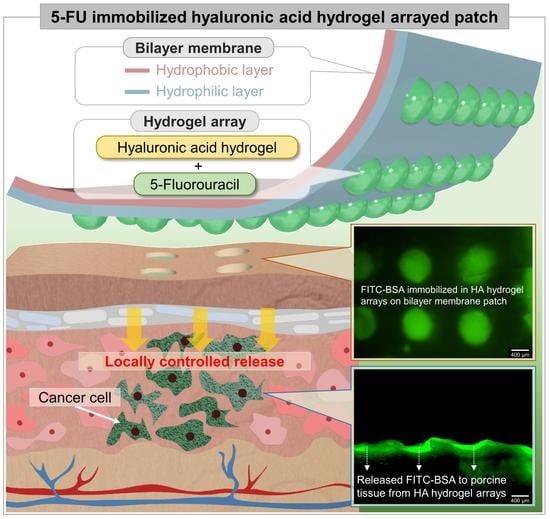
5-Fluorouracil-Immobilized Hyaluronic Acid Hydrogel Arrays on an Electrospun Bilayer Membrane as a Drug Patch
Abstract
1. Introduction
2. Materials and Methods
2.1. Three-Dimensional (3D)-Printing-Based Master Micromold
2.2. Synthesis of HA Hydrogel
2.3. Fabrication of Electrospun Bilayer Membrane
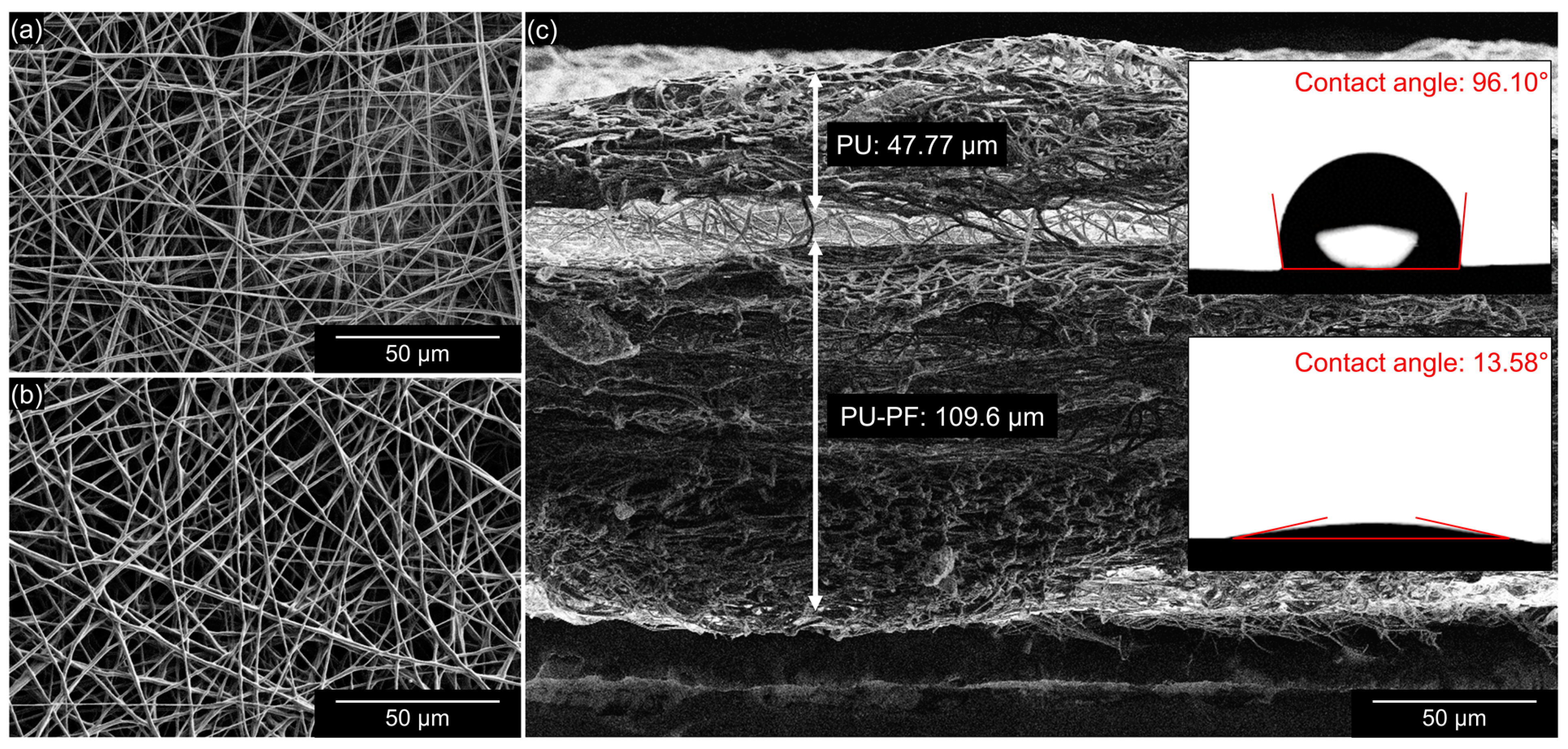
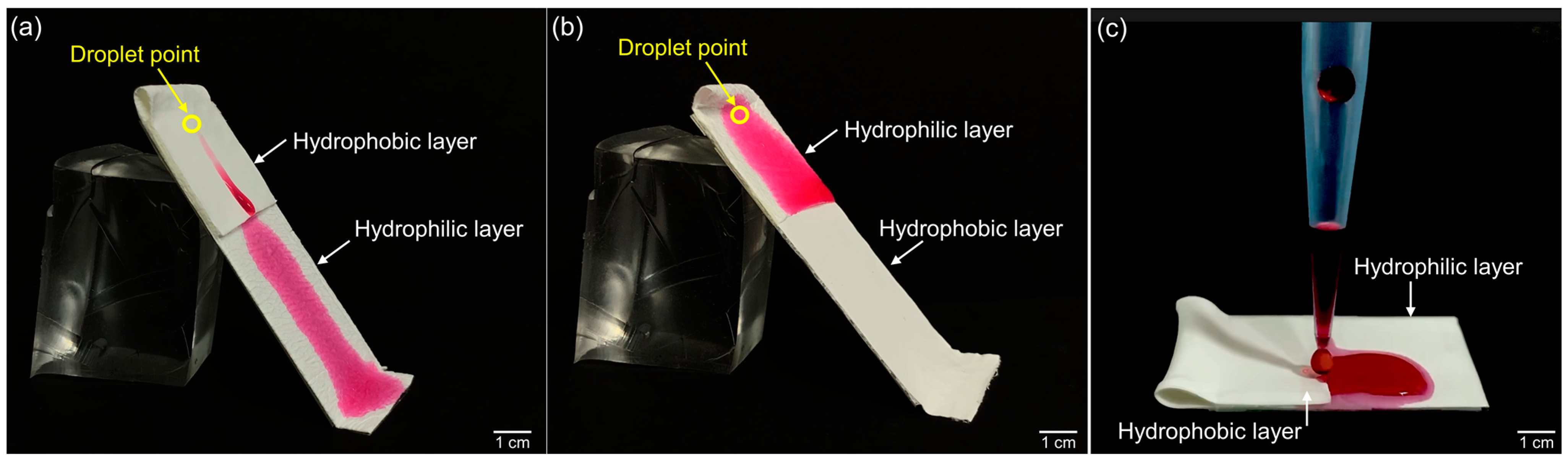
2.4. Characterization of Electrospun Bilayer Membrane
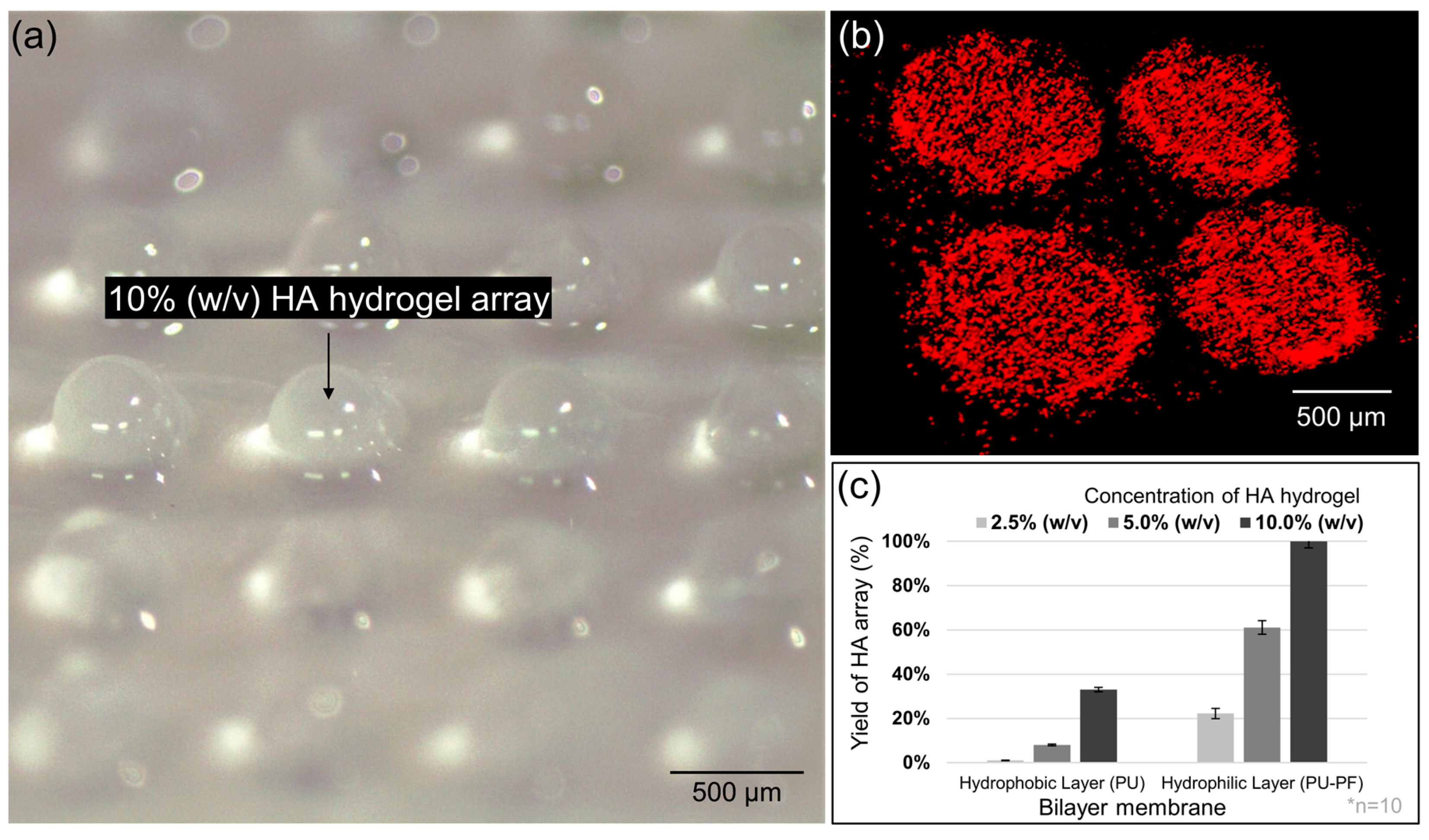
2.5. HA Hydrogel Arraying on Electrospun Bilayer Membrane
2.6. Diffusion Test
2.7. 5-FU Immobilization and Release with YD-10B Cells
3. Results and Discussion
3.1. Electrospun Bilayer Membrane
3.2. HA Hydrogel Array
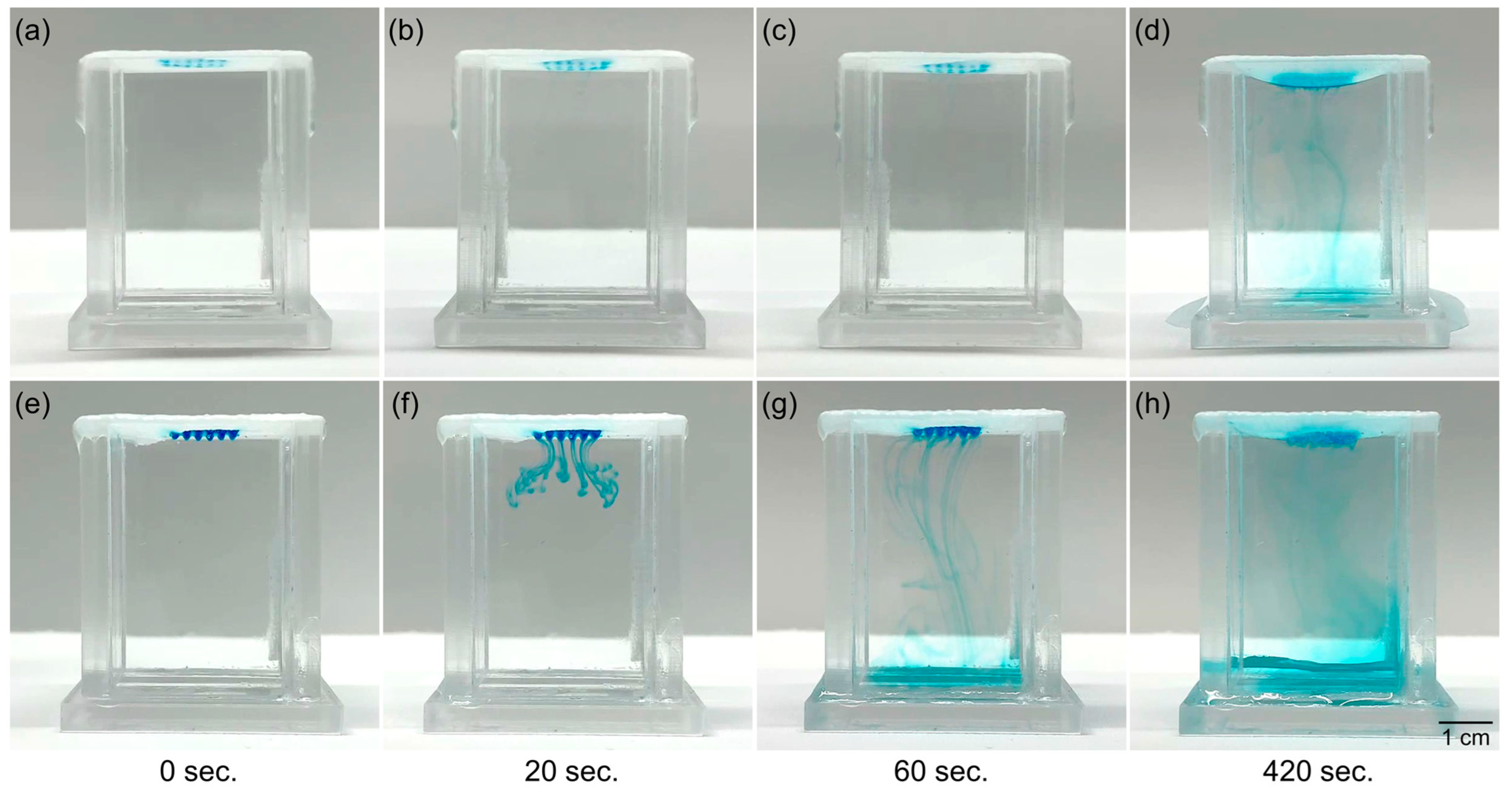
3.3. Diffusion Aspects
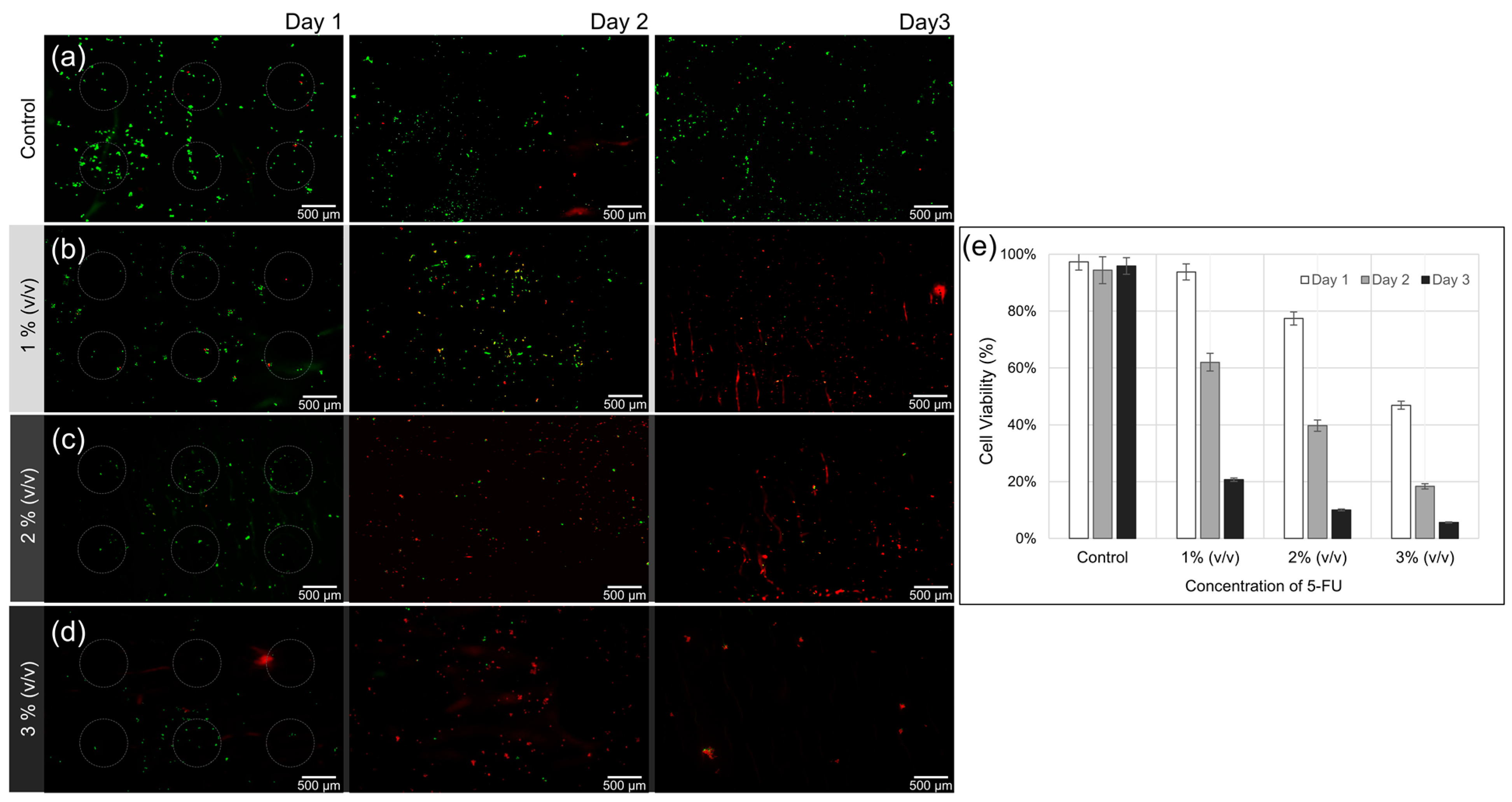
3.4. Effect of 5-FU Local Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kang, S.B.; Hao, J.; Lim, S.B.; Choi, H.S.; Kim, D.W.; Chang, H.J.; Kim, D.Y.; Jung, K.H.; Kim, T.Y.; et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2021, 6, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Sherman, E.J.; Whiting, K.; Ho, M.L.; Shaha, A.R.; Sabra, M.M.; Riaz, N.; Waldenberg, T.E.; Sabol, C.R.; Ganly, I.; et al. Intensity-modulated radiation therapy and doxorubicin in thyroid cancer: A prospective phase 2 trial. Cancer 2021, 127, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Han, S.; Park, S. Recent advances in 3D bioprinted tumor microenvironment. BioChip J. 2020, 14, 137–147. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef]
- Troendle, E.P.; Khan, A.; Searson, P.C.; Ulmschneider, M.B. Predicting drug delivery efficiency into tumor tissues through molecular simulation of transport in complex vascular networks. J. Control. Release 2018, 292, 221–234. [Google Scholar] [CrossRef]
- Jaragh-Alhadad, L.; Behbehani, H.; Karnik, S. Cancer targeted drug delivery using active low-density lipoprotein nanoparticles encapsulated pyrimidines heterocyclic anticancer agents as microtubule inhibitors. Drug Deliv. 2022, 29, 2759–2772. [Google Scholar] [CrossRef]
