.; Cai, L.; Dewi, R.E.; Jha, A.; Ullmann, S.D.; Luong, R.H.; Huang, N.F.; Heilshorn, S.C. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J. Control. Release 2014, 191, 71–81. [Google Scholar] [CrossRef]
- Robinson, S.T.; Douglas, A.M.; Chadid, T.; Kuo, K.; Rajabalan, A.; Li, H.; Copland, I.B.; Barker, T.H.; Galipeau, J.; Brewster, L.P. A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization. Acta Biomater. 2016, 36, 86–98. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Plunkett, N.A.; O’Brien, F.J. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur. Cell Mater. 2010, 20, 218–230. [Google Scholar] [CrossRef]
- Gugala, Z.; Davis, A.R.; Fouletier-Dilling, C.M.; Gannon, F.H.; Lindsey, R.W.; Olmsted-Davis, E.A. Adenovirus BMP2-induced osteogenesis in combination with collagen carriers. Biomaterials 2007, 28, 4469–4479. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Xia, Z.D.; Han, Z.W.; Hulley, P.A.; Triffitt, J.T.; Czernuszka, J.T. Novel 3D collagen scaffolds fabricated by indirect printing technique for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Tierney, C.M.; Haugh, M.G.; Liedl, J.; Mulcahy, F.; Hayes, B.; O’Brien, F.J. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2009, 2, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.T.; Schwarzbauer, J.E. Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing. Mol. Biol. Cell 2019, 30, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Arulmoli, J.; Wright, H.J.; Phan, D.T.T.; Sheth, U.; Que, R.A.; Botten, G.A.; Keating, M.; Botvinick, E.L.; Pathak, M.M.; Zarembinski, T.I.; et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 43, 122–138. [Google Scholar] [CrossRef]
- Chen, W.S.; Guo, L.Y.; Tang, C.C.; Tsai, C.K.; Huang, H.H.; Chin, T.Y.; Yang, M.L.; Chen-Yang, Y.W. The Effect of Laminin Surface Modification of Electrospun Silica Nanofiber Substrate on Neuronal Tissue Engineering. Nanomaterials 2018, 8, 165. [Google Scholar] [CrossRef]
- Garg, K.; Ward, C.L.; Rathbone, C.R.; Corona, B.T. Trans.splantation of devitalized muscle scaffolds is insufficient for appreciable de novo muscle fiber regeneration after volumetric muscle loss injury. Cell Tissue Res. 2014, 358, 857–873. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Ward, C.L.; Garg, K.; Pollot, B.E.; Goldman, S.M.; McKinley, T.O.; Wenke, J.C.; Corona, B.T. Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing. J. Musculoskelet. Neuronal Interact. 2016, 16, 122–134. [Google Scholar]
- Stamati, K.; Priestley, J.V.; Mudera, V.; Cheema, U. Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake. Exp. Cell Res. 2014, 327, 68–77. [Google Scholar] [CrossRef]
- Mokos, Z.B.; Jovic, A.; Grgurevic, L.; Dumic-Cule, I.; Kostovic, K.; Ceovic, R.; Marinovic, B. Current Therapeutic Approach to Hypertrophic Scars. Front. Med. 2017, 4, 83. [Google Scholar] [CrossRef]
- Stuart, K.; Paderi, J.; Snyder, P.W.; Freeman, L.; Panitch, A. Collagen-binding peptidoglycans inhibit MMP mediated collagen degradation and reduce dermal scarring. PLoS ONE 2011, 6, e22139. [Google Scholar] [CrossRef] [PubMed]
- Goldbloom-Helzner, L.; Hao, D.; Wang, A. Developing Regenerative Treatments for Developmental Defects, Injuries, and Diseases Using Extracellular Matrix Collagen-Targeting Peptides. Int. J. Mol. Sci. 2019, 20, 4072. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Liu, R.; Gao, K.; He, C.; He, S.; Zhao, C.; Sun, G.; Farmer, D.L.; Panitch, A.; Lam, K.S.; et al. Developing an Injectable Nanofibrous Extracellular Matrix Hydrogel With an Integrin alphavbeta3 Ligand to Improve Endothelial Cell Survival, Engraftment and Vascularization. Front. Bioeng. Biotechnol. 2020, 8, 890. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Walimbe, T.; Chen, H.; Gao, K.; Kumar, P.; Wei, Y.; Hao, D.; Liu, R.; Farmer, D.L.; Lam, K.S.; et al. Bioactive extracellular matrix scaffolds engineered with proangiogenic proteoglycan mimetics and loaded with endothelial progenitor cells promote neovascularization and diabetic wound healing. Bioact. Mater. 2022, 10, 460–473. [Google Scholar] [CrossRef]
