Morphological Changes of Glial Lamina Cribrosa of Rats Suffering from Chronic High Intraocular Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Model Induction and IOP Measurement
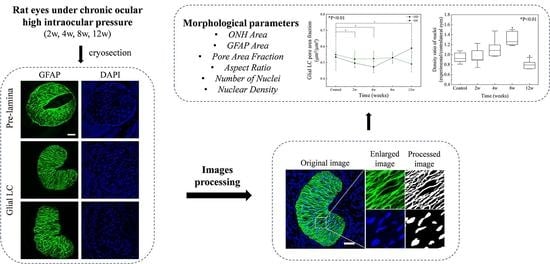
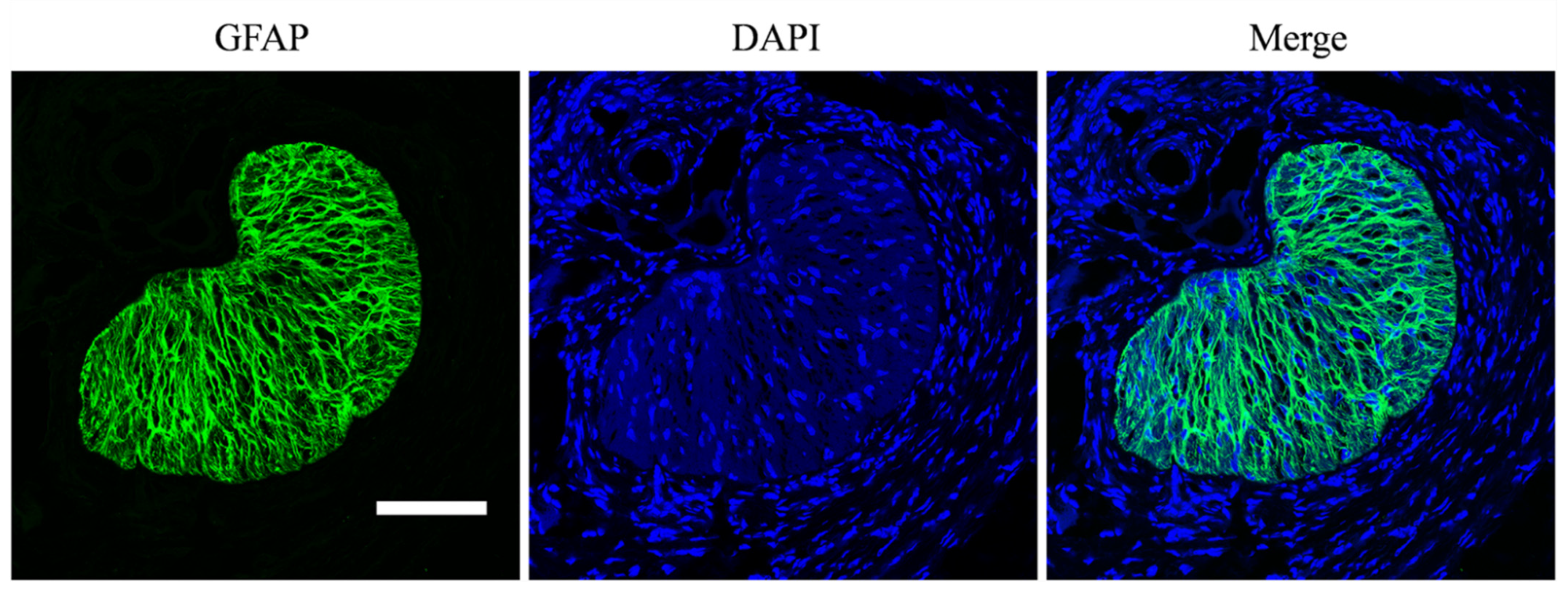
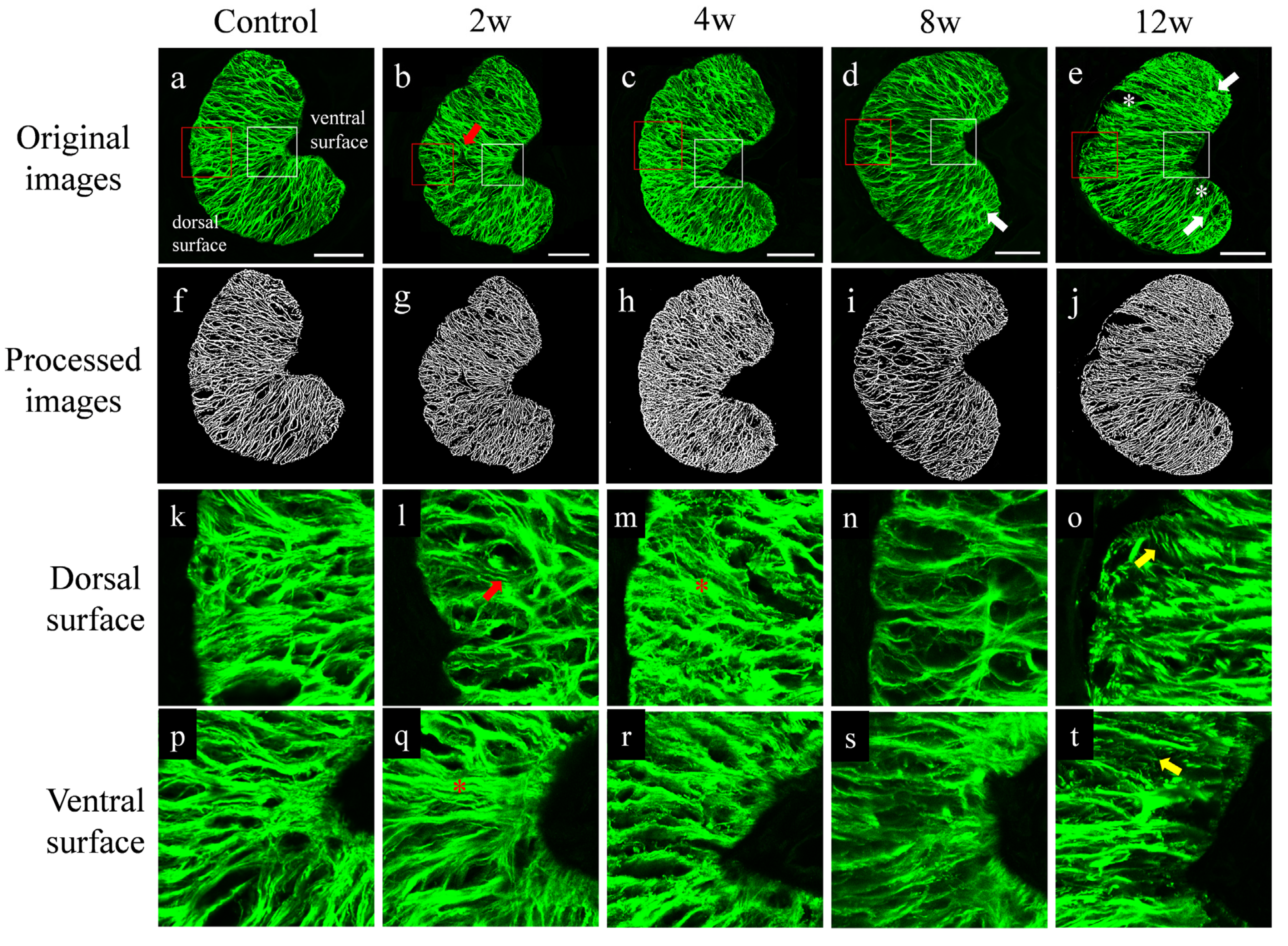
2.3. Staining and Imaging
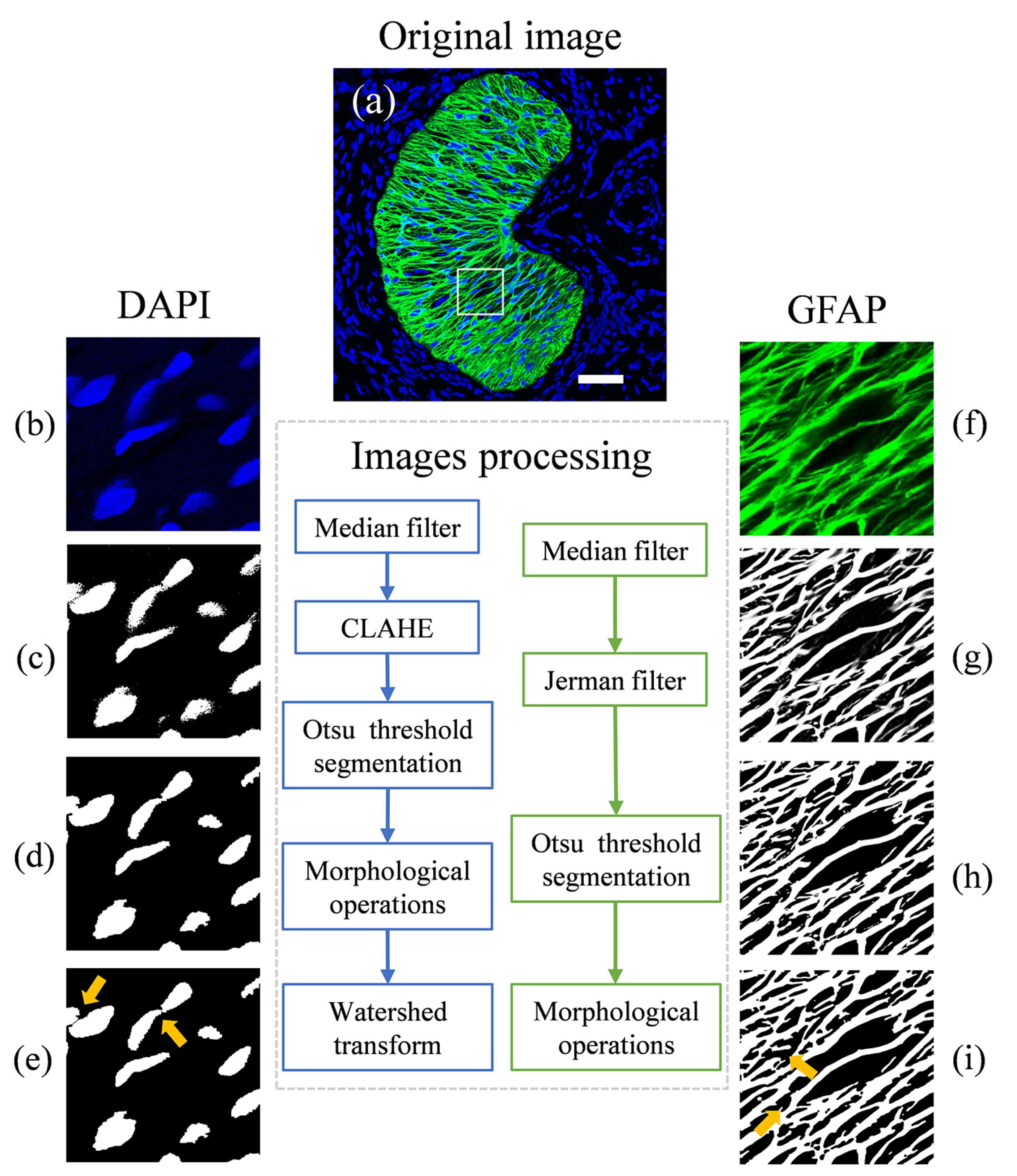
2.4. Image Processing
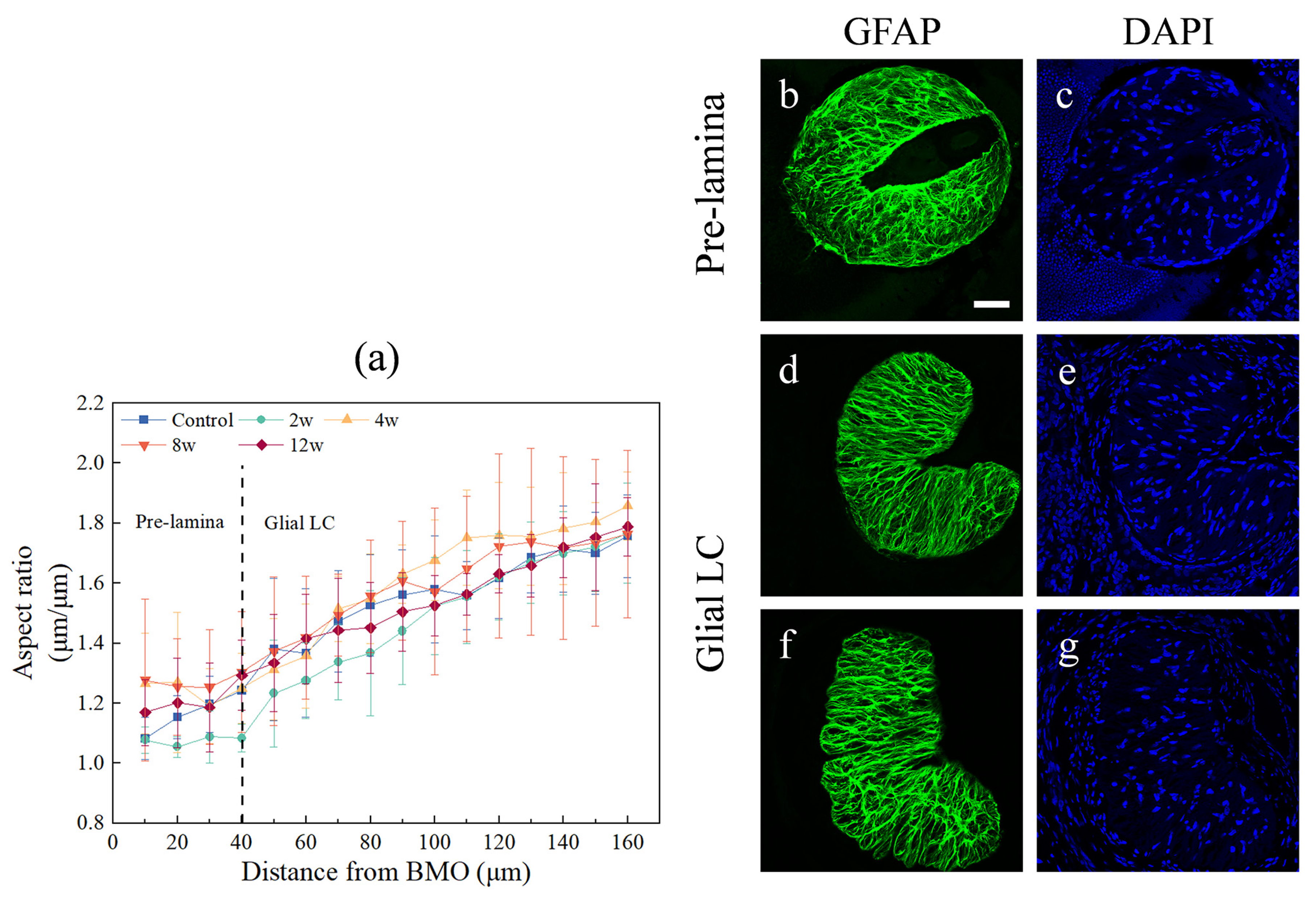
2.5. Structural Measurements
2.6. Regional Division
2.7. Statistical Analysis
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.S. Detecting Optic Nerve Head Deformation and Retinal Nerve Fiber Layer Thinning in Glaucoma Progression. Taiwan J. Ophthalmol. 2015, 5, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.; Koh, V.; Girard, M.J.; Cheng, C.-Y. Imaging of the Lamina Cribrosa and Its Role in Glaucoma: A Review: Imaging the Lamina Cribrosa in Glaucoma. Clin. Experiment. Ophthalmol. 2018, 46, 177–188. [Google Scholar] [CrossRef]
- Ling, Y.T.T.; Pease, M.E.; Jefferys, J.L.; Kimball, E.C.; Quigley, H.A.; Nguyen, T.D. Pressure-Induced Changes in Astrocyte GFAP, Actin, and Nuclear Morphology in Mouse Optic Nerve. Investig. Ophthalmol. Vis. Sci. 2020, 61, 14. [Google Scholar] [CrossRef] [PubMed]
- El Basty, A.; Gaber, R.; Elmaria, A.; Sabry, M.; Hussein, T.R. Correlation Between Changes in Lamina Cribrosa Structure and Visual Field in Primary Open-Angle Glaucoma. Clin. Ophthalmol. 2021, 15, 4715–4722. [Google Scholar] [CrossRef] [PubMed]
- Omodaka, K.; Horii, T.; Takahashi, S.; Kikawa, T.; Matsumoto, A.; Shiga, Y.; Maruyama, K.; Yuasa, T.; Akiba, M.; Nakazawa, T. 3D Evaluation of the Lamina Cribrosa with Swept-Source Optical Coherence Tomography in Normal Tension Glaucoma. PLoS ONE 2015, 10, e0122347. [Google Scholar] [CrossRef]