- Abdulridha, M.K.; Al-Marzoqi, A.H.; Al-Awsi, G.R.L.; Mubarak, S.M.H.; Heidarifard, M.; Ghasemian, A. Anticancer Effects of Herbal Medicine Compounds and Novel Formulations: A Literature Review. J. Gastrointest. Cancer 2020, 51, 765–773. [Google Scholar] [CrossRef]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.C.; Tan, L.T.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Bidwell, G.L., 3rd. Novel Drug Delivery Technologies and Targets for Renal Disease. Hypertension 2022, 79, 1937–1948. [Google Scholar] [CrossRef]
- Qamar, Z.; Qizilbash, F.F.; Iqubal, M.K.; Ali, A.; Narang, J.K.; Ali, J.; Baboota, S. Nano-Based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent Pat. Drug Deliv. Formul. 2019, 13, 246–254. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, K.; Lin, T.; Chen, W.; Yuan, A.; Wu, J.; Hu, Y.; Guo, H. Drug delivery system based on dendritic nanoparticles for enhancement of intravesical instillation. Int. J. Nanomed. 2017, 12, 7365–7374. [Google Scholar] [CrossRef]
- Rudsari, H.K.; Veletic, M.; Bergsland, J.; Balasingham, I. Targeted Drug Delivery for Cardiovascular Disease: Modeling of Modulated Extracellular Vesicle Release Rates. IEEE Trans. Nanobiosci. 2021, 20, 444–454. [Google Scholar] [CrossRef]
- Tang, H.; Xiang, D.; Wang, F.; Mao, J.; Tan, X.; Wang, Y. 5-ASA-loaded SiO2 nanoparticles-a novel drug delivery system targeting therapy on ulcerative colitis in mice. Mol. Med. Rep. 2017, 15, 1117–1122. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Shi, C.; Kaneko, T.; Chen, M. Supramolecular micellar drug delivery system based on multi-arm block copolymer for highly effective encapsulation and sustained-release chemotherapy. J. Mater. Chem. B 2019, 7, 5677–5687. [Google Scholar] [CrossRef]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted Drug Delivery: Trends and Perspectives. Curr. Drug Deliv. 2021, 18, 1435–1455. [Google Scholar] [CrossRef]
- Baveloni, F.G.; Riccio, B.V.F.; Di Filippo, L.D.; Fernandes, M.A.; Meneguin, A.B.; Chorilli, M. Nanotechnology-based Drug Delivery Systems as Potential for Skin Application: A Review. Curr. Med. Chem. 2021, 28, 3216–3248. [Google Scholar] [CrossRef] [PubMed]
