Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials
Abstract
1. Introduction
2. Mechanisms of Action of Stem Cell Transplantation in Ischemic Stroke
3. Features of IA Stem Cell Transplantation
4. Cell Source/Cell Quality
5. Bystander Effects
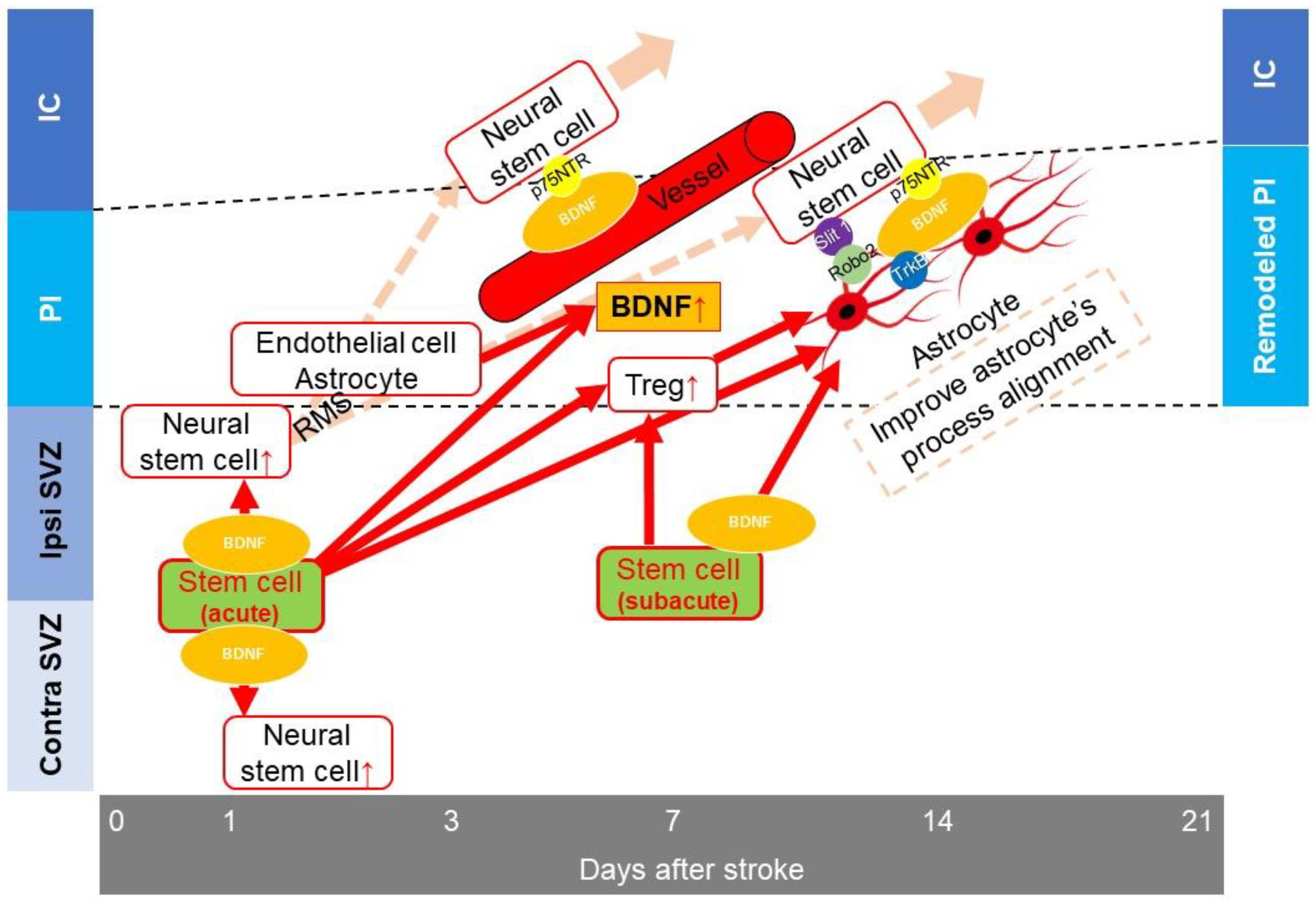
5.1. Angiogenesis
5.2. Neurogenesis
5.3. Anti-Inflammatory Processes
5.4. Enhancement of the Bystander Effect by Gene Modification
5.5. Additional Issues Related to the Bystander Effect
6. Cell Replacement
7. Clinical Trials of IA Stem Cell Transplantation in the Acute and Subacute Phases
8. Future Directions
8.1. Enhancement of Stem Cell Functionality
8.2. Enhancement of Transplanted Stem Cell Migration to an Infarct Site
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent Advances in Cell-Based Therapies for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, B.; Chakrabarti, A.; Medhi, B.; Modi, M.; Radotra, B.D.; Aggarwal, R.; Sinha, V.R. A New Therapeutic Approach for Brain Delivery of Epigallocatechin Gallate: Development and Characterization Studies. Curr. Drug Deliv. 2019, 16, 59–65. [Google Scholar] [CrossRef]
- Ogawa, K.; Kato, N.; Yoshida, M.; Hiu, T.; Matsuo, T.; Mizukami, S.; Omata, D.; Suzuki, R.; Maruyama, K.; Mukai, H.; et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J. Control Release 2022, 348, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Boncoraglio, G.B.; Ranieri, M.; Bersano, A.; Parati, E.A.; Del Giovane, C. Stem cell transplantation for ischemic stroke. Cochrane Database Syst. Rev. 2019, 5, Cd007231. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F., Jr.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients with Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar] [CrossRef]
- Ishizaka, S.; Horie, N.; Satoh, K.; Fukuda, Y.; Nishida, N.; Nagata, I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013, 44, 720–726. [Google Scholar] [CrossRef]
- Yavagal, D.R.; Lin, B.; Raval, A.P.; Garza, P.S.; Dong, C.; Zhao, W.; Rangel, E.B.; McNiece, I.; Rundek, T.; Sacco, R.L.; et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 2014, 9, e93735. [Google Scholar] [CrossRef]
- Fukuda, Y.; Horie, N.; Satoh, K.; Yamaguchi, S.; Morofuji, Y.; Hiu, T.; Izumo, T.; Hayashi, K.; Nishida, N.; Nagata, I. Intra-arterial transplantation of low-dose stem cells provides functional recovery without adverse effects after stroke. Cell Mol. Neurobiol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Horie, N.; Satoh, K.; Ishikawa, T.; Mori, T.; Maeda, H.; Fukuda, Y.; Ishizaka, S.; Hiu, T.; Morofuji, Y.; et al. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1199–1212. [Google Scholar] [CrossRef]
- Namioka, T.; Namioka, A.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Nakazaki, M.; Onodera, R.; Suzuki, J.; Sasaki, Y.; Nagahama, H.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a rat model of chronic cerebral infarction. J. Neurosurg. 2018, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Nito, C.; Sowa, K.; Suda, S.; Nishiyama, Y.; Nakamura-Takahashi, A.; Nitahara-Kasahara, Y.; Imagawa, K.; Hirato, T.; Ueda, M.; et al. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol. Ther. Methods Clin. Dev. 2017, 6, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Horie, N.; Pereira, M.P.; Niizuma, K.; Sun, G.; Keren-Gill, H.; Encarnacion, A.; Shamloo, M.; Hamilton, S.A.; Jiang, K.; Huhn, S.; et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 2011, 29, 274–285. [Google Scholar] [CrossRef]
- Uchida, H.; Niizuma, K.; Kushida, Y.; Wakao, S.; Tominaga, T.; Borlongan, C.V.; Dezawa, M. Human Muse Cells Reconstruct Neuronal Circuitry in Subacute Lacunar Stroke Model. Stroke 2017, 48, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.A.; Leonardo, C.C.; Hall, A.A.; Rowe, D.D.; Collier, L.A.; Benkovic, S.A.; Willing, A.E.; Pennypacker, K.R. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab. Brain Dis. 2012, 27, 131–141. [Google Scholar] [CrossRef]
- Du, S.; Guan, J.; Mao, G.; Liu, Y.; Ma, S.; Bao, X.; Gao, J.; Feng, M.; Li, G.; Ma, W.; et al. Intra-arterial delivery of human bone marrow mesenchymal stem cells is a safe and effective way to treat cerebral ischemia in rats. Cell Transpl. 2014, 23 (Suppl. 1), S73–S82. [Google Scholar] [CrossRef]
