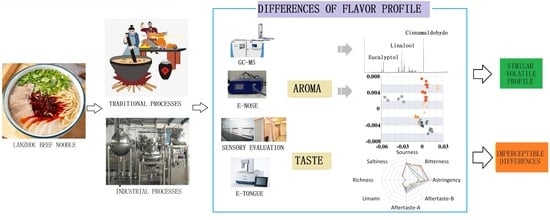
Flavor Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Analysis of Volatile Compounds
2.3. Identification of Volatile Compounds and Data Analysis
2.4. Electronic Nose Analysis
2.5. Electronic Tongue Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compound Analysis
3.1.1. Volatile Substance Contents
Alkenes
Aldehydes
Alcohols
Esters, Phenols and Ketones
Heterocycles, Aromatics, Sulfides and Alkanes
3.1.2. Odor Activity Value Analysis
3.1.3. Principal Component Analysis
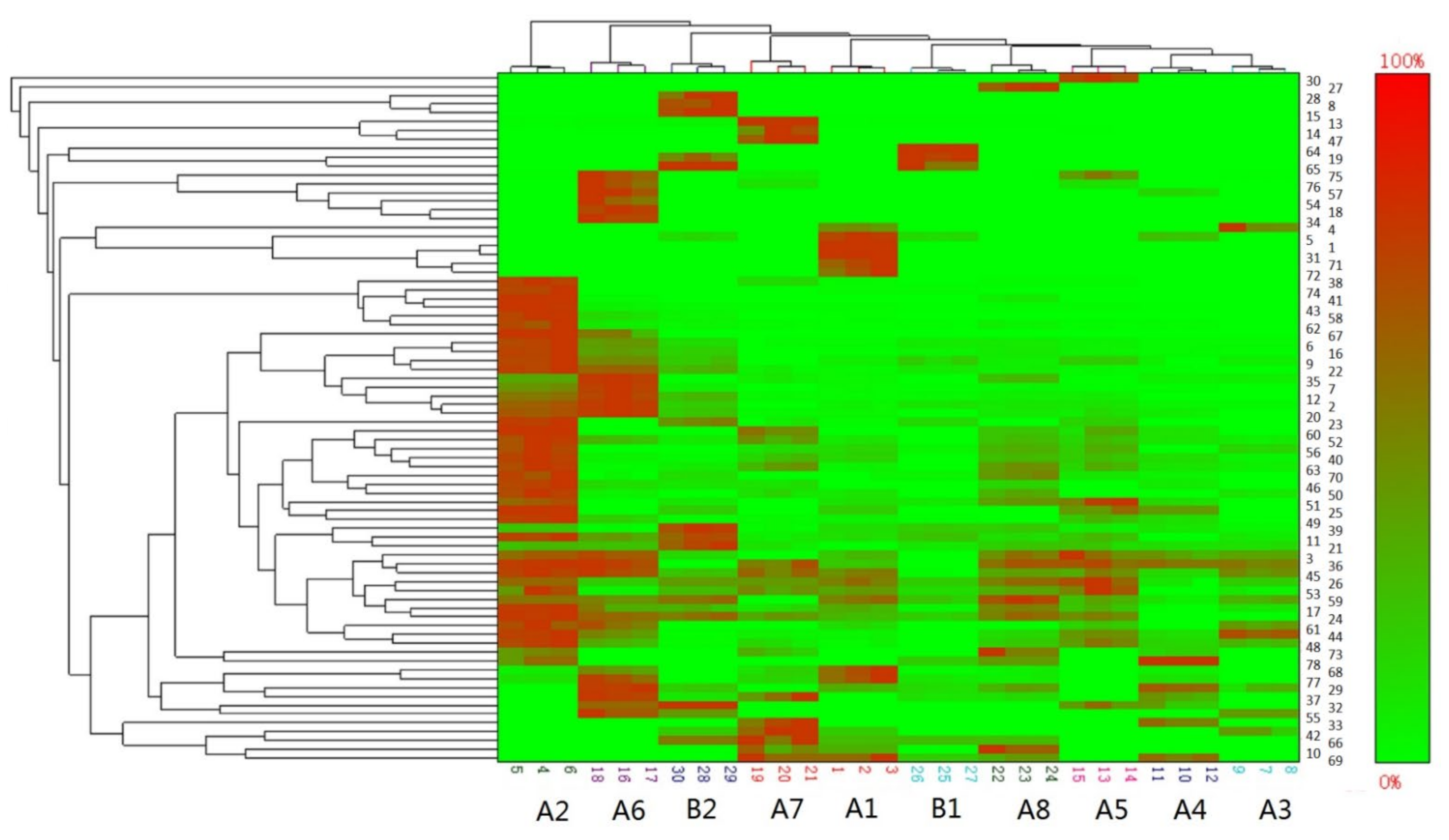
3.1.4. Clustering Analysis
3.2. E-Nose Analysis
3.3. E-Tongue Analysis
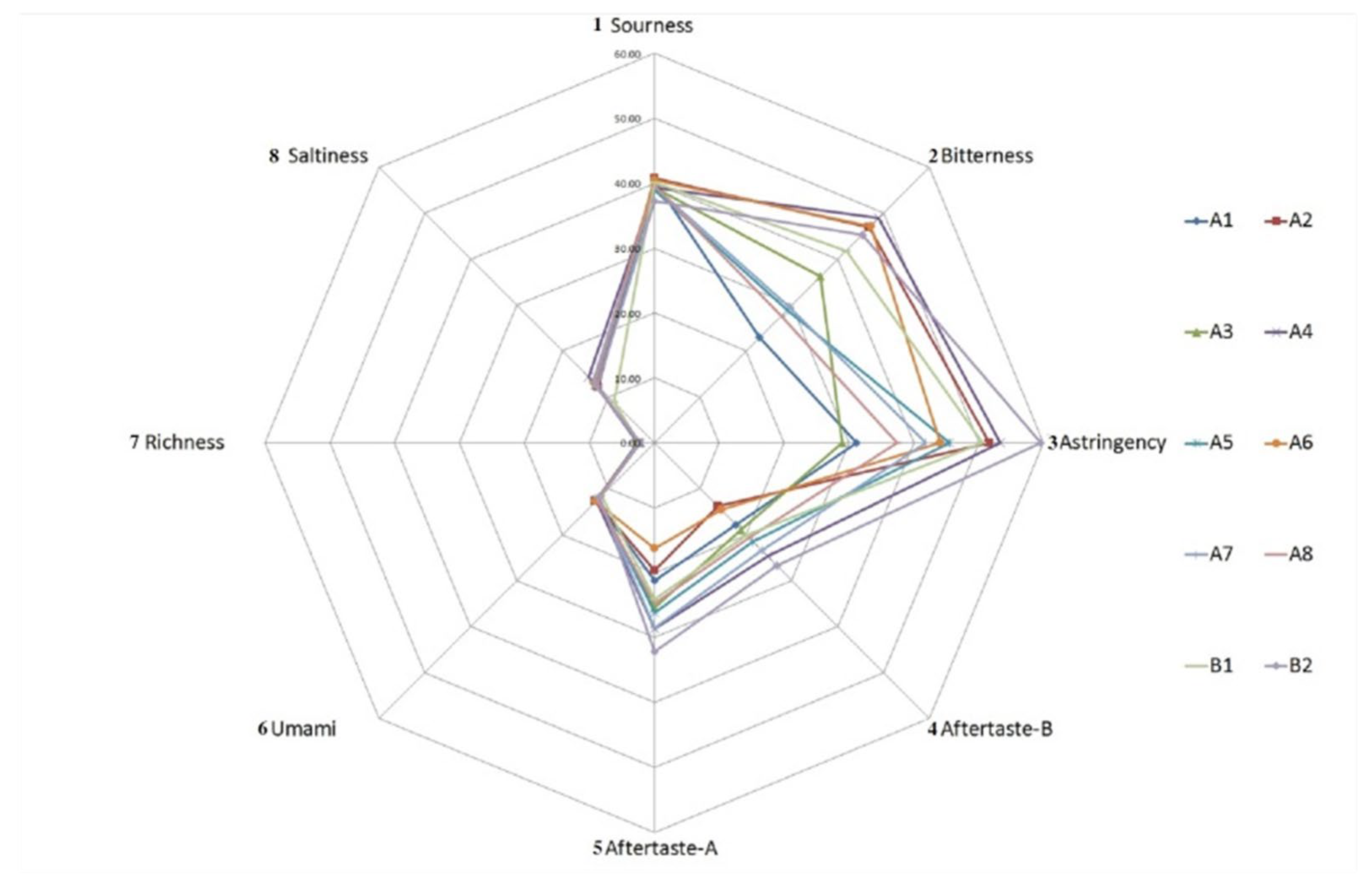
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X. On the cultural characteristics of halal food in Lanzhou and the strategy of building its national brand—Taking Lanzhou beef noodles as an example. Food Saf. Guide 2019, 27, 52–53. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, Y. Investigation and Analysis on Marketing Status of Lanzhou Beef Noodles from the Perspective of the Balance of Supply and Demand. J. HLJ VOCs Inst. Ecol. Eng. 2020, 33, 52–55. [Google Scholar]
- Ma, M. Problems and Countermeasures in the development of Lanzhou beef Ramen industry. Rel. Bel. Nat. Cult. 2018, 11, 199–207. [Google Scholar]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Abrodo, P.A.; Llorente, D.D.; Corujedo, S.J.; de la Fuente, E.D.; Álvarez, M.D.G.; Gomis, D.B. Characterisation of Asturian cider apples on the basis of their aromatic profile by high-speed gas chromatography and solid-phase microextraction. Food Chem. 2010, 121, 1312–1318. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef]
- Wasilewski, T.; Migoń, D.; Gębicki, J.; Kamysz, W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef]
- Zaragozá, P.; Fuentes, A.; Fernández-Segovia, I.; Vivancos, J.L.; Rizo, A.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Evaluation of sea bream (Sparus aurata) shelf life using an optoelectronic nose. Food Chem. 2013, 138, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.R.D.; Leone, F.; Cheli, F.; Chiofaloa, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Chen, Q.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef] [PubMed]
- Warendorf, T.; Belitz, H.D. The Flavor of Bouillon. 1. Quantitative-Analysis of Nonvolatiles. Z. Lebensm. Unters. Forch. 1992, 195, 209–214. [Google Scholar] [CrossRef]
- Warendorf, T.; Belitz, H.D. The Flavor of Bouillon. 2. Sensory Analysis of Nonvolatiles and Imitation of a Bouillon. Z. Lebensm. Unters. Forsch. 1992, 195, 215–223. [Google Scholar] [CrossRef]
- Pereira-Lima, C.I.; Ordoñez, J.A.; de Fernando, G.D.G.; Cambero, M.I. Influence of heat treatment on carnosine, anserine and free amino acid composition of beef broth and its role in flavour development. Eur. Food Res. Technol. 2000, 210, 165–172. [Google Scholar] [CrossRef]