- Ford Versypt, A.N.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—A review. J. Control. Release 2013, 165, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mansor, N.I.; Nordin, N.; Mohamed, F.; Ling, K.H.; Rosli, R.; Hassan, Z. Crossing the Blood-Brain Barrier: A Review on Drug Delivery Strategies for Treatment of the Central Nervous System Diseases. Curr. Drug Deliv. 2019, 16, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipour, F.; Kiani, A.; Amin, A. The High Potency of Polymeric Nanoparticles in the Drug Delivery System for Hypertension Treatment: A Systematic Review. Curr. Hypertens Rev. 2022, 18, 54–63. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Renga, G.; Santarelli, I.; D’Onofrio, F.; Bellet, M.M.; Stincardini, C.; Bartoli, A.; Costantini, C.; Romani, L.; et al. Targeted Drug Delivery Technologies Potentiate the Overall Therapeutic Efficacy of an Indole Derivative in a Mouse Cystic Fibrosis Setting. Cells 2021, 10, 1601. [Google Scholar] [CrossRef]
- Salari, N.; Faraji, F.; Torghabeh, F.M.; Faraji, F.; Mansouri, K.; Abam, F.; Shohaimi, S.; Akbari, H.; Mohammadi, M. Polymer-based drug delivery systems for anticancer drugs: A systematic review. Cancer Treat Res. Commun. 2022, 32, 100605. [Google Scholar] [CrossRef]
- Aberoumandi, S.M.; Mohammadhosseini, M.; Abasi, E.; Saghati, S.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; Davaran, S. An update on applications of nanostructured drug delivery systems in cancer therapy: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1058–1068. [Google Scholar] [CrossRef]
- Daniyal, M.; Liu, B.; Wang, W. Comprehensive Review on Graphene Oxide for Use in Drug Delivery System. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef]
- Dong, P.; Rakesh, K.P.; Manukumar, H.M.; Mohammed, Y.H.E.; Karthik, C.S.; Sumathi, S.; Mallu, P.; Qin, H.L. Innovative nano-carriers in anticancer drug delivery—A comprehensive review. Bioorg. Chem. 2019, 85, 325–336. [Google Scholar] [CrossRef]