- Liu, H.; Honmou, O.; Harada, K.; Nakamura, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 2006, 129, 2734–2745. [Google Scholar] [CrossRef]
- Rosado-de-Castro, P.H.; Schmidt Fda, R.; Battistella, V.; Lopes de Souza, S.A.; Gutfilen, B.; Goldenberg, R.C.; Kasai-Brunswick, T.H.; Vairo, L.; Silva, R.M.; Wajnberg, E.; et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen. Med. 2013, 8, 145–155. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, Z.; Zhang, S.; Yang, H.; Zhang, X.; Pan, J.; Weng, L.; Sha, D.; Zhu, M.; Hu, X.; et al. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res. 2015, 1594, 293–304. [Google Scholar] [CrossRef]
- Chua, J.Y.; Pendharkar, A.V.; Wang, N.; Choi, R.; Andres, R.H.; Gaeta, X.; Zhang, J.; Moseley, M.E.; Guzman, R. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J. Cereb. Blood Flow Metab. 2011, 31, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Kaltwasser, B.; Teli, M.K.; Bretschneider, E.; Bähr, M.; Hermann, D.M. Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice. Cell Death Dis. 2014, 5, e1386. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Choi, S.M.; Kim, H.O.; Kim, E.H.; You, J.; Park, T.; Kim, E.; Kim, H.S. Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience 2013, 238, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.L.; Mesquita, C.T.; Felix, R.M.; Azevedo, J.C.; Barbirato, G.B.; Falcão, C.H.; Gonzalez, C.; Mendonça, M.L.; Manfrim, A.; de Freitas, G.; et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin. Nucl. Med. 2007, 32, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.A.; Martins, M.P.; Araújo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; Sartori El Ammar, J.; Machado, D.C.; Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transpl. 2012, 21 (Suppl. 1), S13–S21. [Google Scholar] [CrossRef]
- Moniche, F.; Gonzalez, A.; Gonzalez-Marcos, J.R.; Carmona, M.; Piñero, P.; Espigado, I.; Garcia-Solis, D.; Cayuela, A.; Montaner, J.; Boada, C.; et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke 2012, 43, 2242–2244. [Google Scholar] [CrossRef]
- Ghali, A.A.; Yousef, M.K.; Ragab, O.A.; ElZamarany, E.A. Intra-arterial Infusion of Autologous Bone Marrow Mononuclear Stem Cells in Subacute Ischemic Stroke Patients. Front. Neurol. 2016, 7, 228. [Google Scholar] [CrossRef]
- Bhatia, V.; Gupta, V.; Khurana, D.; Sharma, R.R.; Khandelwal, N. Randomized Assessment of the Safety and Efficacy of Intra-Arterial Infusion of Autologous Stem Cells in Subacute Ischemic Stroke. Am. J. Neuroradiol. 2018, 39, 899–904. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Ma, T.; Wang, X.; Jiang, D. Immune Tolerance of Mesenchymal Stem Cells and Induction of Skin Allograft Tolerance. Curr. Stem. Cell Res. Ther. 2017, 12, 409–415. [Google Scholar] [CrossRef]
- Casiraghi, F.; Remuzzi, G.; Abbate, M.; Perico, N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev. Rep. 2013, 9, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Casadei, R.; Frabetti, F.; Ventura, C.; Facchin, F.; Canaider, S. Sex-Specific Transcriptome Differences in Human Adipose Mesenchymal Stem Cells. Genes 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Okada, Y.; Turudome, Y.; Ushijima, K. Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Klomjit, N.; Conley, S.M.; Ostlie, M.M.; Jordan, K.L.; Lerman, A.; Lerman, L.O. Impaired immunomodulatory capacity in adipose tissue-derived mesenchymal stem/stromal cells isolated from obese patients. J. Cell Mol. Med. 2021, 25, 9051–9059. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.M.; Carballosa, C.M.; Cheung, H.S. Concise Review: The Deleterious Effects of Cigarette Smoking and Nicotine Usage and Mesenchymal Stem Cell Function and Implications for Cell-Based Therapies. Stem Cells Transl. Med. 2017, 6, 1815–1821. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Hirschi, K.K.; Rohovsky, S.A.; Beck, L.H.; Smith, S.R.; D’Amore, P.A. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 1999, 84, 298–305. [Google Scholar] [CrossRef]