- Keigo, S.; Norihiko, Y.; Ei-ichiro, S.; Tsutomu, H. Novel Brothy Taste Modifier Isolated from Beef Broth. J. Agric. Food Chem. 1998, 4, 1465–1468. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.R.; Lee, H.J.; Choi, H.K.; Lim, T.G.; Yoo, M.; Jang, H.W.; Nam, T.G. Comparison of two commercial solid-phase microextraction fibers for the headspace analysis of volatile compounds in different pork and beef cuts. J. Food Proc. Pres. 2018, 42, e13746.1–e13746.14. [Google Scholar] [CrossRef]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G. Quantification of Aroma Compounds in Parmigiano Reggiano Cheese by a Dynamic Headspace Gas Chromatography-Mass Spectrometry Technique and Calculation of Odor Activity Value. J. Dairy Sci. 2003, 86, 770–776. [Google Scholar] [CrossRef]
- Wu, H.; Yue, T.; Xu, Z.; Zhang, C. Sensor array optimization and discrimination of apple juices according to variety by an electronic nose. Anal. Methods 2017, 9, 921–928. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhang, H.; Wang, Y.; Chen, Q.; Kong, B. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT 2020, 124, 109061. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Hayat, K.; Duhoranimana, E.; Zhang, X.; Xia, S.; Yu, J.; Xing, F. Characterization of odor-active compounds of chicken broth and improved flavor by thermal modulation in electrical stewpots. Food Res. Int. 2018, 109, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Petričević, S.; Marusic Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xiao, H.; Deng, G.; Liu, Y.; Jiang, L.; Li, P.; Wang, J. Based on GC-IMS technology to analyze the difference in flavor composition of different spice boiling liquids. Sci. Technol. Food Ind. 2021, 42, 278–284. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chung, H.Y. Antioxidant and flavor in spices used in the preparation of chinese dishes. In Reference Module in Food Science; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 1–9. [Google Scholar] [CrossRef]
- Liu, M. Effects of Flavor Components and Microbial Community of Spices on the Eating Quality of Roast Chicken. Ph.D. Thesis, Henan University of Technology, Zhengzhou, China, 2014. [Google Scholar]
- Shahidi, F. Flavor of Meat, Meat Products and Seafoods, 2nd ed.; Blackie Academic and Professional: London, UK, 1998; pp. 13–25. [Google Scholar]
- Toldr´a, F. Lawrie’s Meat Science, 8th ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 23–33. [Google Scholar]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefanon, B. Determination of volatile compounds in San Daniele ham using headspace GC–MS. Meat Sci. 2008, 80, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Hancianu, M.; Costache, I.-I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Pham, A.; Schilling, M.; Mikel, W.; Williams, J.; Martin, J.; Coggins, P. Relationships between sensory descriptors, consumer acceptability and volatile flavor compounds of American dry-cured ham. Meat Sci. 2008, 80, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, J.; Zhang, L.; Liu, Y. Research Progress on Flavor Components of Four Spices of Garlic, Onion, Ginger and Chili. Chin. Cond. 2019, 44, 179–185. [Google Scholar] [CrossRef]
- Min, D.B.; Callison, A.L.; Lee, H.O. Singlet Oxygen Oxidation for 2-Pentylfuran and 2-Pentenyfuran Formation in Soybean Oil. J. Food Sci. 2003, 68, 1175–1178. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds Compilations of Odour Threshold Values in Air, Water and Other Media; Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Chen, Q.; Hu, Y.; Wen, R.; Wang, Y.; Qin, L.; Kong, B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef]