- Malcor, J.D.; Hunter, E.J.; Davidenko, N.; Bax, D.V.; Cameron, R.; Best, S.; Sinha, S.; Farndale, R.W. Collagen scaffolds functionalized with triple-helical peptides support 3D HUVEC culture. Regen. Biomater. 2020, 7, 471–482. [Google Scholar] [CrossRef]
- do Amaral, R.; Zayed, N.M.A.; Pascu, E.I.; Cavanagh, B.; Hobbs, C.; Santarella, F.; Simpson, C.R.; Murphy, C.M.; Sridharan, R.; Gonzalez-Vazquez, A.; et al. Functionalising Collagen-Based Scaffolds With Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Zhao, Y.; Ding, W.; Wei, J.; Li, J.; Han, S.; Shang, X.; Wang, B.; Chen, B.; et al. Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater. 2016, 30, 233–245. [Google Scholar] [CrossRef]
- Barthes, J.; Ozcelik, H.; Hindie, M.; Ndreu-Halili, A.; Hasan, A.; Vrana, N.E. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: The recent advances. Biomed. Res. Int. 2014, 2014, 921905. [Google Scholar] [CrossRef]
- Kyburz, K.A.; Anseth, K.S. Synthetic mimics of the extracellular matrix: How simple is complex enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef]
- Lukashev, M.E.; Werb, Z. ECM signalling: Orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998, 8, 437–441. [Google Scholar] [CrossRef]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. J. Biomed. Mater. Res. A 2015, 103, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 586406. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Ma, B.; He, C.; Liu, R.; Farmer, D.L.; Lam, K.S.; Wang, A. Surface modification of polymeric electrospun scaffolds via a potent and high-affinity integrin alpha4beta1 ligand improved the adhesion, spreading and survival of human chorionic villus-derived mesenchymal stem cells: A new insight for fetal tissue engineering. J. Mater. Chem. B 2020, 8, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Fan, Y.; Xiao, W.; Liu, R.; Pivetti, C.; Walimbe, T.; Guo, F.; Zhang, X.; Farmer, D.L.; Wang, F.; et al. Rapid endothelialization of small diameter vascular grafts by a bioactive integrin-binding ligand specifically targeting endothelial progenitor cells and endothelial cells. Acta Biomater. 2020, 108, 178–193. [Google Scholar] [CrossRef]
- Hao, D.; Xiao, W.; Liu, R.; Kumar, P.; Li, Y.; Zhou, P.; Guo, F.; Farmer, D.L.; Lam, K.S.; Wang, F.; et al. Discovery and Characterization of a Potent and Specific Peptide Ligand Targeting Endothelial Progenitor Cells and Endothelial Cells for Tissue Regeneration. ACS Chem. Biol. 2017, 12, 1075–1086. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.L.; Yang, Z.; et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012, 33, 2880–2891. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.; Tang, Z.; Henry, J.; Li-Ping Lee, B.; Zhu, Y.; Yuan, F.; Huang, F.; Li, S. The effect of stromal cell-derived factor-1alpha/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials 2012, 33, 8062–8074. [Google Scholar] [CrossRef]
- Yang, Y.P.; Labus, K.M.; Gadomski, B.C.; Bruyas, A.; Easley, J.; Nelson, B.; Palmer, R.H.; McGilvray, K.; Regan, D.; Puttlitz, C.M.; et al. Osteoinductive 3D printed scaffold healed 5 cm segmental bone defects in the ovine metatarsus. Sci. Rep. 2021, 11, 6704. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Li, K.; Zhu, S.; Lu, B.; Liu, J.; Tang, Y.; Wei, Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact. Mater. 2021, 6, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef] [PubMed]