- Abumiya, T.; Lucero, J.; Heo, J.H.; Tagaya, M.; Koziol, J.A.; Copeland, B.R.; del Zoppo, G.J. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 1038–1050. [Google Scholar] [CrossRef]
- Marti, H.J.; Bernaudin, M.; Bellail, A.; Schoch, H.; Euler, M.; Petit, E.; Risau, W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 2000, 156, 965–976. [Google Scholar] [CrossRef]
- Ogunshola, O.O.; Antic, A.; Donoghue, M.J.; Fan, S.Y.; Kim, H.; Stewart, W.B.; Madri, J.A.; Ment, L.R. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J. Biol. Chem. 2002, 277, 11410–11415. [Google Scholar] [CrossRef]
- Kanazawa, M.; Miura, M.; Toriyabe, M.; Koyama, M.; Hatakeyama, M.; Ishikawa, M.; Nakajima, T.; Onodera, O.; Takahashi, T.; Nishizawa, M.; et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 2017, 7, 42582. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, L.; Huang, S.; Lai, X.; Milner, R.; Li, L. Vascular expression of angiopoietin1, α5β1 integrin and tight junction proteins is tightly regulated during vascular remodeling in the post-ischemic brain. Neuroscience 2017, 362, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Huang, W.Q.; Mallah, J.; Yang, D.; Lippman, M.E.; Li, L.Y. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc. Res. 1999, 58, 224–237. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Tsang, W.; Soltanian-Zadeh, H.; Morris, D.; Zhang, R.; Goussev, A.; Powers, C.; Yeich, T.; Chopp, M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2002, 22, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Zechariah, A.; ElAli, A.; Doeppner, T.R.; Jin, F.; Hasan, M.R.; Helfrich, I.; Mies, G.; Hermann, D.M. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke 2013, 44, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Li, P.C.; Wu, J.H.; Haslam, J.A.; Mao, L.; Xia, Y.P.; He, Q.W.; Wang, X.X.; Lei, H.; Lan, X.L.; et al. Sema3E/PlexinD1 inhibition is a therapeutic strategy for improving cerebral perfusion and restoring functional loss after stroke in aged rats. Neurobiol. Aging 2018, 70, 102–116. [Google Scholar] [CrossRef]
- Vazquez-Liebanas, E.; Nahar, K.; Bertuzzi, G.; Keller, A.; Betsholtz, C.; Mäe, M.A. Adult-induced genetic ablation distinguishes PDGFB roles in blood-brain barrier maintenance and development. J. Cereb. Blood Flow Metab. 2022, 42, 264–279. [Google Scholar] [CrossRef]
- Yang, S.; Jin, H.; Zhu, Y.; Wan, Y.; Opoku, E.N.; Zhu, L.; Hu, B. Diverse Functions and Mechanisms of Pericytes in Ischemic Stroke. Curr. Neuropharmacol. 2017, 15, 892–905. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Carmeliet, P.; Collen, D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. N. Y. Acad. Sci. 2000, 902, 249–262; discussion 262–264. [Google Scholar] [CrossRef]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Wang, X.; Wang, Z.Y.; Wang, Y.Z.; Chen, L.W.; Luo, Z.J. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J. Neurosci. Res. 2013, 91, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, R.; Wu, L.; Chen, Q.; Hu, A.; Zhang, T.; Wang, Z.; Zhu, X. The Regulatory Mechanism of Neurogenesis by IGF-1 in Adult Mice. Mol. Neurobiol. 2015, 51, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Bullock, M.R.; Altememi, N.; Zhou, Z.; Hagood, S.; Rolfe, A.; McGinn, M.J.; Hamm, R.; Colello, R.J. The effect of epidermal growth factor in the injured brain after trauma in rats. J. Neurotrauma 2010, 27, 923–938. [Google Scholar] [CrossRef]
- Osredkar, D.; Sall, J.W.; Bickler, P.E.; Ferriero, D.M. Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol. Dis. 2010, 38, 259–265. [Google Scholar] [CrossRef]
- Yoshimura, S.; Takagi, Y.; Harada, J.; Teramoto, T.; Thomas, S.S.; Waeber, C.; Bakowska, J.C.; Breakefield, X.O.; Moskowitz, M.A. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc. Natl. Acad. Sci. USA 2001, 98, 5874–5879. [Google Scholar] [CrossRef]
- Tang, J.J.; Podratz, J.L.; Lange, M.; Scrable, H.J.; Jang, M.H.; Windebank, A.J. Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain. Mol. Brain 2017, 10, 23. [Google Scholar] [CrossRef]
- Kokaia, Z.; Lindvall, O. Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol. 2003, 13, 127–132. [Google Scholar] [CrossRef]
