Stem Cell- and Cell-Based Therapies for Ischemic Stroke
Abstract
1. Introduction
Search Strategy and Selection Criteria
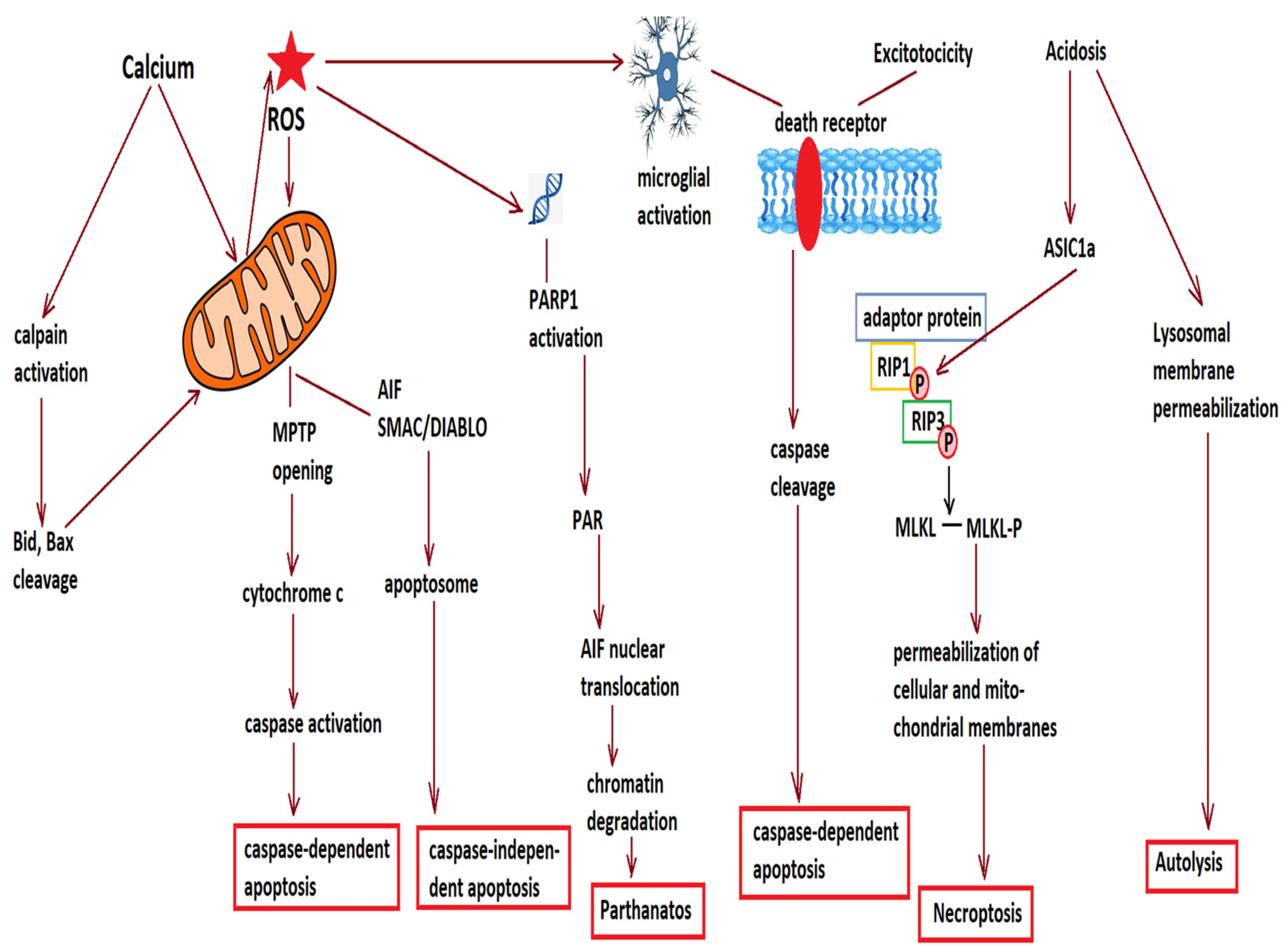
2. Cell Loss after Acute Ischemic Stroke
3. Stem Cell Therapies for Ischemic Stroke
3.1. Types of Stem Cells
3.1.1. Neural Stem Cells
3.1.2. Mesenchymal Stem Cells
3.1.3. Cell-Derived Vesicles
3.2. Mechanisms Involved in the Therapeutic Effects of Stem Cells
3.2.1. Modulation of the Immune Response
3.2.2. Cell Replacement and the Homing of Transplanted Stem Cells
3.2.3. Establishment of Neuron Polarity and Cell Division
3.2.4. Vascular Regeneration
3.2.5. Neuroregeneration and Neurite Growth
3.2.6. Myelination
3.2.7. Synaptic Rewiring and Remodeling of Brain Circuits
3.3. Stem Cells in the Experimental Therapy of Cerebral Ischemia
3.3.1. In Vitro Experimental Studies
3.3.2. Studies on Stem Cell Therapy after Ischemic Stroke in Animal Models
3.3.3. Stem Cell Therapies in Clinical Trials for Ischemic Stroke
3.3.4. Extracellular Vesicles and Exosomes for Ischemic Stroke
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L. Anthology of stroke epidemiology in the 20th and 21st centuries: Assessing the past, the present, and envisioning the future. J. Stroke 2019, 14, 223–237. [Google Scholar] [CrossRef]
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global Stroke Statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, I.A. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J. Integr. Neurosci. 2021, 20, 727–744. [Google Scholar]
- Herpich, F.; Rincon, F. Management of acute ischemic stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- Shafie, M.; Yu, W. Recanalization therapy for acute ischemic stroke with large vessel occlusion: Where we are and what comes next? Transl. Stroke Res. 2021, 12, 369–381. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal cell death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Chu, X.; Fu, X.; Zou, L.; Qi, C.; Li, Z.; Rao, Y.; Ma, K. Oncosis, the possible death pathway in astrocytes after focal cerebral ischemia. Brain Res. 2007, 1149, 157–164. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, A.I. Oxidative stress in ischemia/reperfusion injuries following acute ischemic stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: From pathophysiology to therapeutic strategies. Int. J. Mol. Sci. 2022, 23, 14. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Declerq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Wang, J.J.; Huang, Y.; Liu, F.; Zeng, W.Z.; Li, Y.; Xiong, Z.G.; Zhu, M.X.; Xu, T.L. Tissue acidosis induces neuronal necroptosis via ASIC1a channel independent of its ionic conduction. Elife 2015, 4, e14128. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Kim, N.S.; Yu, S.W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar] [CrossRef]
- Yamashima, T.; Oikawa, S. The role of lysosmal rupture in neuronal death. Prog. Neurobiol. 2009, 89, 343–358. [Google Scholar] [CrossRef]
- Léveillé, F.; Papadia, S.; Fricker, M.; Bell, K.F.; Soriano, F.X.; Martel, M.A.; Puddifoot, C.; Habel, M.; Wyllie, D.J.; Ikonomidou, C.; et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010, 30, 2623–2635. [Google Scholar] [CrossRef]
- Sharp, F.R. Transplants for stroke patients? Ann. Neurol. 1993, 34, 322–323. [Google Scholar] [CrossRef]
- Borlongan, C.V. Concise review: Stem cell therapy for stroke patients: Are we there yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef]
- Surugiu, R.; Olaru, A.; Hermann, D.M.; Glavan, D.; Catalin, B.; Popa-Wagner, A. Recent advances in mono- and combined stem cell therapies of stroke in animal models and humans. Int. J. Mol. Sci. 2019, 20, 6029. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Sajadimajd, S.; Jafari, S.; Izadi, Z.; Sarvari, S.; Sharifi, M.; Falahati, M.; Moakedi, F.; Muganda, W.C.A.; Müller, M.; et al. Novel therapeutic strategies for Alzheimer’s disease: Implications from cell-based therapy and nanotherapy. Nanomedicine 2020, 24, 102149. [Google Scholar] [CrossRef]