- Yan, D.B.; Coloma, F.M.; Metheetrairut, A.; Trope, G.E.; Heathcote, J.G.; Ethier, C.R. Deformation of the Lamina Cribrosa by Elevated Intraocular Pressure. Br. J. Ophthalmol. 1994, 78, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Brazile, B.L.; Yang, B.; Waxman, S.; Lam, P.; Voorhees, A.P.; Hua, Y.; Loewen, R.T.; Loewen, N.A.; Rizzo, J.F.; Jakobs, T.; et al. Lamina Cribrosa Capillaries Straighten as Intraocular Pressure Increases. Investig. Ophthalmol. Vis. Sci. 2020, 61, 2. [Google Scholar] [CrossRef] [PubMed]
- Prada, D.; Harris, A.; Guidoboni, G.; Siesky, B.; Huang, A.M.; Arciero, J. Autoregulation and Neurovascular Coupling in the Optic Nerve Head. Surv. Ophthalmol. 2016, 61, 164–186. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Trinkaus, K.; Wax, M.B. Alterations in the Morphology of Lamina Cribrosa Pores in Glaucomatous Eyes. Br. J. Ophthalmol. 2004, 88, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Omodaka, K.; Maekawa, S.; An, G.; Tsuda, S.; Shiga, Y.; Takada, N.; Kikawa, T.; Takahashi, H.; Yokota, H.; Akiba, M.; et al. Pilot Study for Three-Dimensional Assessment of Laminar Pore Structure in Patients with Glaucoma, as Measured with Swept Source Optical Coherence Tomography. PLoS ONE 2018, 13, e0207600. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kuroda, H.; Suzuki, M.; Ibuki, H.; Araie, M.; Yoneya, S. Glaucomatous Changes in Lamina Pores Shape within the Lamina Cribrosa Using Wide Bandwidth, Femtosecond Mode-Locked Laser OCT. PLoS ONE 2017, 12, e0181675. [Google Scholar] [CrossRef]
- Ma, Y.; Kwok, S.; Sun, J.; Pan, X.; Pavlatos, E.; Clayson, K.; Hazen, N.; Liu, J. IOP-Induced Regional Displacements in the Optic Nerve Head and Correlation with Peripapillary Sclera Thickness. Exp. Eye Res. 2020, 200, 108202. [Google Scholar] [CrossRef]