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006, 24, 739–747. [Google Scholar] [CrossRef]
- Kokovay, E.; Goderie, S.; Wang, Y.; Lotz, S.; Lin, G.; Sun, Y.; Roysam, B.; Shen, Q.; Temple, S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 2010, 7, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Sun, Y.; Xie, L.; Peel, A.; Mao, X.O.; Batteur, S.; Greenberg, D.A. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell Neurosci. 2003, 24, 171–189. [Google Scholar] [CrossRef]
- Saha, B.; Peron, S.; Murray, K.; Jaber, M.; Gaillard, A. Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res. 2013, 11, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Grade, S.; Weng, Y.C.; Snapyan, M.; Kriz, J.; Malva, J.O.; Saghatelyan, A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS ONE 2013, 8, e55039. [Google Scholar] [CrossRef] [PubMed]
- Snapyan, M.; Lemasson, M.; Brill, M.S.; Blais, M.; Massouh, M.; Ninkovic, J.; Gravel, C.; Berthod, F.; Götz, M.; Barker, P.A.; et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 2009, 29, 4172–4188. [Google Scholar] [CrossRef]
- Kaneko, N.; Herranz-Pérez, V.; Otsuka, T.; Sano, H.; Ohno, N.; Omata, T.; Nguyen, H.B.; Thai, T.Q.; Nambu, A.; Kawaguchi, Y.; et al. New neurons use Slit-Robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration. Sci. Adv. 2018, 4, eaav0618. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Lee, S.T.; Bahn, J.J.; Jeon, D.; Kim, J.H.; Kim, S.; Won, C.H.; Kim, M.; Lee, S.K.; et al. Multipotent PDGFRβ-expressing cells in the circulation of stroke patients. Neurobiol. Dis. 2011, 41, 489–497. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Katychev, A.; Wang, X.; Van Buren, E. CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow Metab. 2006, 26, 613–624. [Google Scholar] [CrossRef]
- Nakagomi, T.; Molnár, Z.; Nakano-Doi, A.; Taguchi, A.; Saino, O.; Kubo, S.; Clausen, M.; Yoshikawa, H.; Nakagomi, N.; Matsuyama, T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011, 20, 2037–2051. [Google Scholar] [CrossRef]
- Sato, H.; Ishii, Y.; Yamamoto, S.; Azuma, E.; Takahashi, Y.; Hamashima, T.; Umezawa, A.; Mori, H.; Kuroda, S.; Endo, S.; et al. PDGFR-β Plays a Key Role in the Ectopic Migration of Neuroblasts in Cerebral Stroke. Stem Cells 2016, 34, 685–698. [Google Scholar] [CrossRef]
- Takasawa, K.; Kitagawa, K.; Yagita, Y.; Sasaki, T.; Tanaka, S.; Matsushita, K.; Ohstuki, T.; Miyata, T.; Okano, H.; Hori, M.; et al. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2002, 22, 299–307. [Google Scholar] [CrossRef]
- Namestnikova, D.D.; Gubskiy, I.L.; Revkova, V.A.; Sukhinich, K.K.; Melnikov, P.A.; Gabashvili, A.N.; Cherkashova, E.A.; Vishnevskiy, D.A.; Kurilo, V.V.; Burunova, V.V.; et al. Intra-Arterial Stem Cell Transplantation in Experimental Stroke in Rats: Real-Time MR Visualization of Transplanted Cells Starting with Their First Pass Through the Brain With Regard to the Therapeutic Action. Front. Neurosci. 2021, 15, 641970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Takahashi, H.K.; Liu, K.; Wake, H.; Liu, R.; Maruo, T.; Date, I.; Yoshino, T.; Ohtsuka, A.; Mori, S.; et al. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke 2011, 42, 1420–1428. [Google Scholar] [CrossRef]
- Shichita, T.; Hasegawa, E.; Kimura, A.; Morita, R.; Sakaguchi, R.; Takada, I.; Sekiya, T.; Ooboshi, H.; Kitazono, T.; Yanagawa, T.; et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012, 18, 911–917. [Google Scholar] [CrossRef]
- Kuang, X.; Wang, L.F.; Yu, L.; Li, Y.J.; Wang, Y.N.; He, Q.; Chen, C.; Du, J.R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: Involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 2014, 71, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S.; Schott, E.; Trimbuch, T.; Laubisch, D.; Krueger, C.; Wulczyn, G.; Nitsch, R.; Weber, J.R. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J. Neurosci. 2008, 28, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Zarruk, J.G.; Greenhalgh, A.D.; David, S. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp. Neurol. 2018, 301, 120–132. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; De Simoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Ito, M.; Morita, R.; Komai, K.; Noguchi, Y.; Ooboshi, H.; Koshida, R.; Takahashi, S.; Kodama, T.; Yoshimura, A. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 2017, 23, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Sugiyama, Y.; Ooboshi, H.; Sugimori, H.; Nakagawa, R.; Takada, I.; Iwaki, T.; Okada, Y.; Iida, M.; Cua, D.J.; et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 2009, 15, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Zhong, D.; Yang, L.M.; Sun, B.; Zhong, Z.H.; Yin, Y.H.; Cheng, J.; Yan, B.B.; Li, H.L. Expression of interleukin-17 in ischemic brain tissue. Scand. J. Immunol. 2005, 62, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.; Jander, S.; Witte, O.W.; Stoll, G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J. Neuroimmunol. 1994, 55, 195–203. [Google Scholar] [CrossRef]
- Xie, L.; Li, W.; Hersh, J.; Liu, R.; Yang, S.H. Experimental ischemic stroke induces long-term T cell activation in the brain. J. Cereb. Blood Flow Metab. 2019, 39, 2268–2276. [Google Scholar] [CrossRef]
- Seifert, H.A.; Collier, L.A.; Chapman, C.B.; Benkovic, S.A.; Willing, A.E.; Pennypacker, K.R. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J. Neuroimmune Pharmacol. 2014, 9, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Nguyen, T.D.; Meyermann, R.; Schluesener, H.J. Human focal cerebral infarctions induce differential lesional interleukin-16 (IL-16) expression confined to infiltrating granulocytes, CD8+ T-lymphocytes and activated microglia/macrophages. J. Neuroimmunol. 2001, 114, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Barbieri, I.; Basudev, H.; Brusa, R.; Casati, C.; Lozza, G.; Ongini, E. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur. J. Neurosci. 2000, 12, 2265–2272. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef]
- Liesz, A.; Suri-Payer, E.; Veltkamp, C.; Doerr, H.; Sommer, C.; Rivest, S.; Giese, T.; Veltkamp, R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009, 15, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.F.; Williams, T.M.; Kiewiet, M.B.; Payne, N.L.; Siatskas, C.; Samuel, C.S.; Ricardo, S.D. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am. J. Physiol. Renal. Physiol. 2014, 306, F1222–F1235. [Google Scholar] [CrossRef]
- Wang, L.Q.; Lin, Z.Z.; Zhang, H.X.; Shao, B.; Xiao, L.; Jiang, H.G.; Zhuge, Q.C.; Xie, L.K.; Wang, B.; Su, D.M.; et al. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci. Ther. 2014, 20, 317–326. [Google Scholar] [CrossRef]
- Liu, N.; Chen, R.; Du, H.; Wang, J.; Zhang, Y.; Wen, J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol. Immunol. 2009, 6, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Widera, D.; Holtkamp, W.; Entschladen, F.; Niggemann, B.; Zänker, K.; Kaltschmidt, B.; Kaltschmidt, C. MCP-1 induces migration of adult neural stem cells. Eur. J. Cell Biol. 2004, 83, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.M.; Daruwalla, Z.; Roth, T.M.; Attia, N.P.; Lukacs, N.W.; Richards, A.L.; White, I.O.; Allen, S.J.; Barald, K.F. Immortalized mouse inner ear cell lines demonstrate a role for chemokines in promoting the growth of developing statoacoustic ganglion neurons. J. Assoc. Res. Otolaryngol. 2005, 6, 355–367. [Google Scholar] [CrossRef][Green Version]
- Onda, T.; Honmou, O.; Harada, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 329–340. [Google Scholar] [CrossRef]
- Toyama, K.; Honmou, O.; Harada, K.; Suzuki, J.; Houkin, K.; Hamada, H.; Kocsis, J.D. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp. Neurol. 2009, 216, 47–55. [Google Scholar] [CrossRef]
- Kurozumi, K.; Nakamura, K.; Tamiya, T.; Kawano, Y.; Ishii, K.; Kobune, M.; Hirai, S.; Uchida, H.; Sasaki, K.; Ito, Y.; et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol. Ther. 2005, 11, 96–104. [Google Scholar] [CrossRef]
- Kurozumi, K.; Nakamura, K.; Tamiya, T.; Kawano, Y.; Kobune, M.; Hirai, S.; Uchida, H.; Sasaki, K.; Ito, Y.; Kato, K.; et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol. Ther. 2004, 9, 189–197. [Google Scholar] [CrossRef]