- Svendsen, C.N.; ter Borg, M.G.; Armstrong, R.J.; Rosser, A.E.; Chandran, S.; Ostenfeld, T.; Caldwell, M.A. A new method for the rapid and long-term growth of human neural precursor stem cells. J. Neurosci. Methods 1998, 85, 141–152. [Google Scholar] [CrossRef]
- Conti, L.; Pollard, S.M.; Gorba, T.; Reitano, E.; Toselli, M.; Biella, G.; Sun, Y.; Sanzone, S.; Ying, Q.L.; Cattaneo, E.; et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005, 3, e283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pollard, S.; Conti, L.; Toselli, M.; Biella, G.; Parkin, G.; Willatt, L.; Falk, A.; Cattaneo, E.; Smith, A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol. Cell. Neurosci. 2008, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Golas, M.M. Human cellular models of medium spiny neuron development and Huntington disease. Life Sci. 2018, 209, 179–196. [Google Scholar] [PubMed]
- Jiao, Y.; Liu, Y.W.; Chen, W.G.; Liu, J. Neuroregeneration and functional recovery after stroke: Advancing neural stem cell therapy toward clinical application. Neural Regen. Res. 2021, 16, 80–92. [Google Scholar] [PubMed]
- Morizane, A.; Kikuchi, T.; Hayashi, T.; Mizuma, H.; Takara, S.; Doi, H.; Mawatari, A.; Glasser, M.F.; Shiina, T.; Ishigaki, H.; et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 2017, 8, 385. [Google Scholar]
- Addington, C.P.; Dharmawaj, S.; Heffernan, J.M.; Sirianni, R.W.; Stabenfeldt, S.E. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2017, 60–61, 206–216. [Google Scholar] [CrossRef]
- Imitola, J.; Raddassi, K.; Park, K.I.; Mueller, F.J.; Nieto, M.; Teng, Y.D.; Frenkel, D.; Li, J.; Sidman, R.L.; Walsh, C.A.; et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 18117–18122. [Google Scholar] [CrossRef]
- Baker, E.W.; Kinder, H.A.; West, F.D. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2018, 9, e01214. [Google Scholar] [CrossRef]
- Anderson, L.; Burnstein, R.M.; He, X.; Luce, R.; Furlong, R.; Foltynie, T.; Sykacek, P.; Menon, D.K.; Caldwell, M.A. Gene expression changes in long term expanded human neural progenitor cells passaged by chopping lead to loss of neurogenic potential In Vivo. Exp. Neurol. 2007, 204, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Sinden, J.D.; Hicks, C.; Stroemer, P.; Vishnubhatla, I.; Corteling, R. Human neural stem cell therapy for chronic ischemic stroke: Charting progress from laboratory to patients. Stem Cells Dev. 2017, 26, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Reuss, J.L.; Liu, N.; Wu, C.; Li, J.; Xu, S.; Wang, F.; Hazel, T.G.; Cunningham, M.; et al. Stable intracerebral transplantation of neural stem cells for the treatment of paralysis due to ischemic stroke. Stem Cells Transl. Med. 2019, 8, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Jurcău, M.C.; Andronie-Cioara, F.L.; Jurcău, A.; Marcu, F.; Ţiț, D.M.; Pașcalău, N.; Nistor-Cseppentö, D.C. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants 2022, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef]
- Karow, M.; Camp, J.G.; Falk, S.; Gerber, T.; Pataskar, A.; Gac-Santel, M.; Kageyama, J.; Brazovskaja, A.; Garding, A.; Fan, W.; et al. Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat. Neurosci. 2018, 21, 932–940. [Google Scholar] [CrossRef]
- An, N.; Xu, H.; Gao, W.Q.; Yang, H. Direct conversion of somatic cells into induced neurons. Mol. Neurobiol. 2018, 55, 642–651. [Google Scholar] [CrossRef]
- Kondziolka, D.; Wechsler, L.; Goldstein, S.; Meltzer, C.; Thulborn, K.R.; Gebel, J.; Jannetta, P.; DeCesare, S.; Elder, E.M.; McGrogan, M.; et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000, 55, 565–569. [Google Scholar] [CrossRef]
- Gnecchi, M.; Melo, L.G. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009, 482, 281–294. [Google Scholar] [PubMed]