- Yang, B.; Brazile, B.; Jan, N.-J.; Hua, Y.; Wei, J.; Sigal, I.A. Structured Polarized Light Microscopy for Collagen Fiber Structure and Orientation Quantification in Thick Ocular Tissues. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, Q.; Yang, H.; Chen, J.D.; Wang, N.; Jonas, J.B. Lamina Cribrosa Pore Movement during Acute Intraocular Pressure Rise. Br. J. Ophthalmol. 2020, 104, 800–806. [Google Scholar] [CrossRef]
- Voorhees, A.P.; Jan, N.-J.; Austin, M.E.; Flanagan, J.G.; Sivak, J.M.; Bilonick, R.A.; Sigal, I.A. Lamina Cribrosa Pore Shape and Size as Predictors of Neural Tissue Mechanical Insult. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5336. [Google Scholar] [CrossRef]
- Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Morrison, J.C.; Johnson, E.C. Early Optic Nerve Head Glial Proliferation and Jak-Stat Pathway Activation in Chronic Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 921–932. [Google Scholar] [CrossRef]
- Reynaud, J.; Lockwood, H.; Gardiner, S.K.; Williams, G.; Yang, H.; Burgoyne, C.F. Lamina Cribrosa Microarchitecture in Monkey Early Experimental Glaucoma: Global Change. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3451. [Google Scholar] [CrossRef][Green Version]
- Pang, I.-H.; Clark, A.F. Inducible Rodent Models of Glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Khaw, P.T.; Yin, Z.Q.; Li, D.; Raisman, G.; Li, Y. Structural Basis of Glaucoma: The Fortified Astrocytes of the Optic Nerve Head Are the Target of Raised Intraocular Pressure. Glia 2012, 60, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.T.T.; Shi, R.; Midgett, D.E.; Jefferys, J.L.; Quigley, H.A.; Nguyen, T.D. Characterizing the Collagen Network Structure and Pressure-Induced Strains of the Human Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2406. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, T.; Li, L.; Qian, X.; Liu, Z. A Feasible Method for Independently Evaluating the Mechanical Properties of Glial LC and RGC Axons by Combining Atomic Force Microscopy Measurement with Image Segmentation. J. Mech. Behav. Biomed. Mater. 2022, 126, 105041. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. The Morphology and Spatial Arrangement of Astrocytes in the Optic Nerve Head of the Mouse. J. Comp. Neurol. 2009, 516, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ma, L.; Liu, L.; Li, L.; Qian, X. Preliminary Study on the Blockade of Axonal Transport by Activated Astrocytes in Optic Nerve Head under Chronic Ocular Hypertension. J. Mech. Med. Biol. 2019, 19, 1940040. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Liu, Z. Time Course Changes of the Mechanical Properties of the Iris Pigment Epithelium in a Rat Chronic Ocular Hypertension Model. BioMed Res. Int. 2018, 2018, 4862309. [Google Scholar] [CrossRef]
- Jerman, T.; Pernus, F.; Likar, B.; Spiclin, Z. Enhancement of Vascular Structures in 3D and 2D Angiographic Images. IEEE Trans. Med. Imaging 2016, 35, 2107–2118. [Google Scholar] [CrossRef]
- Jerman, T.; Pernus, F.; Likar, B.; Spiclin, Z. Blob Enhancement and Visualization for Improved Intracranial Aneurysm Detection. IEEE Trans. Visual. Comput. Graph. 2016, 22, 1705–1717. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Meyer, F. Topographic Distance and Watershed Lines. Signal Process. 1994, 38, 113–125. [Google Scholar] [CrossRef]