- Chang, D.J.; Lee, N.; Choi, C.; Jeon, I.; Oh, S.H.; Shin, D.A.; Hwang, T.S.; Lee, H.J.; Kim, S.U.; Moon, H.; et al. Therapeutic effect of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) in a rodent model of middle cerebral artery occlusion. Cell Transpl. 2013, 22, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Sowa, K.; Nito, C.; Nakajima, M.; Suda, S.; Nishiyama, Y.; Sakamoto, Y.; Nitahara-Kasahara, Y.; Nakamura-Takahashi, A.; Ueda, M.; Kimura, K.; et al. Impact of Dental Pulp Stem Cells Overexpressing Hepatocyte Growth Factor after Cerebral Ischemia/Reperfusion in Rats. Mol. Ther. Methods Clin. Dev. 2018, 10, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yamashita, T.; Takizawa, S.; Kuroda, S.; Kinouchi, H.; Kawahara, N. Stem cell therapy for cerebral ischemia: From basic science to clinical applications. J. Cereb. Blood Flow Metab. 2012, 32, 1317–1331. [Google Scholar] [CrossRef]
- Yao, H.W.; Kuan, C.Y. Early neutrophil infiltration is critical for inflammation-sensitized hypoxic-ischemic brain injury in newborns. J. Cereb. Blood Flow Metab. 2020, 40, 2188–2200. [Google Scholar] [CrossRef]
- McColgan, P.; Sharma, P.; Bentley, P. Stem cell tracking in human trials: A meta-regression. Stem Cell Rev. Rep. 2011, 7, 1031–1040. [Google Scholar] [CrossRef]
- Tan, C.; Shichinohe, H.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Hokari, M.; Hamauchi, S.; Houkin, K. Short-, middle- and long-term safety of superparamagnetic iron oxide-labeled allogeneic bone marrow stromal cell transplantation in rat model of lacunar infarction. Neuropathology 2015, 35, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Palma-Tortosa, S.; Coll-San Martin, B.; Kokaia, Z.; Tornero, D. Neuronal Replacement in Stem Cell Therapy for Stroke: Filling the Gap. Front Cell Dev. Biol. 2021, 9, 662636. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.M.; Andres, R.H.; Steinberg, G.K. Optimizing the success of cell transplantation therapy for stroke. Neurobiol. Dis. 2010, 37, 275–283. [Google Scholar] [CrossRef]
- Shen, L.H.; Ye, M.; Ding, X.S.; Han, Q.; Zhang, C.; Liu, X.F.; Huang, H.; Wu, E.B.; Huang, H.F.; Gu, X.S. Protective effects of MCI-186 on transplantation of bone marrow stromal cells in rat ischemic stroke model. Neuroscience 2012, 223, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kitada, M.; Wakao, S.; Nishikawa, K.; Tanimura, Y.; Makinoshima, H.; Goda, M.; Akashi, H.; Inutsuka, A.; Niwa, A.; et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc. Natl. Acad. Sci. USA 2010, 107, 8639–8643. [Google Scholar] [CrossRef]
- Wakao, S.; Oguma, Y.; Kushida, Y.; Kuroda, Y.; Tatsumi, K.; Dezawa, M. Phagocytosing differentiated cell-fragments is a novel mechanism for controlling somatic stem cell differentiation within a short time frame. Cell Mol. Life Sci. 2022, 79, 542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, W.; Zhu, J.; Wu, L.; Xu, G.; Liu, X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transpl. 2013, 22, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bentley, P.; Hamady, M.; Marley, S.; Davis, J.; Shlebak, A.; Nicholls, J.; Williamson, D.A.; Jensen, S.L.; Gordon, M.; et al. Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem Cells Transl. Med. 2014, 3, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.B.; Dutton, K.; Purdy, P.D. Assessment of Complication Types and Rates Related to Diagnostic Angiography and Interventional N euroradiologic Procedures. A Four Year Review (1993–1996). Interv. Neuroradiol. 1998, 4, 27–37. [Google Scholar] [CrossRef]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mohanty, S.; Bhatia, R.; Srivastava, M.V.; Garg, A.; Srivastava, A.; Goyal, V.; Tripathi, M.; Kumar, A.; Bal, C.; et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J. Med. Res. 2012, 136, 221–228. [Google Scholar]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef]