- Yasuhara, T.; Hara, K.; Maki, M.; Mays, R.W.; Deans, R.J.; Hess, D.C.; Carroll, J.E.; Borlongan, C.V. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J. Cereb. Blood Flow Metab. 2008, 28, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Matsukawa, N.; Hara, K.; Maki, M.; Ali, M.M.; Yu, S.J.; Bae, E.; Yu, G.; Xu, L.; McGrogan, M.; et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 2009, 18, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Morita, T.; Niizuma, K.; Kushida, Y.; Kuroda, Y.; Wakao, S.; Sakata, H.; Matsuzaka, Y.; Mushiake, H.; Tominaga, T.; et al. Transplantation of unique subpopulation of fibroblasts, Muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 2016, 34, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Haller, E.; Lin, R.; Borlongan, C.V. Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity towards BBB repair in ischemic stroke rats. Stem Cells 2017, 35, 1246–1258. [Google Scholar] [CrossRef]
- Li, Z.; Ye, H.; Cai, X.; Sun, W.; He, B.; Yang, Z.; Xu, P. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Brain Res. Bull. 2019, 153, 324–333. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takagi, T.; Kuramoto, Y.; Tatebayashi, K.; Shirakawa, M.; Yamahara, K.; Doe, N.; Yoshimura, S. Intravenous administration of human amniotic mesenchymal stem cells in the subacute phase of cerebral infarction in a mouse model ameliorates neurological disturbance by suppressing blood brain barrier disruption and apoptosis via immunomodulation. Cell Transplant. 2021, 30, 9636897211024184. [Google Scholar] [CrossRef]
- Namioka, T.; Namioka, A.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Nakazaki, M.; Onodera, R.; Suzuki, J.; Sasaki, Y.; Nagahama, H.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a rat model of chronic cerebral infarction. J. Neurosurg. 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Daniele, C.; Gamez, C.; Medina Balbuena, S.; Pastene, D.O.; Nardozi, D.; Brenna, C.; Yard, B.; Gretz, N.; Bieback, K. Stem/stromal cells for treatment of kidney injuries with focus on preclinical models. Front. Med. (Lausanne) 2018, 5, 179. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, L. Ischemic brain extracts induce human marrow stromal cell growth factor production. Neuropathology 2002, 22, 275–279. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Y.; Li, D.; Gou, H.F.; Wang, W. Improved therapeutic potential of MSCs by genetic modification. Gene Ther. 2018, 25, 538–547. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.; Li, W.; Tang, G.; Ma, Y.; Liu, Y.; Yuan, F.; Zhang, Z.; Yang, G.Y.; Wang, Y. CXCL12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res. Ther. 2018, 9, 139. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Shen, L.; Ding, W.; Chen, X.; Wu, E.; Cai, K.; Wang, G. Hypoxic preconditioning augments the therapeutic efficacy of bone marrow stromal cells in a rat ischemic stroke model. Cell. Mol. Neurobiol. 2017, 37, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.; Yousefi, M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. J. Cell. Physiol. 2019, 235, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.J.; Gu, L.; Sims, B.; Matthews, Q.L. Exosome biogenesis and biological function in response to viral infections. Open Virol. J. 2018, 12, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflammation 2019, 16, 178. [Google Scholar] [CrossRef]
- Avena-Koenigsberger, A.; Misic, B.; Sporns, O. Communication dynamics in complex brain networks. Nat. Rev. Neurosci. 2017, 19, 17–33. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Bähr, M.; Giebel, B.; Hermann, D.M. Immunological and non-immunological effects of stem cell-derived extracellular vesicles on the ischaemic brain. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418789326. [Google Scholar] [CrossRef]
- Buller, B.; Chopp, M.; Ueno, Y.; Zhang, L.; Zhang, R.L.; Morris, D.; Zhang, Y.; Zhang, Z.G. Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Glia 2012, 60, 1906–1914. [Google Scholar] [CrossRef]