- Dong, Y.; Fu, Y.; Qian, X.; Lin, L.; Yuan, Y.; Li, Y.; Shao, W.; Gao, Q. Optic Nerve Head Astrocytes Contribute to Vascular Associated Effects. Front. Med. 2022, 9, 943986. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, G.; Ebneter, A.; Wood, J.P.M.; Casson, R.J. The Optic Nerve Head Is the Site of Axonal Transport Disruption, Axonal Cytoskeleton Damage and Putative Axonal Regeneration Failure in a Rat Model of Glaucoma. Acta Neuropathol. 2011, 121, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic Nerve Damage in Human Glaucoma. II. The Site of Injury and Susceptibility to Damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Garcia-Herranz, D.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Bravo-Osuna, I.; Carretero, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.E.; et al. Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines 2021, 9, 682. [Google Scholar] [CrossRef]
- Biswas, S.; Wan, K.H. Review of Rodent Hypertensive Glaucoma Models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef]
- Tehrani, S.; Johnson, E.C.; Cepurna, W.O.; Morrison, J.C. Astrocyte Processes Label for Filamentous Actin and Reorient Early within the Optic Nerve Head in a Rat Glaucoma Model. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6945–6952. [Google Scholar] [CrossRef]
- Tehrani, S.; Davis, L.; Cepurna, W.O.; Delf, R.K.; Lozano, D.C.; Choe, T.E.; Johnson, E.C.; Morrison, J.C. Optic Nerve Head Astrocytes Display Axon-Dependent and -Independent Reactivity in Response to Acutely Elevated Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2019, 60, 312–321. [Google Scholar] [CrossRef]
- Kim, Y.W.; Jeoung, J.W.; Kim, Y.K.; Park, K.H. Clinical Implications of In Vivo Lamina Cribrosa Imaging in Glaucoma. J. Glaucoma 2017, 26, 753–761. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration Severity Can Be Predicted from Early Microglia Alterations Monitored in Vivo in a Mouse Model of Chronic Glaucoma. Dis. Models Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Andrade, J.C.F.; Kanadani, F.N.; Furlanetto, R.L.; Lopes, F.S.; Ritch, R.; Prata, T.S. Elucidation of the Role of the Lamina Cribrosa in Glaucoma Using Optical Coherence Tomography. Surv. Ophthalmol. 