- Kakkar, V.; Verma, M.K.; Saini, K.; Kaur, I.P. Nano Drug Delivery in Treatment of Oral Cancer, A Review of the Literature. Curr. Drug Targets 2019, 20, 1008–1017. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, W.; Che, C.; Liu, J.; Si, M.; Gong, Z.; Gao, R.; Yang, G. A Targeted Nano Drug Delivery System of AS1411 Functionalized Graphene Oxide Based Composites. Chem. Open 2021, 10, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Meschi, S.S.; Farghadan, A.; Arzani, A. Flow topology and targeted drug delivery in cardiovascular disease. J. Biomech. 2021, 119, 110307. [Google Scholar] [CrossRef]
- Pinelli, F.; Ortola, O.F.; Makvandi, P.; Perale, G.; Rossi, F. In vivo drug delivery applications of nanogels: A review. Nanomedicine 2020, 15, 2707–2727. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Patro, C.S. Solid Lipid Nanoparticles for Efficient Oral Delivery of Tyrosine Kinase Inhibitors: A Nano Targeted Cancer Drug Delivery. Adv. Pharm. Bull. 2022, 12, 298–308. [Google Scholar] [CrossRef]
- Soica, C.; Trandafirescu, C.; Danciu, C.; Muntean, D.; Dehelean, C.; Simu, G. New improved drug delivery technologies for pentacyclic triterpenes: A review. Protein Pept. Lett. 2014, 21, 1137–1145. [Google Scholar] [CrossRef]
- Gorantla, S.; Batra, U.; Puppala, E.R.; Waghule, T.; Naidu, V.G.M.; Singhvi, G. Emerging trends in microneedle-based drug delivery strategies for the treatment of rheumatoid arthritis. Expert Opin. Drug Deliv. 2022, 19, 395–407. [Google Scholar] [CrossRef]
- Gadzinski, P.; Froelich, A.; Wojtylko, M.; Bialek, A.; Krysztofiak, J.; Osmalek, T. Microneedle-based ocular drug delivery systems—Recent advances and challenges. Beilstein J. Nanotechnol. 2022, 13, 1167–1184. [Google Scholar] [CrossRef]
- Nazary Abrbekoh, F.; Salimi, L.; Saghati, S.; Amini, H.; Fathi Karkan, S.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390. [Google Scholar] [CrossRef]
- Arauna, D.; Vijayakumar, S.; Duran-Lara, E. Latest Advances in Hydrogel-Based Drug Delivery Systems for Optimization of Metabolic Syndrome Treatment. Curr. Med. Chem. 2021, 28, 6274–6286. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.; Bhosale, R.R.; Shivakumar, H.G.; Kulkarni, P.K. Current Perspectives on Novel Drug Delivery Systems and Approaches for Management of Cervical Cancer: A Comprehensive Review. Curr. Drug Targets 2016, 17, 337–352. [Google Scholar] [CrossRef]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Hsu, X.L.; Wu, L.C.; Hsieh, J.Y.; Huang, Y.Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Sonker, M.; Bajpai, S.; Khan, M.A.; Yu, X.; Tiwary, S.K.; Shreyash, N. Review of Recent Advances and Their Improvement in the Effectiveness of Hydrogel-Based Targeted Drug Delivery: A Hope for Treating Cancer. ACS Appl. Bio. Mater. 2021, 4, 8080–8109. [Google Scholar] [CrossRef]
- Villalba-Rodriguez, A.M.; Parra-Saldivar, R.; Ahmed, I.; Karthik, K.; Malik, Y.S.; Dhama, K.; Iqbal, H.M.N. Bio-Inspired Biomaterials and their Drug Delivery Perspectives—A Review. Curr. Drug Metab. 2017, 18, 893–904. [Google Scholar] [CrossRef]
- Wani, S.U.D.; Gautam, S.P.; Qadrie, Z.L.; Gangadharappa, H.V. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems—A review. Int. J. Biol. Macromol. 2020, 163, 2145–2161. [Google Scholar] [CrossRef]
- Gao, N.; You, H. Recent applications of point-of-care devices for glucose detection on the basis of stimuli-responsive volume phase transition of hydrogel. BioChip J. 2021, 15, 23–41. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Wu, Z.; Xu, X.; Ma, H.; Hou, J.; Xu, Q.; Yang, R.; Zhang, K.; Zhang, M. Robust PEDOT: PSS-Based hydrogel for highly efficient interfacial solar water purification. Chem. Eng. J. 2022, 442, 136284. [Google Scholar] [CrossRef]
- Abbasalizadeh, F.; Alizadeh, E.; Bagher Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Anticancer Effect of Alginate-chitosan Hydrogel Loaded with Curcumin and Chrysin on Lung and Breast Cancer Cell Lines. Curr. Drug Deliv. 2022, 19, 600–613. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Dulong, V.; Le Cerf, D.; Cazin, L.; Lamacz, M.; Vannier, J.P. Hyaluronan hydrogel: An appropriate three-dimensional model for evaluation of anticancer drug sensitivity. Acta Biomater. 2008, 4, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Xu, H.; Zhao, Y.; Li, Z.; Li, J.; Wang, H.; Zhuge, D.; Guo, X.; Xu, H.; et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 2018, 8, 4429–4446. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Ondeck, M.G.; Engler, A.J. Mechanical Characterization of a Dynamic and Tunable Methacrylated Hyaluronic Acid Hydrogel. J. Biomech. Eng. 2016, 138, 021003. [Google Scholar] [CrossRef]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 279–291. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft. Matter. 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lee, S.Y.; Kim, S.; Koo, J.S.; Seo, J.H.; Jeong, D.I.; Hwang, C.; Lee, J.; Cho, H.J. Selenium and dopamine-crosslinked hyaluronic acid hydrogel for chemophotothermal cancer therapy. J. Control. Release 2020, 324, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.; Choi, W.; Kim, J.; Yu, H.-Y.; Choi, N.; Cho, I.-J. Fabrication of multi-layered macroscopic hydrogel scaffold composed of multiple components by precise control of UV energy. BioChip J. 2018, 12, 280–286. [Google Scholar] [CrossRef]
- Yi, M.H.; Lee, J.E.; Kim, C.B.; Lee, K.W.; Lee, K.H. Locally Controlled Diffusive Release of Bone Morphogenetic Protein-2 Using Micropatterned Gelatin Methacrylate Hydrogel Carriers. BioChip J. 2020, 14, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T. Cellular Biophysics: Transport; MIT Press: Cambridge, UK, 1996. [Google Scholar]
- Pal, P.; Pandey, J.P.; Sen, G. Sesbania gum based hydrogel as platform for sustained drug delivery: An ‘in vitro’ study of 5-Fu release. Int. J. Biol. Macromol. 2018, 113, 1116–1124. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, C.; Yang, L.; Wu, Q.; Shi, S.; Shi, H.; Qian, Z.; Wei, Y. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer 2010, 10, 402. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, S.; Wu, M.; Cui, H.; Wu, R.; Chen, S.; Xu, C.; Lu, X.; Feng, S. Self-assembling hydrogel loaded with 5-FU PLGA microspheres as a novel vitreous substitute for proliferative vitreoretinopathy. J. Biomed. Mater. Res. A 2020, 108, 2435–2446. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Lopez-Cervantes, M.