- Piening, L.M.; Wachs, R.A. Matrix Bound Nanovesicles: What are they and what do they do? Cells Tissues Organs 2022, 211, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Swindell, H.S.; Ramasubramanian, L.; Liu, R.; Lam, K.S.; Farmer, D.L.; Wang, A. Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified With Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front. Bioeng. Biotechnol. 2020, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Koda, I.; Song, M.; Chen, G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 2011, 32, 9658–9666. [Google Scholar] [CrossRef]
- Litowczenko, J.; Wozniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef]
- Wu, R.; Gao, W.; Yao, K.; Ge, J. Roles of Exosomes Derived From Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019, 10, 648. [Google Scholar] [CrossRef]
- Kucuk, N.; Primozic, M.; Knez, Z.; Leitgeb, M. Exosomes Engineering and Their Roles as Therapy Delivery Tools, Therapeutic Targets, and Biomarkers. Int. J. Mol. Sci. 2021, 22, 9543. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Yu, C.C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Johnson, K.W.; de Paz, N.; Dooner, M.S.; Puente, N.; Ayala, C.; Brilliant, K.; Berz, D.; Lee, D.; et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp. Hematol. 2010, 38, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.L.; Schmittgen, T.D.; et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Rani, S.; Ritter, T. The Exosome—A Naturally Secreted Nanoparticle and its Application to Wound Healing. Adv. Mater. 2016, 28, 5542–5552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Kim, S.H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; He, T.; Jiang, J.; Li, Y.; Chen, P. Osteogenic Effect of tsRNA-10277-Loaded Exosome Derived from Bone Mesenchymal Stem Cells on Steroid-Induced Osteonecrosis of the Femoral Head. Drug Des. Devel. Ther. 2020, 14, 4579–4591. [Google Scholar] [CrossRef]
- Won Lee, G.; Thangavelu, M.; Joung Choi, M.; Yeong Shin, E.; Sol Kim, H.; Seon Baek, J.; Woon Jeong, Y.; Eun Song, J.; Carlomagno, C.; Miguel Oliveira, J.; et al. Exosome mediated transfer of miRNA-140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. J. Cell Biochem. 2020, 121, 3642–3652. [Google Scholar] [CrossRef]
- Dorairaj, V.; Sulaiman, S.A.; Abu, N.; Abdul Murad, N.A. Extracellular Vesicles in the Development of the Non-Alcoholic Fatty Liver Disease: An Update. Biomolecules 2020, 10, 1494. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Control. Release 2016, 238, 166–175. [Google Scholar] [CrossRef]
- Oh, S.; Jung, J.H.; Ahn, K.J.; Jang, A.Y.; Byun, K.; Yang, P.C.; Chung, W.J. Stem Cell and Exosome Therapy in Pulmonary Hypertension. Korean Circ. J. 2022, 52, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, D.S. Illuminating the physiology of extracellular vesicles. Stem Cell Res. Ther. 2016, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Kato, K.; Yamashita, T.; Imai, T.; Saji, H.; Takakura, Y. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci. 2015, 104, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.V.; Mor, S.; Wang, D.; Goldbloom-Helzner, L.; Clark, K.; Hao, D.; Farmer, D.L.; Wang, A. Engineering extracellular vesicles for Alzheimer’s disease: An emerging cell-free approach for earlier diagnosis and treatment. WIRes. Mech. Dis. 2022, 14, e1541. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Evers, M.J.W.; van de Wakker, S.I.; de Groot, E.M.; de Jong, O.G.; Gitz-Francois, J.J.J.; Seinen, C.S.; Sluijter, J.P.G.; Schiffelers, R.M.; Vader, P. Functional siRNA Delivery by Extracellular Vesicle-Liposome Hybrid Nanoparticles. Adv. Healthc. Mater. 2022, 11, e2101202. [Google Scholar] [CrossRef]