2022, 67, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Takusagawa, H.L.; Hoguet, A.; Junk, A.K.; Nouri-Mahdavi, K.; Radhakrishnan, S.; Chen, T.C. Swept-Source OCT for Evaluating the Lamina Cribrosa: A Report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Nadler, Z.; Wang, B.; Schuman, J.S.; Ferguson, R.D.; Patel, A.; Hammer, D.X.; Bilonick, R.A.; Ishikawa, H.; Kagemann, L.; Sigal, I.A.; et al. In Vivo Three-Dimensional Characterization of the Healthy Human Lamina Cribrosa with Adaptive Optics Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- Kawano, J. Chemoarchitecture of Glial Fibrillary Acidic Protein (GFAP) and Glutamine Synthetase in the Rat Optic Nerve: An Immunohistochemical Study. Okajimas Folia Anat. Jpn. 2015, 92, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Takushima, A. The Aspect Ratio of the Palpebral Fissure as a New Blepharoptosis Parameter. J. Plast. Surg. Hand Surg. 2022, 56, 111–114. [Google Scholar] [CrossRef]
- Yoshii, Y.; Tung, W.; Ishii, T. Strain and Morphological Changes of Median Nerve After Carpal Tunnel Release. J. Ultrasound Med. 2017, 36, 1153–1159. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Morrison, J.C.; Dorman-Pease, M.E.; Dunkelberger, G.R.; Quigley, H.A. Optic Nerve Head Extracellular Matrix in Primary Optic Atrophy and Experimental Glaucoma. Arch. Ophthalmol. 1990, 108, 1020–1024. [Google Scholar] [CrossRef]
- Midgett, D.; Liu, B.; Ling, Y.T.T.; Jefferys, J.L.; Quigley, H.A.; Nguyen, T.D. The Effects of Glaucoma on the Pressure-Induced Strain Response of the Human Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 41. [Google Scholar] [CrossRef]