- Zhang, Y.; Ueno, Y.; Liu, X.L.; Buller, B.; Wang, X.; Chopp, M.; Zhang, Z.G. The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J. Neurosci. 2013, 33, 6885–6894. [Google Scholar] [CrossRef] [PubMed]
- Remus, E.W.; Sayeed, I.; Won, S.; Lyle, A.N.; Stein, D.G. Progesterone protects endothelial cells after cerebrovascular occlusion by decreasing MCP-1 and CXCL1-mediated macrophage infiltration. Exp. Neurol. 2015, 271, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.-P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Huang, L.; Gonzalez, R.; Kim, H.-S.; Hamblin, M.H.; Lee, J.-P. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cell Transl. Med. 2015, 4, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, Y.-J.; Roh, J.; Kim, E.-C.; Lee, H.J.; Kim, S.U.; Yoon, B.W. Long-term effects of magnetically targeted ferumoxide-labeled human neural stem cells in focal cerebral ischemia. Cell Transplant. 2015, 24, 183–190. [Google Scholar] [CrossRef]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780–792. [Google Scholar]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, X.; Chen, L.; Li, B.; Gu, B.; Wang, H.; Wu, G.; Kong, J.; Chen, W.; Yu, Y. Interferon-γ promotes neuronal repair by transplanted neural stem cells in ischemic rats. Stem Cells Dev. 2018, 27, 355–366. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Gójska-Grymajło, A.; Zieliński, M.; Wardowska, A.; Gąsecki, D.; Pikuła, M.; Karaszewski, B. CXCR7+ and CXCR4+ stem cells and neuron specific enolase in acute ischemic stroke patients. Neurochem. Int. 2018, 120, 134–139. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, S.H.; Kim, S.U.; Yoon, B.W. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in the ischemic rat brain. Neural Regen. Res. 2016, 11, 298–304. [Google Scholar] [PubMed]
- Rahman, A.A.; Amruta, N.; Pinteaux, E.; Bix, G.J. Neurogenesis after stroke: A therapeutic perspective. Transl. Stroke Res. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lazutkin, A.; Podgorny, O.; Enikolopov, G. Modes of division and differentiation of neural stem cells. Behav. Brain Res. 2019, 374, 112118. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.G.; Subramanian, L.; Salma, J.; Kriegstein, A.R. How mechanisms of stem cell polarity shape the human cerebral cortex. Nat. Rev. Neurosci. 2022, 23, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Sekine, H.; Homma, J.; Kobayashi, T.; Kobayashi, E.; Kawamata, T.; Shimizu, T. Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J. Neurosurg. 2019, 132, 442–455. [Google Scholar] [CrossRef]
- Sakata, H.; Niizuma, K.; Wakai, T.; Narasimhan, P.; Maier, C.M.; Chan, P.H. Neural stem cells genetically modified to overexpress Cu/Zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke 2012, 43, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Stevanato, L.; Stroemer, R.P.; Tang, E.; Richardson, S.; Sinden, J.D. In Vivo and In Vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013, 22, 1541–1552. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, L.; Huang, X.; Zhao, T.; Wu, L.Y.; Liu, Z.H.; Ding, X.; Liu, S.; Wu, Y.; Zhao, Y.; et al. miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res. 2013, 11, 657–667. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, Y.; Wang, F.; Cheng, X.; Zhao, T.; Zhao, Y.; Fan, M.; Zhu, L. Neural progenitor cells-secreted exosomal miR-210 induced by hypoxia influences cell viability. Neuroreport 2020, 31, 798–805. [Google Scholar] [CrossRef]
- Cohen, E.J.; Quarta, E.; Bravi, R.; Granato, A.; Miniacchi, D. Neural plasticity and network remodeling: From concepts to pathology. Neuroscience 2017, 344, 326–345. [Google Scholar] [CrossRef]
- Sherman, S.P.; Bang, A.G. High-throughput screen for compounds that modulate neurite growth of human induced pluripotent stem cell-derived neurons. Dis. Model Mech. 2018, 11, dmm031906. [Google Scholar] [CrossRef]