- Lazar, S.; Mor, S.; Chen, J.; Hao, D.; Wang, A. Bioengineered extracellular vesicle-loaded bioscaffolds for therapeutic applications in regenerative medicine. Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Nasirishargh, A.; Kumar, P.; Ramasubramanian, L.; Clark, K.; Hao, D.; Lazar, S.V.; Wang, A. Exosomal microRNAs from mesenchymal stem/stromal cells: Biology and applications in neuroprotection. World J. Stem Cells 2021, 13, 776–794. [Google Scholar] [CrossRef]
- Hao, D.; He, C.; Ma, B.; Lankford, L.; Reynaga, L.; Farmer, D.L.; Guo, F.; Wang, A. Hypoxic Preconditioning Enhances Survival and Proangiogenic Capacity of Human First Trimester Chorionic Villus-Derived Mesenchymal Stem Cells for Fetal Tissue Engineering. Stem Cells Int. 2019, 2019, 9695239. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hao, D.; Jiang, H.; Huang, M.; Du, Q.; Lin, Y.; Liu, F.; Chen, B. Nrf2 Regulates CHI3L1 to Suppress Inflammation and Improve Post-Traumatic Osteoarthritis. J. Inflamm. Res. 2021, 14, 4079–4088. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, F.; Chai, R.; Zhou, W.; Hu, M.; Liu, B.; Chen, X.; Liu, M.; Xu, Q.; Liu, N.; et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J. Cell Mol. Med. 2019, 23, 7617–7631. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, J.; Tan, W.L.W.; Jiang, Y.; Wang, S.; Li, Q.; Yu, X.; Tan, J.; Liu, S.; Zhang, P.; et al. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 2020, 11, 354. [Google Scholar] [CrossRef]
- Gong, X.H.; Liu, H.; Wang, S.J.; Liang, S.W.; Wang, G.G. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J. Cell Physiol. 2019, 234, 13878–13893. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv. Healthc. Mater. 2019, 8, e1801271. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wu, J.Y.; Liu, J.; Xu, W.; Qiu, X.; Huang, S.; Hu, X.B.; Xiang, D.X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnology 2021, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Xue, V.W.; Wong, S.C.C.; Song, G.; Cho, W.C.S. Promising RNA-based cancer gene therapy using extracellular vesicles for drug delivery. Expert Opin. Biol. Ther. 2020, 20, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.Y.; Cho, R.; Shin, D.M.; Lee, S.W.; Oh, Y.M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017, 49, e284. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kumar, H.; Jo, M.J.; Kim, J.; Yoon, J.K.; Lee, J.R.; Kang, M.; Choo, Y.W.; Song, S.Y.; Kwon, S.P.; et al. Therapeutic Efficacy-Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018, 18, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chu, D.; Wang, Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J. Control. Release 2016, 224, 208–216. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Wang, J.M.; Li, Y.J.; Wu, J.Y.; Cai, J.X.; Wen, J.; Xiang, D.X.; Hu, X.B.; Li, W.Q. Comparative evaluation of methods for isolating small extracellular vesicles derived from pancreatic cancer cells. Cell Biosci. 2021, 11, 37. [Google Scholar] [CrossRef]
- Staufer, O.; Dietrich, F.; Rimal, R.; Schroter, M.; Fabritz, S.; Boehm, H.; Singh, S.; Moller, M.; Platzman, I.; Spatz, J.P. Bottom-up assembly of biomedical relevant fully synthetic extracellular vesicles. Sci. Adv. 2021, 7, eabg6666. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; Garcia-Alvarez, F.; Basanez, G.; Alegre-Aguaron, E.; Desportes, P.; Larrad, L.; Naval, J.; Martinez-Lorenzo, M.J.; Anel, A. Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2272–2282. [Google Scholar] [CrossRef]