| No. | Sensor Name | Representative Material Species | Description(s) |
|---|---|---|---|
| 1 | W1C | Aromatic | Sensitive to aromatic constituents, benzene |
| 2 | W5S | Broad range | Sensitive to nitrogen oxides |
| 3 | W3C | Aromatic | Sensitive to aroma, ammonia |
| 4 | W6S | Hydrogen | Sensitive to hydrides |
| 5 | W5C | Arom-aliph | Sensitive to short-chain alkane aromatic component |
| 6 | W1S | Broad-methane | Sensitive to methyl |
| 7 | W1W | Sulphur-organic | Sensitive to sulfides |
| 8 | W2S | Broad-alcohol | Sensitive to alcohols, aldehydes and ketones |
| 9 | W2W | Sulf-chlor | Sensitive to organic sulfides |
| 10 | W3S | Methane-aliph | Sensitive to long-chain alkanes |
| Attributes | Definition with Reference Material |
|---|---|
| Overall acceptability | - |
| Beef like | Cooked beef |
| Fat like | Warmed beef tallow |
| Spice like | 0.3% Cinnamon oil solution in water |
| Salty taste | Sodium chloride 0.5% solution in water |
| Astringency taste | Aluminum sulfate 0.02‰ solution in water |
| Sour taste | Citric acid 0.3 g/L solution in water |
| Bitter taste | Quinine chloride 0.05% solution in water |
| Umami taste | Monosodium glutamate, 0.05% solution in water |
| 1 Compound | Cas | 2 A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | B1 | B2 | RI | 3 RI (Reference) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde | |||||||||||||
| n-Pentanal | 110-62-3 | 1.10 ± 0.10 a | — | — | — | — | — | — | — | — | — | — | 929 |
| n-Hexanal | 66-25-1 | 14.17 ± 0.48 a | — | — | 1.51 ± 0.05 b | — | — | — | — | 0.91 ± 0.04 c | 0.88 ± 0.07 c | 1079 | 1083 |
| (E)-2-methyl-2-butenal | 1115-11-3 | 3.03 ± 0.18 a | — | — | — | — | — | — | — | — | — | 1093 | 1095 |
| Heptanal | 111-71-7 | — | — | — | — | — | — | — | — | — | 0.51 ± 0.03 a | 1186 | 1188 |
| Octanal | 124-13-0 | 3.54 ± 0.18 e | 3.39 ± 0.05 a | — | — | 2.05 ± 0.12 c | 2.59 ± 0.07 b | 1.66 ± 0.15 d | 2.43 ± 0.23 b | 0.86 ± 0.06 f | 2.44 ± 0.20 b | 1288 | 1287 |
| 1-Nonanal | 124-19-6 | 11.40 ± 0.75 c,d | — | 3.12 ± 1.18 d,e | 8.07 ± 0.33 b | — | 9.96 ± 0.21 a | — | 4.60 ± 0.57 c | 1.79 ± 0.15 f | 2.73 ± 0.20 e | 1393 | 1390 |
| (E)-2-octenal | 2548-87-0 | — | — | — | — | 0.88 ± 0.05 a | — | — | — | — | — | 1430 | 1434 |
| Decanal | 112-31-2 | — | — | — | 2.96 ± 0.48 c | — | 6.34 ± 0.18 a | 4.93 ± 1.35 b | 1.08 ± 0.12 d | 0.92 ± 0.11 d | 0.79 ± 0.07 d | 1498 | 1498 |
| Benzaldehyde | 100-52-7 | 72.27 ± 1.93 c | 42.08 ± 0.73 b | 5.58 ± 0.30 e | 18.72 ± 0.63 c,d | 12.65 ± 1.86 d | 1.33 ± 0.15 e | 3.89 ± 0.81 e | 40.75 ± 2.79 b | 36.54 ± 1.30 b | 140.38 ± 8.61 a | 1526 | 1527 |
| (E)-2-decenal | 3913-81-3 | — | 9.42 ± 0.38 a | — | 0.97 ± 0.01 e | — | 1.68 ± 0.15 c | 1.46 ± 0.18 c,d | 2.01 ± 0.08 b | — | 1.26 ± 0.08 d,e | 1644 | 1642 |
| (Z)-citral | 106-26-3 | 7.59 ± 0.66 c | 19.33 ± 0.85 a | 2.42 ± 0.18 c | — | — | 1.28 ± 0.14 d | 3.31 ± 0.88 c | 8.28 ± 0.64 b | — | — | 1682 | 1688 |
| 1-Dodecanal | 112-54-9 | — | — | — | — | — | 3.06 ± 0.52 a | — | — | — | — | 1711 | 1709 |