- Hopkins, A.A.; Murphy, R.; Irnaten, M.; Wallace, D.M.; Quill, B.; O’Brien, C. The Role of Lamina Cribrosa Tissue Stiffness and Fibrosis as Fundamental Biomechanical Drivers of Pathological Glaucoma Cupping. Am. J. Physiol. -Cell Physiol. 2020, 319, C611–C623. [Google Scholar] [CrossRef]
- Sun, D.; Qu, J.; Jakobs, T.C. Reversible Reactivity by Optic Nerve Astrocytes. Glia 2013, 61, 1218–1235. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. Structural Remodeling of Fibrous Astrocytes after Axonal Injury. J. Neurosci. 2010, 30, 14008–14019. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Seifert, P.; Jakobs, T.C. Astrocytes in the Optic Nerve Head of Glaucomatous Mice Display a Characteristic Reactive Phenotype. Investig. Ophthalmol. Vis. Sci. 2017, 58, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Lye-Barthel, M.; Sun, D.; Jakobs, T.C. Morphology of Astrocytes in a Glaucomatous Optic Nerve. Investig. Ophthalmol. Vis. Sci. 2013, 54, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Cone, F.E.; Steinhart, M.R.; Oglesby, E.N.; Kalesnykas, G.; Pease, M.E.; Quigley, H.A. The Effects of Anesthesia, Mouse Strain and Age on Intraocular Pressure and an Improved Murine Model of Experimental Glaucoma. Exp. Eye Res. 2012, 99, 27–35. [Google Scholar] [CrossRef]
- Hernandez, M.R. The Optic Nerve Head in Glaucoma: Role of Astrocytes in Tissue Remodeling. Prog. Retin. Eye Res. 2000, 19, 297–321. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Lu, Q.; Qing, G.; Wang, N.; Wang, Y.; Li, S.; Yang, D.; Yan, F. Detection of Early Neuron Degeneration and Accompanying Glial Responses in the Visual Pathway in a Rat Model of Acute Intraocular Hypertension. Brain Res. 2009, 1303, 131–143. [Google Scholar] [CrossRef]