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Li, W.; Hu, Y.; Shi, L.; Zhang, X.; Xiong, L.; Zhang, W.; Ullah, I. Electrospinning of Polycaprolactone/Pluronic F127 dissolved in glacial acetic acid: Fibrous scaffolds fabrication, characterization and in vitro evaluation. J. Biomater. Sci. Polym. Ed. 2018, 29, 1155–1167. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Ribeiro, M.E.; Ricardo, N.M.; Souza, T.V.; Moura, C.L.; Chaibundit, C.; Yeates, S.G.; Nixon, K.; Attwood, D. The effect of water-soluble polymers, PEG and PVP, on the solubilisation of griseofulvin in aqueous micellar solutions of Pluronic F127. Int. J. Pharm. 2011, 421, 252–257. [Google Scholar] [CrossRef]
- Quintans-Junior, L.J.; Brito, R.G.; Quintans, J.S.S.; Santos, P.L.; Camargo, Z.T.; Barreto, P.A.; Arrigoni-Blank, M.F.; Lucca-Junior, W.; Scotti, L.; Scotti, M.T.; et al. Nanoemulsion Thermoreversible Pluronic F127-Based Hydrogel Containing Hyptis pectinata (Lamiaceae) Leaf Essential Oil Produced a Lasting Anti-hyperalgesic Effect in Chronic Noninflammatory Widespread Pain in Mice. Mol. Neurobiol. 2018, 55, 1665–1675. [Google Scholar] [CrossRef]
- Rill, R.; Liu, Y.; Ramey, B.; Van Winkle, D.; Locke, B. Capillary gel electrophoresis of nucleic acids in pluronic F127 copolymer liquid crystals. Chromatographia 1999, 49, S65–S71. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Ren, T.; Gong, H.; Gou, J.; Zhang, Y.; Cai, C.; Tang, X. Effects of Pluronic F127-PEG multi-gel-core on the release profile and pharmacodynamics of Exenatide loaded in PLGA microspheres. Colloids Surf. B Biointerfaces 2016, 147, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yuan, S.; Hao, J.; Fang, X. Mechanism of inhibition of P-glycoprotein mediated efflux by Pluronic P123/F127 block copolymers: Relationship between copolymer concentration and inhibitory activity. Eur. J. Pharm. Biopharm. 2013, 83, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, C.; Wei, F.; Lv, H. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Shams Ardekani, M.R.; Sharifzadeh, M.; Khanavi, M. Preparation of Polyurethane/Pluronic F127 Nanofibers Containing Peppermint Extract Loaded Gelatin Nanoparticles for Diabetic Wounds Healing: Characterization, In Vitro, and In Vivo Studies. Evid. Based Complement. Altern. Med. 2021, 2021, 6646702. [Google Scholar] [CrossRef]