| (E)-citral | 141-27-5 | 17.77 ± 1.70 c | 27.88 ± 1.61 a | 6.10 ± 0.31 c | — | 10.44 ± 2.25 b | — | 4.78 ± 0.61 c | 11.63 ± 0.63 b | 1.32 ± 0.07 e | — | 1734 | 1733 |
| 4-Isopropylbenzaldehyde | 122-03-2 | 16.58 ± 1.43 b | 5.43 ± 0.18 b | 3.11 ± 0.24 d | 0.87 ± 0.07 f | 3.19 ± 0.51 d | 4.38 ± 0.28 c | — | 7.32 ± 0.50 a | 2.24 ± 0.17 e | 5.61 ± 0.41 b | 1782 | 1785 |
| Trans-2-dodecenal | 4826-62-4 | — | — | — | 3.48 ± 0.28 a | — | 1.96 ± 0.25 b | — | 1.26 ± 0.14 c | — | — | 1854 | 1849 |
| 4-Propylbenzaldehyde | 28785-06-0 | 14.25 ± 0.95 d,e | 21.32 ± 0.87 b | 3.86 ± 0.26 e | 0.98 ± 0.06 f | — | 6.73 ± 2.00 d | 19.06 ± 1.30 c | 1.20 ± 0.11 f | 31.53 ± 1.94 a | 1871 | — | |
| o-Anisaldehyde | 135-02-4 | 4.02 ± 0.95 c | — | — | — | — | — | 4.60 ± 0.96 a | 1.16 ± 0.09 c | 1.06 ± 0.08 c | 3.12 ± 0.13 b | 1966 | 1941 |
| 2-Propenal | 101-39-3 | 6.87 ± 0.71 e | 16.32 ± 0.17 a | 2.72 ± 0.20 e | 0.94 ± 0.06 f | 3.58 ± 0.59 e | 5.92 ± 0.72 d | 2.40 ± 0.27 e | 11.40 ± 1.04 c | 3.39 ± 0.42 e | 12.81 ± 1.29 b | 1984 | 1992 |
| 4-Methoxybenzaldehyde | 123-11-5 | 5.35 ± 0.53 a | — | — | 1.49 ± 0.14 b | — | — | 1.81 ± 0.31 a | 0.97 ± 0.14 c | 0.55 ± 0.06 e | — | 2029 | 2027 |
| Cinnamaldehyde | 14371-10-9 | 86.22 ± 10.13 e,f | 1010.89 ± 78.50 a | 21.44 ± 2.00 e,f | 70.35 ± 7.13 d,e | 108.51 ± 10.94 d | 26.05 ± 3.43 e,f | 5.04 ± 1.08 f | 610.60 ± 30.81 b | 42.80 ± 7.12 e,f | 183.05 ± 17.62 c | 2044 | 2040 |
| Alkane | |||||||||||||
| n-Decane | 124-18-5 | — | — | — | 0.97 ± 0.14 c | — | 2.08 ± 0.06 a | — | 1.41 ± 0.14 b | 1.44 ± 0.05 b | — | — | 980 |
| Alkenes | |||||||||||||
| 2-Pinene | 80-56-8 | 5.55 ± 1.12 e | 22.94 ± 0.92 b | 0.81 ± 0.04 f | 1.44 ± 0.13 e,f | 3.70 ± 0.17 d | 27.68 ± 0.48 a | — | 3.28 ± 0.32 d | 3.76 ± 0.21 d | 7.02 ± 0.37 c | 1014 | 1015 |
| 3-Thujene | 2867-05-2 | 2.61 ± 0.20 d | 4.13 ± 0.06 b | — | — | — | 5.02 ± 0.02 a | — | — | — | 1.08 ± 0.10 c | 1021 | 1022 |
| Comphene | 79-92-5 | 4.46 ± 1.05 d | 12.42 ± 0.61 a | — | — | 1.79 ± 0.20 d | 4.39 ± 0.46 b | — | 1.63 ± 0.12 d | 3.37 ± 0.25 c | 4.80 ± 0.38 b | 1055 | 1060 |
| β-Pinene | 127-91-3 | 5.12 ± 0.82 e,f | 38.29 ± 1.79 a | 1.23 ± 0.04 f | 0.96 ± 0.08 f | 2.96 ± 0.40 e | 20.47 ± 0.38 b | 0.74 ± 0.27 f | 4.52 ± 0.21 d | 4.38 ± 0.23 d | 7.26 ± 0.39 c | 1090 | 1094 |
| Sabinen | 3387-41-5 | 5.67 ± 0.80 d | 25.76 ± 1.04 b | — | 0.80 ± 0.05 d | 0.91 ± 0.13 d | 45.11 ± 1.33 a | — | 1.85 ± 0.24 d | 0.81 ± 0.04 d | 3.45 ± 0.21 c | 1107 | 1109 |
| 3-Carene | 13466-78-9 | 23.96 ± 3.39 e | 86.19 ± 3.86 a | 6.55 ± 0.57 e,f | 3.80 ± 0.44 f,g | 24.61 ± 1.80 c | 54.89 ± 1.23 b | 1.04 ± 0.21 g | 10.03 ± 0.77 e | 19.44 ± 0.73 d | 26.15 ± 1.68 c | 1132 | 1135 |
| α-Phellandrene | 99-83-2 | 8.72 ± 0.45 e,f | 13.27 ± 0.49 a | 2.13 ± 0.25 e,f | 0.72 ± 0.10 g | 4.90 ± 0.49 d | 7.11 ± 0.59 c | 2.06 ± 0.27 e,f | 2.97 ± 0.19 e | 4.66 ± 0.07 d | 11.91 ± 0.76 b | 1152 | 1155 |
| β-Myrcene | 123-35-3 | 9.15 ± 0.54 e | 69.03 ± 2.00 b | — | — | 8.88 ± 0.57 d | 94.90 ± 4.07 a | 3.91 ± 0.23 e | 7.92 ± 0.46 d | 3.61 ± 0.22 e | 34.99 ± 2.60 c | 1156 | 1160 |
| α-Terpilene | 99-86-5 | 5.89 ± 0.76 e | 3.91 ± 0.17 d | 1.05 ± 0.14 f | — | — | 6.11 ± 0.21 c | 241.31 ± 10.83 a | 1.35 ± 0.02 e,f | 3.31 ± 0.13 d | 11.48 ± 0.80 b | 1168 | 1172 |