- Zhao, H.; Zuo, X.; Ren, L.; Li, Y.; Tai, H.; Du, J.; Xie, X.; Zhang, X.; Han, Y.; Wu, Y.; et al. Combined use of bFGF/EGF and all-trans-retinoic acid cooperatively promotes neuronal differentiation and neurite outgrowth in neural stem cells. Neurosci. Lett. 2019, 690, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bierlein De La Rosa, M.; Sharma, A.D.; Mallapragada, S.K.; Sakaguchi, D.S. Transdifferentiation of brain-derived neurotrophic factor (BDNF)-secreting mesenchymal stem cells significantly enhance BDNF secretion and Schwann cell marker proteins. J. Biosci. Bioeng. 2017, 124, 572–582. [Google Scholar] [CrossRef]
- Kondiles, B.R.; Horner, P.J. Myelin plasticity, neural activity, and traumatic neural injury. Dev. Neurobiol. 2018, 78, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.H.; Ravanelli, A.M.; Schwindt, R.; Scott, E.K.; Appel, B. Neuronal activity biases axon selection for myelination In Vivo. Nat. Neurosci. 2015, 18, 683–689. [Google Scholar] [CrossRef]
- Letellier, M.; Levet, F.; Thoumine, O.; Goda, Y. Differential role of pre- and postsynaptic neurons in the activity-dependent control of synaptic strengths across dendrites. PLoS Biol. 2019, 17, e2006223. [Google Scholar] [CrossRef]
- Tornero, D.; Tsupykov, O.; Granmo, M.; Rodriguez, C.; Gronning-Hansen, M.; Thelin, J.; Smozhanik, E.; Laterza, C.; Wattananit, S.; Ge, R.; et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain 2017, 140, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Li, L.; Gou, X.; Xu, H.; Zhao, Z.; Wang, Q.; Xu, L. TAT-PEP enhanced neurobehavioral functional recovery by facilitating axonal regeneration and corticospinal tract projection after stroke. Mol. Neurobiol. 2018, 55, 652–667. [Google Scholar] [CrossRef]
- Huang, P.; Gebhart, N.; Richelson, E.; Brott, T.G.; Meschia, J.F.; Zubair, A.C. Mechanism of mesenchymal stem-cell induced neuron recovery and anti-inflammation. Cythotherapy 2014, 16, 1336–1344. [Google Scholar] [CrossRef]
- Egashira, Y.; Sugitani, S.; Suzuki, Y.; Mishiro, K.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res. 2012, 146, 87–95. [Google Scholar] [CrossRef]
- Jingli, Y.; Jing, W.; Saeed, Y. Ischemic brain stroke and mesenchymal stem cells: An overview of molecular mechanisms and therapeutic potential. Stem Cells Int. 2022, 2022, 5930244. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Datta, S.R.; Greenberg, M.E. Transcription-dependent and –independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001, 11, 297–305. [Google Scholar] [CrossRef]
- Huang, W.; Lv, B.; Zeng, H.; Shi, D.; Liu, Y.; Chen, F.; Li, F.; Liu, X.; Zhu, R.; Yu, L.; et al. Paracrine factors secreted by MSCs promote astrocyte survival associated with GFAP downregulation after ischemic stroke via p38 MAPK and JNK. J. Cell. Physiol. 2015, 230, 2461–2475. [Google Scholar] [CrossRef]
- Jean LeBlanc, N.; Menet, R.; Picard, K.; Parent, G.; Tremblay, M.È.; ElAli, A. Canonical Wnt pathway maintains blood-brain barrier integrity upon ischemic stroke and its activation ameliorates tissue plasminogen activator therapy. Mol. Neurobiol. 2019, 56, 6521–6538. [Google Scholar] [CrossRef]
- Huang, H.; Qian, K.; Han, X.; Li, X.; Zheng, Y.; Chen, Z.; Huang, X.; Chen, H. Intraparenchymal neural stem/progenitor cell transplantation for ischemic stroke animals: A meta-analysis and systematic review. Stem Cell Int. 2018, 2018, 4826407. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, B.; Chhatbar, P.Y.; Dong, Y.; Alawieh, A.; Lowe, F.; Hu, X.; Feng, W. Mesenchymal stem cell therapy in stroke: A systematic review of literature in pre-clinical and clinical research. Cell Transplant. 2018, 27, 1723–1730. [Google Scholar] [CrossRef]
- Sakata, H.; Niizuma, K.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Narasimhan, P.; Maier, C.M.; Nishiyama, Y.; Chan, P.H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012, 32, 3462–3473. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Gu, Y.; Guo, X. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Sci Rep. 2016, 6, 32291. [Google Scholar] [CrossRef]