- Lopez, N.N.; Clark, A.F.; Tovar-Vidales, T. Isolation and Characterization of Human Optic Nerve Head Astrocytes and Lamina Cribrosa Cells. Exp. Eye Res. 2020, 197, 108103. [Google Scholar] [CrossRef]




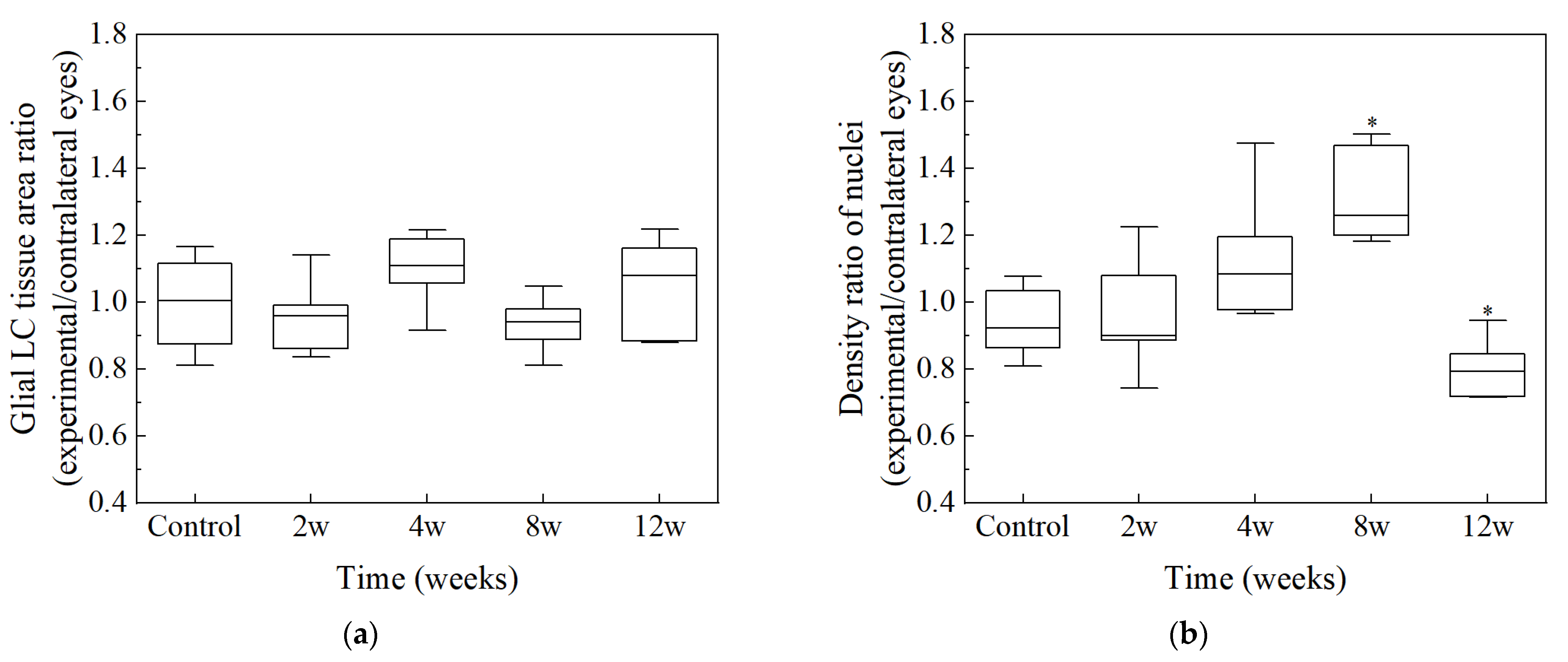
| Groups | Number of Nuclei | Glial LC Tissue Area (mm2) | GFAP Area (mm2) | Density of Nuclei (n × 103/mm2) |
|---|---|---|---|---|
| Control | 164 ± 21 ab | 0.0660 ± 0.00911 | 0.0271 ± 0.00487 | 2.67 ± 0.392 b |
| 2 w | 176 ± 18 ab | 0.0648 ± 0.00679 | 0.0320 ± 0.00495 | 2.76 ± 0.241 ab |
| 4 w | 169 ± 28 ab | 0.0595 ± 0.00613 | 0.0296 ± 0.00657 | 3.00 ± 0.403 ab |
| 8 w | 201 ± 7 a | 0.0703 ± 0.00811 | 0.0313 ± 0.00414 | 3.43 ± 0.467 a |
| 12 w | 162 ± 17 b | 0.0612 ± 0.00521 | 0.0234 ± 0.00710 | 2.57 ± 0.300 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Liu, L.; Li, L.; Qian, X. Morphological Changes of Glial Lamina Cribrosa of Rats Suffering from Chronic High Intraocular Pressure. Bioengineering 2022, 9, 741. https://doi.org/10.3390/bioengineering9120741
Zhang J, Liu Y, Liu L, Li L, Qian X. Morphological Changes of Glial Lamina Cribrosa of Rats Suffering from Chronic High Intraocular Pressure. Bioengineering. 2022; 9(12):741. https://doi.org/10.3390/bioengineering9120741
Chicago/Turabian StyleZhang, Jingxi, Yushu Liu, Liu Liu, Lin Li, and Xiuqing Qian. 2022. "Morphological Changes of Glial Lamina Cribrosa of Rats Suffering from Chronic High Intraocular Pressure" Bioengineering 9, no. 12: 741. https://doi.org/10.3390/bioengineering9120741
APA StyleZhang, J., Liu, Y., Liu, L., Li, L., & Qian, X. (2022). Morphological Changes of Glial Lamina Cribrosa of Rats Suffering from Chronic High Intraocular Pressure. Bioengineering, 9(12), 741. https://doi.org/10.3390/bioengineering9120741