| Limonene | 138-86-3 | 23.48 ± 1.83 g,h | 314.22 ± 10.35 a | 8.32 ± 0.69 g,h | 12.36 ± 0.76 f,g | 23.08 ± 1.52 e | 156.00 ± 5.89 b | 2.45 ± 0.17 h | 36.76 ± 3.23 d | 19.91 ± 0.38 e,f | 78.46 ± 5.09 c | 1188 | 1186 |
| β-Phellandrene | 555-10-2 | — | — | — | — | — | 63.26 ± 2.72 a | — | — | — | — | 1207 | 1208 |
| (Z)-β-ocimene | 3338-55-4 | — | 23.83 ± 0.79 b | 0.82 ± 0.07 d | 2.18 ± 0.18 c,d | 3.50 ± 0.15 c | 44.95 ± 1.29 a | 1.35 ± 0.25 d | 3.77 ± 0.45 c | 0.51 ± 0.01 d | 24.98 ± 2.01 b | 1234 | 1234 |
| γ-Terpinene | 99-85-4 | 10.32 ± 0.36 f,g | 59.72 ± 1.47 b | — | 5.86 ± 0.50 e,f | 9.82 ± 3.25 d | 69.57 ± 1.47 a | 2.51 ± 0.38 g | 7.57 ± 0.51 d,e | — | 19.50 ± 1.33 c | 1239 | 1238 |
| β-Ocimene | 13877-91-3 | 2.00 ± 0.10 f | 39.06 ± 1.51 a | — | 2.50 ± 0.21 e | 4.98 ± 0.85 d | 24.38 ± 0.88 b | 2.34 ± 0.45 e | 4.75 ± 0.41 d | — | 13.18 ± 1.04 c | 1249 | 1251 |
| Styrene | 100-42-5 | 2.39 ± 0.13 e | 5.23 ± 0.28 b | 2.87 ± 0.22 d | 2.82 ± 0.17 d | 4.06 ± 0.83 c | 4.85 ± 0.11 b,c | 2.39 ± 0.42 d | 4.34 ± 0.36 b,c | 2.21 ± 0.11 d | 12.99 ± 1.18 a | 1257 | 1254 |
| Terpinolene | 586-62-9 | 1.82 ± 0.02 d | 15.05 ± 0.37 a | — | — | 3.45 ± 0.22 c | — | 1.05 ± 0.87 d | — | 2.78 ± 0.06 c | 9.30 ± 0.72 b | 1277 | 1279 |
| Perillene | 539-52-6 | — | — | — | — | — | — | — | — | 1.49 ± 0.15 a | 1.59 ± 0.14 a | 1422 | 1431 |
| Dehydro-p-cymene | 1195-32-0 | — | — | — | 1.21 ± 0.14 c | 2.22 ± 0.29 b | 2.24 ± 0.10 b | — | — | — | 3.45 ± 0.18 a | 1443 | 1438 |
| α-Cubebene | 17699-14-8 | 3.36 ± 0.13 e | 8.15 ± 0.48 a | 4.18 ± 0.14 c | 2.73 ± 0.23 d | 2.31 ± 0.18 d | 6.85 ± 0.42 b | — | 2.68 ± 0.21 d | — | 0.62 ± 0.05 f | 1452 | 1453 |
| p-Menth-3-ene | 20307-84-0 | 13.88 ± 0.65 e | 41.92 ± 1.86 a | 36.45 ± 2.61 b | 12.02 ± 1.04 c | 9.62 ± 0.84 c,d | 7.74 ± 0.78 d | 2.32 ± 0.29 e | 10.73 ± 1.20 c | 3.54 ± 0.10 e | 3.81 ± 0.28 e | 1466 | 1467 |
| (+)-Cyclosativene | 22469-52-9 | — | 8.00 ± 0.40 a | 1.09 ± 0.09 e | 5.29 ± 0.44 c | 6.11 ± 0.32 b | — | — | 3.74 ± 0.21 d | — | — | 1475 | 1477 |
| Ylangene | 14912-44-8 | — | 3.36 ± 0.15 a | — | 1.15 ± 0.07 c | 0.88 ± 0.05 d | — | — | 1.43 ± 0.04 b | — | — | 1478 | 1481 |
| Copaene | 3856-25-5 | 18.06 ± 0.13 f | 96.03 ± 4.62 b | 57.77 ± 3.14 d | 125.69 ± 10.07 a | 19.42 ± 1.01 e | 25.28 ± 2.25 e | 2.91 ± 0.16 f | 75.66 ± 5.70 c | 3.79 ± 0.19 f | 3.94 ± 0.36 f | 1485 | 1488 |
| β-Cubebene | 13744-15-5 | — | 6.07 ± 0.21 a | 2.22 ± 0.12 b | — | — | — | — | — | — | — | 1535 | 1537 |
| Trans-α-bergamotene | 13474-59-4 | — | — | — | 1.30 ± 0.13 b | 2.41 ± 0.32 a | — | — | — | — | — | 1556 | 1561 |
| Isocaryophyllene | 118-65-0 | — | — | 13.14 ± 0.81 a | 1.98 ± 0.05 c,d | 2.05 ± 0.34 c,d | 1.52 ± 0.11 d,e | 3.36 ± 0.28 b | 2.64 ± 0.21 c | — | 1.30 ± 0.10 e | 1572 | 1572 |
| β-Elemene | 515-13-9 | 5.42 ± 0.89 f | 17.25 ± 0.53 a | 9.67 ± 0.53 c | 7.58 ± 0.55 d | 17.19 ± 1.51 a | 11.98 ± 0.97 b | 1.63 ± 0.44 f | 4.46 ± 0.38 e | 1.08 ± 0.02 f | 3.30 ± 0.25 e | 1587 | 1586 |
| β-Caryophyllene | 87-44-5 | 192.43 ± 20.52 e,f | 802.49 ± 18.77 a | 695.22 ± 37.07 b | 151.39 ± 10.43 d | 458.50 ± 17.35 c | 463.56 ± 46.55 c | 76.95 ± 9.20 e | 173.35 ± 12.14 d | 21.21 ± 0.48 f | 35.84 ± 1.97 e,f | 1592 | 1588 |