- Saad, N.M.; Zubir, S.A. Palm kernel oil polyol-based polyurethane as shape memory material: Effect of polyol molar ratio. J. Phys. Sci. 2019, 30, 77–89. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, Y.H.; Liu, C.J.; Chung, R.J. Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with beta-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery. Pharmaceutics 2018, 10, 37. [Google Scholar] [CrossRef]
- Rodrigues, A.; Brito, A.; Janknecht, P.; Proenca, M.F.; Nogueira, R. Quantification of humic acids in surface water: Effects of divalent cations, pH, and filtration. J. Environ. Monit. 2009, 11, 377–382. [Google Scholar] [CrossRef]



| Polymer | Solvent | Concentration | Total Volume |
|---|---|---|---|
| Polyurethane (PU) | Dimethylformamide:Tetrahydrofuran = 1:1.5 (v/v) | 10% (w/v) | 10 mL |
| PU-Pluronic F-127 (PU-PF) | PU: 10% (w/v) PU-PF: 10% (w/v) | 5 mL | |
| Voltage | Tip-to-collector distance | Flow rate | Needle gauge |
| 13.5 kV | 40 cm | 0.4 mL/h | 23 G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Lee, S.-M.; Kim, C.-B.; Lee, K.-H. 5-Fluorouracil-Immobilized Hyaluronic Acid Hydrogel Arrays on an Electrospun Bilayer Membrane as a Drug Patch. Bioengineering 2022, 9, 742. https://doi.org/10.3390/bioengineering9120742
Lee J-E, Lee S-M, Kim C-B, Lee K-H. 5-Fluorouracil-Immobilized Hyaluronic Acid Hydrogel Arrays on an Electrospun Bilayer Membrane as a Drug Patch. Bioengineering. 2022; 9(12):742. https://doi.org/10.3390/bioengineering9120742
Chicago/Turabian StyleLee, Ji-Eun, Seung-Min Lee, Chang-Beom Kim, and Kwang-Ho Lee. 2022. "5-Fluorouracil-Immobilized Hyaluronic Acid Hydrogel Arrays on an Electrospun Bilayer Membrane as a Drug Patch" Bioengineering 9, no. 12: 742. https://doi.org/10.3390/bioengineering9120742
APA StyleLee, J.-E., Lee, S.-M., Kim, C.-B., & Lee, K.-H. (2022). 5-Fluorouracil-Immobilized Hyaluronic Acid Hydrogel Arrays on an Electrospun Bilayer Membrane as a Drug Patch. Bioengineering, 9(12), 742. https://doi.org/10.3390/bioengineering9120742