- Taguchi, A.; Sakai, C.; Soma, T.; Kasahara, Y.; Stern, D.M.; Kajimoto, K.; Ihara, M.; Daimon, T.; Yamahara, K.; Doi, K.; et al. Intravenous Autologous Bone Marrow Mononuclear Cell Transplantation for Stroke: Phase1/2a Clinical Trial in a Homogeneous Group of Stroke Patients. Stem Cells Dev. 2015, 24, 2207–2218. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Bennett, E.R.; Durham, R.J.; Volpi, J.J.; Wiese, J.R.; Frankel, M.; Shpall, E.; Wilson, J.M.; Troy, J.; Kurtzberg, J. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Transl. Med. 2018, 7, 521–529. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Tan, S.; Li, Z.; Xie, H.; Chen, P.; Wang, K.; He, Z.; He, P.; Ke, Y.; et al. Autologous Endothelial Progenitor Cells Transplantation for Acute Ischemic Stroke: A 4-Year Follow-Up Study. Stem Cells Transl. Med. 2019, 8, 14–21. [Google Scholar] [CrossRef]
- Ito, M.; Aswendt, M.; Lee, A.G.; Ishizaka, S.; Cao, Z.; Wang, E.H.; Levy, S.L.; Smerin, D.L.; McNab, J.A.; Zeineh, M.; et al. RNA-Sequencing Analysis Revealed a Distinct Motor Cortex Transcriptome in Spontaneously Recovered Mice After Stroke. Stroke 2018, 49, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Chen, W.; Li, W.; Huang, Y.; Duan, D.; Ge, L.; He, J.; Liu, J.; Hu, Z.; Lu, M. Ischemic-hypoxic preconditioning enhances the mitochondrial function recovery of transplanted olfactory mucosa mesenchymal stem cells via miR-181a signaling in ischemic stroke. Aging 2021, 13, 11234–11256. [Google Scholar] [CrossRef]
- Sakata, H.; Niizuma, K.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Narasimhan, P.; Maier, C.M.; Nishiyama, Y.; Chan, P.H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012, 32, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Bliss, T.M.; Hua, T.; Lee, A.; Oh, B.; Levinson, A.; Mehta, S.; Sun, G.; Steinberg, G.K. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials 2017, 142, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Wei, Z.; Wei, L. Preconditioning strategy in stem cell transplantation therapy. Transl. Stroke Res. 2013, 4, 76–88. [Google Scholar] [CrossRef]
- Yu, S.P.; Tung, J.K.; Wei, Z.Z.; Chen, D.; Berglund, K.; Zhong, W.; Zhang, J.Y.; Gu, X.; Song, M.; Gross, R.E.; et al. Optochemogenetic Stimulation of Transplanted iPS-NPCs Enhances Neuronal Repair and Functional Recovery after Ischemic Stroke. J. Neurosci. 2019, 39, 6571–6594. [Google Scholar] [CrossRef]
- Daadi, M.M.; Klausner, J.Q.; Bajar, B.; Goshen, I.; Lee-Messer, C.; Lee, S.Y.; Winge, M.C.; Ramakrishnan, C.; Lo, M.; Sun, G.; et al. Optogenetic Stimulation of Neural Grafts Enhances Neurotransmission and Downregulates the Inflammatory Response in Experimental Stroke Model. Cell Transpl. 2016, 25, 1371–1380. [Google Scholar] [CrossRef]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef]
- Korshunova, I.; Rhein, S.; García-González, D.; Stölting, I.; Pfisterer, U.; Barta, A.; Dmytriyeva, O.; Kirkeby, A.; Schwaninger, M.; Khodosevich, K. Genetic modification increases the survival and the neuroregenerative properties of transplanted neural stem cells. JCI Insight 2020, 5, 126268. [Google Scholar] [CrossRef]
- Gonzales-Portillo, G.S.; Sanberg, P.R.; Franzblau, M.; Gonzales-Portillo, C.; Diamandis, T.; Staples, M.; Sanberg, C.D.; Borlongan, C.V. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transpl. 2014, 23, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Chen, J.; Shen, H.C.; Ye, M.; Liu, X.F.; Ding, W.S.; Sheng, Y.F.; Ding, X.S. Possible Mechanism of Therapeutic Effect of 3-Methyl-1-phenyl-2-pyrazolin-5-one and Bone Marrow Stromal Cells Combination Treatment in Rat Ischemic Stroke Model. Chin. Med. J. 2016, 129, 1471–1476. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; MacIntosh, B.J.; Shirzadi, Z.; Kiss, A.; Bethune, A.; Heyn, C.; Mithani, K.; Hamani, C.; Black, S.E.; Hynynen, K.; et al. Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood-brain barrier opening in patients with Alzheimer’s disease. Neuroimage 2019, 200, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Fomenko, A.; Lozano, A.M. Magnetic Resonance-Guided Focused Ultrasound: Current Status and Future Perspectives in Thermal Ablation and Blood-Brain Barrier Opening. J. Korean Neurosurg. Soc. 2019, 62, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, Q.; Xie, Q.; Liu, Z.; Gao, Y.; He, Y.; Tan, X.; Xu, Y. Low intensity ultrasound targeted microbubble destruction assists MSCs delivery and improves neural function in brain ischaemic rats. J. Drug Target 2020, 28, 320–329. [Google Scholar] [CrossRef]