| (−)-Alloaromadendrene | 25246-27-9 | 3.14 ± 0.54 d | 2.20 ± 0.05 c | — | 2.81 ± 0.18 b | 5.22 ± 0.79 a | 1.97 ± 0.24 c | 1.23 ± 0.21 d | — | — | — | 1640 | 1642 |
| (+)-Aromadendrene | 489-39-4 | — | 16.68 ± 0.81 a | — | — | — | — | 2.01 ± 0.41 c | 4.45 ± 0.30 b | — | 1.20 ± 0.06 d | 1641 | 1637 |
| Humulene | 6753-98-6 | 9.63 ± 2.65 f | 53.46 ± 2.26 a | 20.12 ± 1.09 c | 12.71 ± 0.65 d,e | 33.77 ± 4.72 b | 21.67 ± 2.43 c | 10.06 ± 2.69 e | 14.78 ± 1.32 d | 1.63 ± 0.02 f | 2.31 ± 0.15 f | 1665 | 1673 |
| γ-Muurolene | 30021-74-0 | 8.88 ± 2.38 e | 44.92 ± 1.49 a | — | 10.09 ± 0.53 c | 10.20 ± 3.18 c | 6.36 ± 0.34 d | — | 16.93 ± 1.31 b | 2.24 ± 0.08 e | 2.97 ± 0.16 e | 1685 | 1681 |
| Guaiene | 88-84-6 | 7.75 ± 0.71 c | 14.36 ± 0.99 a | — | 3.02 ± 0.31 c | 15.18 ± 2.90 a | — | — | 9.01 ± 0.63 b | 1.94 ± 0.06 c | 2.06 ± 0.09 c | 1687 | 1671 |
| Germacrene D | 23986-74-5 | — | 12.26 ± 0.48 a | — | 3.89 ± 0.29 c | 4.21 ± 0.14 c | 6.22 ± 0.66 b | — | 2.48 ± 0.22 d | — | 1.36 ± 0.05 e | 1705 | 1710 |
| β-Cadinene | 523-47-7 | — | — | — | — | 4.24 ± 0.44 a | — | — | — | — | — | 1709 | — |
| Borneol | 507-70-0 | 37.20 ± 3.11 b,c | 20.24 ± 5.57 a | 2.17 ± 0.16 e | — | 20.60 ± 3.80 a | 0.83 ± 0.01 e | 13.59 ± 1.75 b | 11.08 ± 0.72 b,c | 4.37 ± 0.17 d,e | 8.24 ± 0.17 c,d | 1700 | 1700 |
| Longifolene-(v4) | 61262-67-7 | 18.77 ± 3.16 e | 49.54 ± 2.21 a | 10.20 ± 0.59 d | 16.82 ± 1.32 c | 40.86 ± 4.86 b | — | 4.16 ± 0.88 e | 16.90 ± 1.15 c | 3.12 ± 0.15 e | 3.56 ± 0.34 e | 1713 | — |
| Eremophilene | 10219-75-7 | — | — | — | — | — | 8.83 ± 1.07 a | — | — | — | — | 1715 | 1710 |
| Zingiberene | 495-60-3 | 82.36 ± 16.43 c,d | 87.02 ± 3.53 b | 17.71 ± 1.28 c,d | 34.69 ± 2.58 c,d | 286.21 ± 38.44 a | 10.00 ± 1.06 d | 22.27 ± 3.95 c,d | 41.26 ± 2.07 c | 18.63 ± 0.42 c,d | 28.79 ± 2.28 c,d | 1719 | 1713 |
| α-Muurolene | 10208-80-7 | — | 27.00 ± 1.27 a | — | — | — | — | — | — | — | — | 1722 | 1720 |
| β-Bisabolene | 495-61-4 | 84.82 ± 11.50 c | 125.04 ± 4.82 a | — | 44.65 ± 2.76 c | 104.15 ± 10.82 b | — | 25.04 ± 2.34 c | 23.21 ± 1.58 c | 12.38 ± 0.09 d | — | 1725 | 1722 |
| Azulene | 275-51-4 | 3.99 ± 0.51 b | — | — | — | — | — | 1.95 ± 0.25 a | — | 1.24 ± 0.14 b | — | 1743 | 1746 |
| (Z,E)-α-farnesene | 26560-14-5 | 15.59 ± 3.50 c | 28.35 ± 1.04 b | 2.06 ± 0.25 d,e | 11.15 ± 0.52 c | 36.98 ± 7.72 a | 3.75 ± 0.47 d,e | 3.37 ± 1.08 d,e | 7.55 ± 0.45 c,d | 1.32 ± 0.03 e | 2.08 ± 0.11 d,e | 1749 | 1721 |
| (+)-δ-Cadinene | 483-76-1 | 19.14 ± 8.04 d | 11.15 ± 0.51 c,d | 23.21 ± 1.08 c | 34.54 ± 7.62 b | — | 13.44 ± 3.00 c | 43.03 ± 3.25 a | 5.61 ± 0.11 d | — | 1755 | 1753 | |
| Sesquiphellandrene | 20307-83-9 | 69.90 ± 14.66 c,d e | 104.50 ± 4.68 b | 5.73 ± 0.38 e | 37.61 ± 2.51 c | 129.88 ± 22.84 a | 10.19 ± 1.18 d,e | 24.47 ± 2.72 c,d | 30.84 ± 2.12 c | 7.30 ± 0.20 d,e | 9.85 ± 0.50 d,e | 1768 | 1764 |
| α-Curcumene | 644-30-4 | 97.18 ± 16.75 d,e | 193.35 ± 7.60 a | 9.86 ± 0.67 e | 69.85 ± 4.65 b | 194.58 ± 29.70 a | 25.71 ± 2.72 d,e | 41.94 ± 1.63 c,d | 62.07 ± 3.90 b,c | 17.30 ± 0.13 e | 19.06 ± 1.62 e | 1773 | 1770 |
| Germacrene b | 15423-57-1 | — | — | — | — | 2.61 ± 0.40 a | — | — | 1.02 ± 0.11 b | — | — | 1825 | 1823 |
| α-Calacorene | 21391-99-1 | — | 3.70 ± 0.08 a | — | — | — | — | — | — | — | — | 1915 | 1916 |