- Lees, J.S.; Sena, E.S.; Egan, K.J.; Antonic, A.; Koblar, S.A.; Howells, D.W.; Macleod, M.R. Stem cell-based therapies for experimental stroke: A systematic review and meta-analysis. Int. J. Stroke 2012, 7, 582–588. [Google Scholar] [CrossRef]
- Mochizuki, N.; Takagi, N.; Kurokawa, K.; Onozato, C.; Moriyama, Y.; Tanonaka, K.; Takeo, S. Injection of neural progenitor cells improved learning and memory dysfunction after cerebral ischemia. Exp. Neurol. 2008, 211, 194–202. [Google Scholar] [CrossRef]
- Somaa, F.A.; Wang, T.Y.; Niclis, J.C.; Bruggeman, K.F.; Kauhausen, J.A.; Guo, H.; McDougall, S.; Williams, R.J.; Nisbet, D.R.; Thompson, L.H.; et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017, 20, 1964–1977. [Google Scholar] [CrossRef]
- Mack, G.S. ReNeuron and StemCells get green light for neural stem cell trials. Nat. Biotechnol. 2011, 9, 95–96. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, Y.; Zeng, H.; Chen, G. Progress in mesenchymal stem cell therapy for ischemic stroke. Stem Cells Int. 2021, 2021, 9923566. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Cui, L.L.; Kerkelä, E.; Bakreen, A.; Nitzsche, F.; Andrzejewska, A.; Nowakowski, A.; Janowski, M.; Walczak, P.; Boltze, J.; Lukomska, B.; et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res. Ther. 2015, 6, 11. [Google Scholar] [CrossRef]
- Donega, V.; van Velthoven, C.T.; Nijboer, C.H.; van Bel, F.; Kas, M.J.; Kavelaars, A.; Heijnen, C.J. Intranasal mesenchymal stem cell treatment for neonatal brain damage: Long-term cognitive and sensorimotor improvement. PLoS ONE. 2013, 8, e51253. [Google Scholar] [CrossRef]
- Lalu, M.M.; Montroy, J.; Dowlatshahi, D.; Hutton, B.; Juneau, P.; Wesch, N.; Zhang, S.Y.; McGinn, R.; Corbett, D.; Stewart, D.J.; et al. From the lab to patients: A systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Transl. Stroke Res. 2020, 11, 345–364. [Google Scholar] [CrossRef]
- Clinicaltrials.Gov, Homepage on the Internet. Available online: ClinicalTrials.gov (accessed on 15 October 2022).
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell 2018, 22, 941–950. [Google Scholar] [CrossRef]
- Meneghini, V.; Frati, G.; Sala, D.; De Cicco, S.; Luciani, M.; Cavazzini, C.; Paulis, M.; Mentzen, W.; Morena, F.; Gianelli, S.; et al. Generation of human induced pluripotent stem cell-derived bona fide neural stem cells for ex vivo gene therapy of metachromatic leukodystrophy. Stem Cells Transl. Med. 2017, 6, 352–368. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, Y.; Li, J.; Xu, Y.; Zhou, J.; Qiu, C.; Zhang, S.; He, Y.; Yu, L. Therapeutic effect of biomimetic scaffold loaded with human amniotic epithelial cell-derived neural-like cells for spinal cord injury. Bioengineering 2022, 9, 535. [Google Scholar] [CrossRef]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bentley, P.; Hamady, M.; Marley, S.; Davis, J.; Shlebak, A.; Nicholls, J.; Williamson, D.A.; Jensen, S.L.; Gordon, M.; et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl. Med. 2014, 3, 1322–1330. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Oxidative stress in the pathogenesis of Alzheimer’s disease and cerebrovascular disease with therapeutic implications. CNS Neurol. Disord. Drug Targets 2020, 19, 94–108. [Google Scholar]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef]
- Frolova, L.; Li, I.T.S. Targeting capabilities of native and bioengineered extracellular vesicles for drug delivery. Bioengineering 2022, 9, 496. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma In Vitro and In Vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Suire, C.N.; Hade, M.D. Extracellular vesicles in type 1 diabetes: A versatile tool. Bioengineering 2022, 9, 105. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Labriola, N.R.; Azagury, A.; Gutierrez, R.; Mathiowitz, E.; Darling, E.M. Concise review: Fabrication, customization, and application of cell mimicking microparticles in stem cell science. Stem Cells Transl. Med. 2018, 7, 232–240. [Google Scholar]