- Ahmed, N.; Gandhi, D.; Melhem, E.R.; Frenkel, V. MRI Guided Focused Ultrasound-Mediated Delivery of Therapeutic Cells to the Brain: A Review of the State-of-the-Art Methodology and Future Applications. Front. Neurol. 2021, 12, 669449. [Google Scholar] [CrossRef]



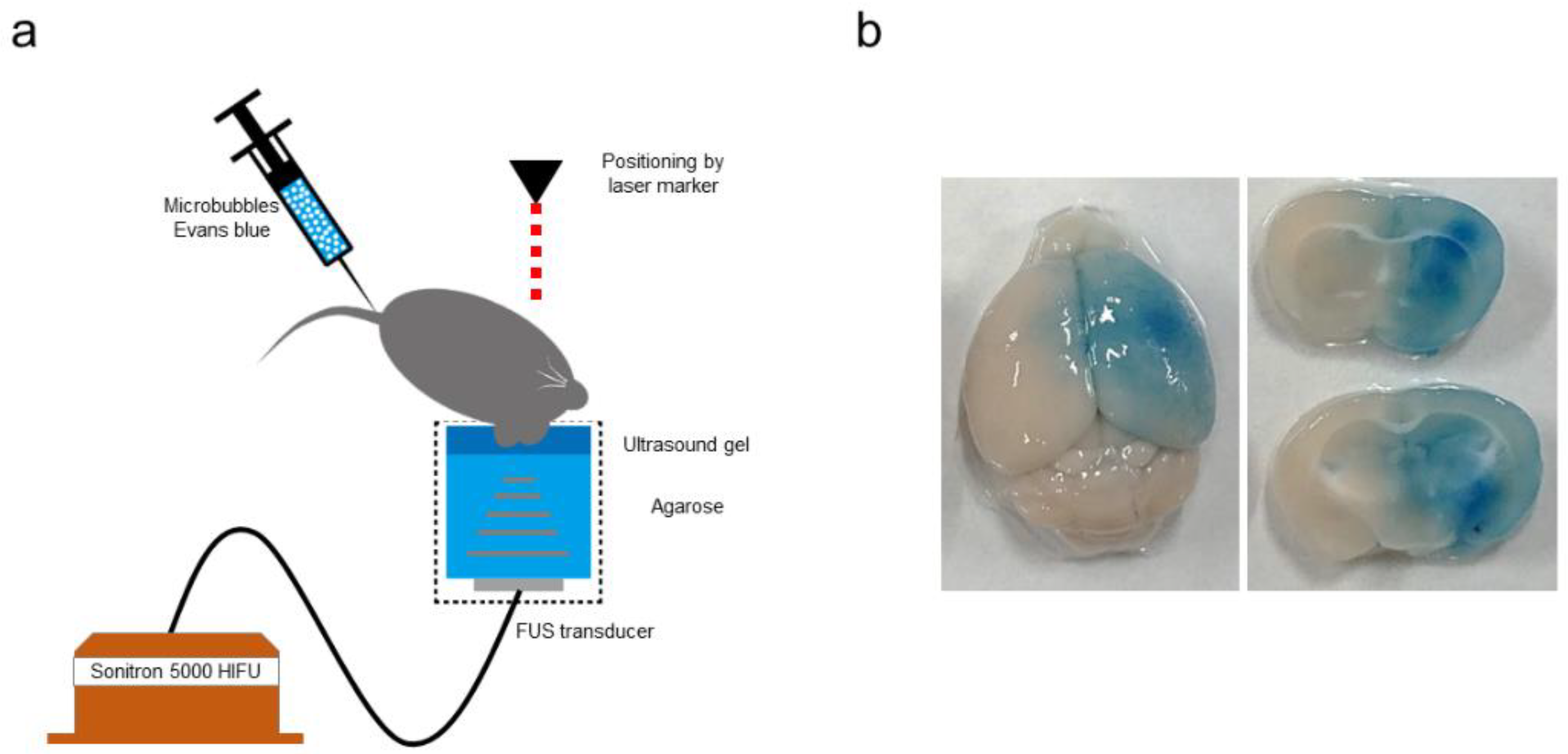
| Reference | Cell Type | Cell Source | Cell Dose/Total Volume | Infusion Speed | Infarct Area | Infusion Site | Timing of Transplantation Mean (Min–Max) | No. of Treated Patients /No. of Controls | Adverse Effects (Procedure-Related and Delayed Complication) | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Correa, P. L. 2007 | Autologous | BMMC | 3.0 × 107/NR | NR | MCA | MCA | 9 days | 1/0 | None | NR |
| Friedrich, M. A. 2012 | Autologous | BMMC | Mean 22.08 × 107 (5.1–60 × 107) /15 mL | 0.5 mL/min | MCA | MCA | 6 ± 1.8 days (3–10) | 20/0 | None | There was significant reduction in the median pretreatment NIHSS score over the 180 days post transplantation. |
| Moniche, F. et al., 2012 | Autologous | BMMC | Mean 1.59 × 108/NR | 0.5–1.0 mL/min | MCA | M1 | 6.4 ± 1.3 (5–9 days) | 10/10 | No procedure-related adverse effects. Two seizures. | No significant differences in neurological function between the treatment and control groups. The better BI at one month correlated with a higher number of CD34+ cells. |
| Jiang, Y. et al., 2013 | Allogenic | UCMSC | 2.0 × 107/20 mL | 1 mL/min | MCA | M1 | 17.3 ± 5.7 (11–19 days) | 3/0 | None | Improvement in mRS was seen in 2/3 patients at 90 and 180 days. |
| Rosado-de-Castro, P. H. et al., 2013 | Autologous | BMMC | Mean 2.80 × 108 (1.25–5×108)/10 mL | 1 mL/min | MCA | MCA | Mean 62 ± 20.4 (19–82 days) | 7/0 | No procedure-related adverse effects. Two had seizures. | No patients with worsened scores of BI, mRS, or NIHSS. |
| Banerjee, S. et al., 2014 | Autologous | CD34+ stem cells | Mean 2.2 × 106 (1.2–2.79 × 106)/NR | NR/10 min | MCA | MCA | Within 7 days | 5/0 | No procedure-related adverse effects. One had renal dysfunction. | There was a significant difference in mean NIHSS and mRS before and 180 days after transplantation. No reduction of the mean infarct volume. No new lesions on MRI. |
| Ghali, A. A. 2016 | Autologous | BMMC | About 1 × 106/100 mL | NR | MCA | ICA | Mean 22 (12–32 days) | 21/18 | None | There were no significant differences in the improvement of NIHSS and BI between the stem cell and control groups at 12 months. |
| Bhatia, V. et al., 2018 | Autologous | BMMC | Mean 6.1 × 108 (maximum 5 × 108)/mean 5 mL | Mean 0.5 mL/min | MCA | M1 | Mean 10 (8–15 days) | 10/10 | No procedure-related adverse effects. | Compared with controls, there was increase in the incidence of good outcomes. |
| Savitz, S. I. et al., 2019 | Autologous | BMMC | Mean 3.08 × 106 (1.6 × 105–7.5 × 107)/2.7 ± 0.8 mL | 2.7 ± 0.8 mL/2 to 3 min | MCA | ICA | 13–19 days | 29/19 | Four asymptomatic infarctions. | There was no significant efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, S.; Yoshida, M.; Horie, N.; Satoh, K.; Fukuda, Y.; Ishizaka, S.; Ogawa, K.; Morofuji, Y.; Hiu, T.; Izumo, T.; et al. Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials. Bioengineering 2023, 10, 33. https://doi.org/10.3390/bioengineering10010033
Yamaguchi S, Yoshida M, Horie N, Satoh K, Fukuda Y, Ishizaka S, Ogawa K, Morofuji Y, Hiu T, Izumo T, et al. Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials. Bioengineering. 2023; 10(1):33. https://doi.org/10.3390/bioengineering10010033
Chicago/Turabian StyleYamaguchi, Susumu, Michiharu Yoshida, Nobutaka Horie, Katsuya Satoh, Yuutaka Fukuda, Shunsuke Ishizaka, Koki Ogawa, Yoichi Morofuji, Takeshi Hiu, Tsuyoshi Izumo, and et al. 2023. "Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials" Bioengineering 10, no. 1: 33. https://doi.org/10.3390/bioengineering10010033
APA StyleYamaguchi, S., Yoshida, M., Horie, N., Satoh, K., Fukuda, Y., Ishizaka, S., Ogawa, K., Morofuji, Y., Hiu, T., Izumo, T., Kawakami, S., Nishida, N., & Matsuo, T. (2023). Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials. Bioengineering, 10(1), 33. https://doi.org/10.3390/bioengineering10010033