| Caryophyllene oxide | 1139-30-6 | 2.33 ± 0.34 c | 6.32 ± 0.28 a | 0.87 ± 0.06 c | — | — | 3.16 ± 0.38 b | — | — | 0.61 ± 0.07 c | 0.73 ± 0.07 c | 1979 | 1976 |
| 1-Methylnaphthalene | 90-12-0 | — | — | — | — | — | — | — | — | 0.65 ± 0.09 a | — | 1888 | 1891 |
| Aromatic | |||||||||||||
| Toluene | 108-88-3 | 2.47 ± 0.09 c,d | 2.09 ± 0.07 a,b | 1.20 ± 0.05 c | 1.18 ± 0.15 c | 2.00 ± 0.52 a,b | 2.27 ± 0.13 a | — | 1.75 ± 0.24 b | — | 0.54 ± 0.03 d | 1037 | 1043 |
| Ethylbenzene | 100-41-4 | — | 2.61 ± 0.09 b | 1.84 ± 0.12 b | 2.40 ± 0.03 b | 2.94 ± 0.37 b | 3.80 ± 0.30 b | 390.06 ± 51.23 a | 2.32 ± 0.14 b | — | — | 1181 | 1175 |
| o-Cymene | 527-84-4 | 7.85 ± 0.50 f,g | 31.95 ± 1.02 a | 1.53 ± 0.09 g | 2.03 ± 0.15 g | 2.69 ± 0.19 f,g | 26.28 ± 0.35 b | 3.54 ± 0.83 e,f | 3.99 ± 0.27 d,e | 4.78 ± 0.21 d | 13.20 ± 0.90 c | 1266 | 1274 |
| Elemicin | 487-11-6 | 16.04 ± 2.44 a | 3.93 ± 0.10 b | — | 1.10 ± 0.10 d | — | 3.41 ± 0.56 b,c | 3.19 ± 0.33 c | — | — | 0.82 ± 0.06 d | 2230 | 2232 |
| Sulfide | |||||||||||||
| Dimethyl disulfide | 624-92-0 | 5.91 ± 0.34 b | — | 2.63 ± 0.75 a | — | — | — | — | — | — | — | 1070 | 1071 |
| Heterocyclic | |||||||||||||
| 2-Butylfuran | 4466-24-4 | — | — | — | — | — | — | — | — | — | 1.05 ± 0.09 a | 1132 | 1130 |
| 2-Pentylfuran | 3777-69-3 | — | — | — | — | — | — | — | — | 0.81 ± 0.04 a | 0.55 ± 0.08 b | 1233 | 1229 |
| 1,3-Dithiane | 505-23-7 | 21.09 ± 0.29 c | — | — | — | — | 15.54 ± 0.19 a | 5.60 ± 0.65 d | 11.92 ± 0.72 b | — | — | 1280 | 1296 |
| 2-Hexylfuran | 3777-70-6 | — | — | — | — | — | — | — | — | — | 2.19 ± 0.25 a | 1332 | 1329 |
| Linalyl oxide | 5989-33-3 | 2.30 ± 0.32 a | — | — | — | — | — | — | — | — | — | 1441 | 1443 |
| Cosmene | 460-01-5 | — | — | — | — | — | 1.89 ± 0.14 a | — | — | — | — | 1452 | 1460 |
| Ethers | |||||||||||||
| Diallyl sulfide | 592-88-1 | 1.97 ± 0.08 c | — | — | — | — | — | 1.00 ± 0.17 b | 1.44 ± 0.03 a | — | — | 1141 | 1148 |
| Dimethyl trisulfide | 3658-80-8 | — | — | — | — | — | — | — | 0.96 ± 0.09 a | — | — | 1380 | 1376 |
| Diallyl disulfide | 2179-57-9 | 37.10 ± 2.19 e | 110.96 ± 0.85 b | 21.50 ± 0.58 d | 19.58 ± 1.05 d | 2.80 ± 0.26 f | 252.00 ± 5.33 a | 25.05 ± 5.56 d | 88.14 ± 5.69 c | — | — | 1481 | 1480 |
| Estragole | 140-67-0 | 33.68 ± 1.48 e | 166.66 ± 4.52 a | 29.13 ± 1.96 d | 53.89 ± 9.02 b | 41.00 ± 2.96 c | 6.41 ± 0.77 e,f | — | 2.58 ± 0.31 f | 2.22 ± 0.04 f | 1672 | 1671 | |
| Diallyl trisulfide | 2050-87-5 | 38.16 ± 2.36 c | 60.73 ± 1.76 a | 6.02 ± 0.47 d | 4.69 ± 0.29 d,e | 1.60 ± 0.35 f | — | 2.26 ± 0.27 e,f | 37.46 ± 3.78 b | — | — | 1790 | 1789 |
| Anethole | 104-46-1 | 26.19 ± 1.25 c,d | 364.58 ± 26.66 a | 4.99 ± 0.86 d | 12.23 ± 1.19 c,d | 11.37 ± 1.04 c,d | 40.32 ± 5.67 b | 12.80 ± 1.43 c,d | 27.56 ± 5.97 b,c | 9.71 ± 1.55 c,d | 23.61 ± 2.98 b,c d | 1830 | 1834 |
| Methylisoeugenol | 93-16-3 | 8.39 ± 1.35 a | — | — | — | — | 2.71 ± 0.50 a | 0.78 ± 0.07 b | 0.84 ± 0.05 b | — | — | 2186 | 2185 |
| Myristicin | 607-91-0 | 31.11 ± 4.14 a | 1.89 ± 0.01 b,c | — | 1.61 ± 0.10 b,c | — | 10.11 ± 1.13 a | 2.51 ± 0.13 b | 0.95 ± 0.16 c | 1.34 ± 0.04 b,c | — | 2267 | 2257 |
| Alcohols | |||||||||||||