- Chen, B.; Li, Q.; Zhao, B.; Wang, Y. Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Transl. Med. 2017, 6, 1753–1758. [Google Scholar] [CrossRef]
- Incontri Abraham, D.; Gonzales, M.; Ibarra, A.; Borlongan, C.V. Stand alone or join forces? Stem cell therapy for stroke. Expert Opin. Biol. Ther. 2019, 19, 25–33. [Google Scholar] [CrossRef]

| Characteristic | Possible Variants | Number of Studies |
|---|---|---|
| Stroke model | transient | 46 |
| permanent | 16 | |
| Animals used in the trial | rats | 40 |
| mice | 19 | |
| Mongolian gerbil | 2 | |
| pigs | 1 | |
| Gender | male | 54 |
| female | 1 | |
| not stated | 7 | |
| Source of NSPCs | human | 28 |
| rat | 14 | |
| mouse | 20 | |
| Use of immunosuppressors | yes | 21 |
| no | 24 | |
| not stated | 14 |
| Characteristics | Possible Variants | Number of Studies |
|---|---|---|
| Stroke model | permanent | 34 |
| transient | 34 | |
| two arms | 4 | |
| Animal species | rat | 61 |
| mouse | 9 | |
| dog (Beagle) | 1 | |
| monkey (Macaca fascicularis) | 1 | |
| Type of MSCs delivered | bone marrow-derived MSCs | 62 |
| adipose tissue-derived MSCs | 2 | |
| umbilical cord- or placenta-derived MSCs | 8 | |
| Delivery route | intracerebral | 37 |
| intravenous | 26 | |
| intra-arterial | 4 | |
| intranasal | 1 | |
| mixed delivery routes | 4 |
| Trial Identifier | Phase | Status | Stem Cell Types Used | Protocol |
|---|---|---|---|---|
| Neural stem cells | ||||
| NCT01151124 (PISCES) | 1 | active, not recruiting | NSCs, CTX0E03 line | Intracerebral delivery of increasing doses 6 months to 5 years post-stroke |
| NCT02117635 (PISCES-II) | 2 | completed | Allogeneic NSCs, CTX-derived precursor cells | Intracerebral transplantation of 20 × 106 cells 2–3 months post-stroke |
| NCT04631406 | 1 | recruiting | Human ESC-derived NR1 cells | Intracerebral graft of increasing doses of cells 6 to 60 months post-stroke |
| Mesenchymal stem cells | ||||
| NCT03080571 | 1 | completed | Autologous BM-derived MSCs | Intra-arterial delivery of an unspecified number of MSCs within 15 days post-stroke |
| NCT00859014 | 1 | completed | Autologous mononuclear BM-derived stem cells | Intravenous delivery of 10 × 106 cells within 24–72 h post-stroke |
| NCT004097652 | 1 | completed | Allogeneic UC-derived MSCs | Intravenous delivery of 3 different doses of cells within 48–168 h post-stroke |
| NCT00473057 | 1 | completed | Autologous BM-MSCs | 500 × 106 cells delivered IA in up to 10 patients and IV in up to 5 patients within 3–90 days post-stroke |
| NCT04434768 | 1 | recruiting | Allogeneic UC-MSCs (UMSC01) | Two arms: intravenous or intravenous and intra-arterial delivery following thrombolysis within 36 h after stroke onset |
| NCT02397018 | 1 | completed | Allogeneic cord blood infusion | 0.5–5 × 107 cells/kg within 3–10 days post-stroke |
| NCT02433509 | 1 | recruiting | Allogeneic human UC-derived monocytes | 200–500 × 106 cells delivered as IV infusion within 10 days from stroke onset |
| NCT01297413 | 1/2 | completed | Allogeneic BM-MSCs | 0.5–1.5 × 106 cells/kg delivered IV within 6 months |
| NCT05292625 | 1/2 | recruiting | Allogeneic UC-MSCs | 1.5 × 106 cells/kg delivered IV or intrathecal in stroke patients within 24 months post-stroke, repeated after 3 months |
| NCT01287936 | 1/2 | completed | Allogeneic modified stem cells (SB623 cell line) | Three arms with doses ranging between 2.5–5 × 106 cells with stereotactic intracerebral delivery 6 to 60 months post-stroke |
| NCT02605707 | 1/2 | completed | Autologous endothelial progenitor cells | IV, 6 to 60 months post-stroke, number of cells not stated |
| NCT00535197 | 1/2 | completed | Autologous CD34+ BM-MSCs | Intra-arterial delivery into the ipsilateral MCA within 7 days post-stroke; non-specified number of cells |