- Vazquez-Rios, A.J.; Molina-Crespo, A.; Bouzo, B.L.; Lopez-Lopez, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, K.M.; Seeliger, M.; Musaev, T.; Macri, L.K.; Clark, R.A. Fibronectin Interaction and Enhancement of Growth Factors: Importance for Wound Healing. Adv. Wound Care 2015, 4, 469–478. [Google Scholar] [CrossRef]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, D.; Chakrabarty, S. Growth factors in tumor microenvironment. Front. Biosci. 2010, 15, 151–165. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, L.; Zhao, Y.; Sun, W.; Chen, B.; Lin, H.; Wang, X.; Zhang, L.; Xu, B.; Dai, J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation 2009, 119, 1776–1784. [Google Scholar] [CrossRef]
- Sun, J.; Mou, C.; Shi, Q.; Chen, B.; Hou, X.; Zhang, W.; Li, X.; Zhuang, Y.; Shi, J.; Chen, Y.; et al. Controlled release of collagen-binding SDF-1alpha from the collagen scaffold promoted tendon regeneration in a rat Achilles tendon defect model. Biomaterials 2018, 162, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.; Weisel, R.D.; Li, R.K.; Radisic, M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 15, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Growth Factor-Extracellular Matrix Interactions Regulate Wound Repair. Adv. Wound Care 2012, 1, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gresham, R.C.H.; Bahney, C.S.; Leach, J.K. Growth factor delivery using extracellular matrix-mimicking substrates for musculoskeletal tissue engineering and repair. Bioact. Mater. 2021, 6, 1945–1956. [Google Scholar] [CrossRef]
- Facca, S.; Cortez, C.; Mendoza-Palomares, C.; Messadeq, N.; Dierich, A.; Johnston, A.P.; Mainard, D.; Voegel, J.C.; Caruso, F.; Benkirane-Jessel, N. Active multilayered capsules for in vivo bone formation. Proc. Natl. Acad. Sci. USA 2010, 107, 3406–3411. [Google Scholar] [CrossRef]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am. J. Pathol. 2005, 167, 1575–1586. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-beta in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef]
- Novais, A.; Chatzopoulou, E.; Chaussain, C.; Gorin, C. The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review. Cells 2021, 10, 932. [Google Scholar] [CrossRef]
- Leonard, A.; Koria, P. Growth factor functionalized biomaterial for drug delivery and tissue regeneration. J. Bioact. Compat. Polym. 2017, 32, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Seims, K.B.; Hunt, N.K.; Chow, L.W. Strategies to Control or Mimic Growth Factor Activity for Bone, Cartilage, and Osteochondral Tissue Engineering. Bioconjug Chem. 2021, 32, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, J.L.; Vranckx, J.J. State of the Art of Clinical Applications of Tissue Engineering in 2021. Tissue Eng. Part B Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, D.; Lopez, J.-M.; Chen, J.; Iavorovschi, A.M.; Lelivelt, N.M.; Wang, A. Engineering Extracellular Microenvironment for Tissue Regeneration. Bioengineering 2022, 9, 202. https://doi.org/10.3390/bioengineering9050202
Hao D, Lopez J-M, Chen J, Iavorovschi AM, Lelivelt NM, Wang A. Engineering Extracellular Microenvironment for Tissue Regeneration. Bioengineering. 2022; 9(5):202. https://doi.org/10.3390/bioengineering9050202
Chicago/Turabian StyleHao, Dake, Juan-Maria Lopez, Jianing Chen, Alexandra Maria Iavorovschi, Nora Marlene Lelivelt, and Aijun Wang. 2022. "Engineering Extracellular Microenvironment for Tissue Regeneration" Bioengineering 9, no. 5: 202. https://doi.org/10.3390/bioengineering9050202
APA StyleHao, D., Lopez, J.-M., Chen, J., Iavorovschi, A. M., Lelivelt, N. M., & Wang, A. (2022). Engineering Extracellular Microenvironment for Tissue Regeneration. Bioengineering, 9(5), 202. https://doi.org/10.3390/bioengineering9050202