| NCT04608838 (J-REPAIR) | 1/2 | completed | Allogeneic dental pulp stem cells | 1 or 3 × 108 cells delivered IV within 48 h from stroke onset |
| NCT01468064 (AMETIS) | 1/2 | completed | Autologous BM and endothelial progenitor cells (EPCs) | Either 2.5 × 106 BM-MSCs or 2.5 × 106 EPCs delivered IV within 4 weeks after stroke onset |
| NCT04590118 (ASSiST) | 1/2 | recruiting | Allogeneic human MSCs | 0.5 × 106, 1 × 106, or 2 × 106 MSCs delivered IV more than 6 months after stroke onset |
| NCT03915431 | 1/2 | recruiting | Allogeneic BM-MSCs (NCS-01) | Various number of cells delivered IV within 24 h after stroke onset |
| NCT04093336 | 1/2 | recruiting | Allogeneic human UC-MSCs | 2 × 106 cells/kg transplanted IV within 24 h post-stroke onset |
| NCT05008588 | 1/2 | recruiting | UC-MSCs + conditioned medium | Intranasal delivery of conditioned medium for 3 days followed by intraparenchymal transplant of 20 × 106 MSCs or just intraparenchymal transplant in ischemic stroke patients (time window not specified) |
| NCT04811651 | 2 | recruiting | UC-MSCs | IV delivery of 100 × 106 cells in 5 groups: between 6 and 24 h from stroke onset; 1–3 days post-stroke; 4–7 days post-stroke; 1–4 weeks post-stroke; 1–6 months post-stroke |
| NCT01678534 (AMASCIS-01 | 2 | completed | Allogeneic adipose tissue-derived MSCs | 106/kg delivered IV within 2 weeks from stroke onset |
| NCT02178657 | 2 | active, not recruiting | Autologous BM-MSCs | 2 × 106 and 5 × 106 cells/kg delivered IA within 7 days from stroke onset |
| NCT04280003 | 2 | recruiting | Allogeneic adipose tissue- derived MSCs | 106 cells/kg delivered IV within 4 days from stroke onset |
| NCT02448641 (ACTISSIMA) | 2 | completed | Modified stem cells (SB623 cell line) | Intraparenchimatous implant of 2.5 × 106 and 5 × 106 cells, 6 to 90 months post-stroke |
| NCT01501773 | 2 | completed | Autologous BM-MSCs | 30–500 × 106 mononuclear cells delivered IV within 7–30 days post-stroke |
| NCT02425670 | 2 | completed | Autologous BM-MSCs | 30–500 × 106 cells injected IV within 7–30 days from stroke onset |
| NCT03004976(CoBIS2) | 2 | completed | Allogeneic umbilical cord blood | 0.5–5 × 107 cells/kg delivered IV within 3–10 days from stroke onset |
| NCT00875654 (ISIS) | 2 | completed | Autologous MSCs | IV delivery within 6 weeks from stroke onset; number of cells not stated, 2 different doses will be used |
| NCT01436487 | 2 | completed | MULTISTEM investigational adult stem cells | Three different doses of cells delivered IV within 1–2 days from stroke onset |
| NCT02961504 (TREASURE) | 2/3 | active, not recruiting | Regenerative cell elements (HLCM051) | 1.2 × 109 cells delivered IV 18–36 h after stroke onset |
| NCT03545607 (MASTERS-2) | 3 | recruiting | Allogeneic adult stem cells (MULTISTEM) | 1.2 × 109 cells infused IV within 18–36 h after stroke onset |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor-Cseppentö, D.C.; Jurcău, M.C.; Jurcău, A.; Andronie-Cioară, F.L.; Marcu, F. Stem Cell- and Cell-Based Therapies for Ischemic Stroke. Bioengineering 2022, 9, 717. https://doi.org/10.3390/bioengineering9110717
Nistor-Cseppentö DC, Jurcău MC, Jurcău A, Andronie-Cioară FL, Marcu F. Stem Cell- and Cell-Based Therapies for Ischemic Stroke. Bioengineering. 2022; 9(11):717. https://doi.org/10.3390/bioengineering9110717
Chicago/Turabian StyleNistor-Cseppentö, Delia Carmen, Maria Carolina Jurcău, Anamaria Jurcău, Felicia Liana Andronie-Cioară, and Florin Marcu. 2022. "Stem Cell- and Cell-Based Therapies for Ischemic Stroke" Bioengineering 9, no. 11: 717. https://doi.org/10.3390/bioengineering9110717
APA StyleNistor-Cseppentö, D. C., Jurcău, M. C., Jurcău, A., Andronie-Cioară, F. L., & Marcu, F. (2022). Stem Cell- and Cell-Based Therapies for Ischemic Stroke. Bioengineering, 9(11), 717. https://doi.org/10.3390/bioengineering9110717