| Eucalyptol | 470-82-6 | 342.20 ± 21.98 c,d | 249.86 ± 2.90 a | 52.20 ± 1.83 e,f | 22.93 ± 1.18 g | 96.20 ± 12.46 d | 135.62 ± 30.74 c | 58.34 ± 10.23 e | 165.33 ± 4.28 b | 28.78 ± 0.69 f,g | 103.08 ± 13.46 d | 1199 | 1199 |
| 2-Heptanol | 543-49-7 | 2.04 ± 0.16 c | 2.78 ± 0.10 a | — | 1.11 ± 0.07 b | 1.29 ± 0.29 b | — | — | — | — | 0.49 ± 0.01 c | 1320 | 1319 |
| 1-Octen-3-ol | 3391-86-4 | — | — | — | 0.87 ± 0.08 b | — | — | 1.10 ± 0.19 a | — | — | — | 1453 | 1456 |
| Cis-(+/-)-4-Thujanol | 15537-55-0 | 13.03 ± 1.40 d | 14.29 ± 0.35 b | 1.35 ± 0.09 e | — | — | 19.86 ± 0.49 a | — | 6.47 ± 0.30 c | — | — | 1464 | 1469 |
| 2-Ethylhexan-1-ol | 104-76-7 | 3.17 ± 0.36 a | — | — | — | — | — | — | — | — | — | 1490 | 1491 |
| Linalool | 78-70-6 | 606.79 ± 38.51 c | 886.80 ± 26.90 a | 51.11 ± 2.08 d,e | 163.34 ± 3.52 c | 267.01 ± 29.23 b | 66.75 ± 3.54 d | 270.53 ± 61.92 b | 306.29 ± 13.14 b | 7.43 ± 0.15 e | 32.81 ± 1.44 d,e | 1549 | 1552 |
| 1-Octanol | 111-87-5 | 2.13 ± 0.20 d | — | 1.60 ± 0.15 b | — | — | — | 3.67 ± 0.41 a | — | — | 1.30 ± 0.08 c | 1558 | 1555 |
| 2-Cyclohexen-1-ol | 29803-81-4 | 24.44 ± 2.05 b | 6.60 ± 0.25 c | 6.54 ± 0.39 c | 2.31 ± 0.11 f | 5.04 ± 0.56 d | 11.75 ± 0.54 a | 2.65 ± 0.31 f | 4.16 ± 0.45 e | — | 3.82 ± 0.19 e | 1562 | 1557 |
| Terpinen-4-ol | 562-74-3 | 397.81 ± 31.89 b,c | 198.66 ± 7.06 a | 120.39 ± 6.49 c,d | 48.95 ± 1.14 f | 116.44 ± 19.79 c,d | 189.98 ± 11.42 a | 150.89 ± 15.92 b | 101.39 ± 5.30 d | 15.70 ± 0.43 g | 71.11 ± 2.95 e | 1601 | 1606 |
| 1-Nonanol | 143-08-8 | — | — | — | — | — | — | 2.04 ± 0.29 a | — | — | — | 1663 | 1666 |
| α-Terpineol | 98-55-5 | 138.41 ± 13.74 d | 253.68 ± 15.58 a | 36.22 ± 2.17 d | 50.03 ± 1.30 d | 84.38 ± 15.37 c | 100.72 ± 8.06 c | 138.18 ± 19.49 b | 96.46 ± 6.05 c | 14.75 ± 0.35 e | 40.89 ± 1.98 d | 1697 | 1697 |
| (+)-Trans-piperitenol | 16721-39-4 | 4.47 ± 0.75 c | 2.04 ± 0.17 b | 1.91 ± 0.12 b | — | — | 2.47 ± 0.17 a | — | — | — | 1.06 ± 0.12 d | 1745 | 1742 |
| Nerol | 106-25-2 | — | 19.33 ± 0.47 a | 1.05 ± 0.17 e | 4.18 ± 0.18 d | 6.73 ± 1.91 c | — | 13.24 ± 0.88 b | 6.25 ± 0.51 c | — | 0.70 ± 0.06 e | 1797 | 1794 |
| (−)-Trans-carveol | 1197-07-5 | — | — | 0.61 ± 0.06 a | — | — | — | — | — | — | — | 1836 | 1836 |
| Geraniol | 106-24-1 | 15.94 ± 2.49 d,e | 65.49 ± 3.29 a | 6.75 ± 0.57 d,e | 10.33 ± 0.37 d | 23.52 ± 6.23 c | 2.86 ± 0.34 e | 33.05 ± 5.48 b | 37.12 ± 2.39 b | 1.26 ± 0.03 e | 7.53 ± 0.53 d,e | 1848 | 1853 |
| 2-(4-Methylphenyl)propan-2-ol | 1197-01-9 | 5.16 ± 0.64 a | — | 1.22 ± 0.15 b | — | — | 1.58 ± 0.05 a | — | — | — | — | 1852 | 1844 |
| 2-Phenylethanol | 60-12-8 | — | — | — | — | — | — | — | — | 1.42 ± 0.07 b | 1.81 ± 0.14 a | 1913 | 1912 |
| Cinnamyl alcohol | 104-54-1 | — | 1.97 ± 0.04 b | — | 3.22 ± 0.19 a | — | — | — | 1.42 ± 0.05 c | 0.67 ± 0.05 d | — | 2286 | 2286 |
| Ketone | |||||||||||||
| 6-Methyl-5-hepten-2-one | 110-93-0 | 12.68 ± 0.49 b | 4.99 ± 0.06 a | 2.23 ± 0.06 d | 1.06 ± 0.06 e | 5.33 ± 0.45 a | 2.22 ± 0.04 d | 3.36 ± 0.24 c | 4.52 ± 0.27 b | 2.27 ± 0.03 d | 3.26 ± 0.10 c | 1338 | 1341 |
| 2-Nonanone | 821-55-6 | — | — | — | — | — | — | — | — | — | 1.12 ± 0.11 a | 1389 | 1386 |
| 2-Decanone | 693-54-9 | 9.15 ± 0.28 d | 11.04 ± 0.17 a | 7.21 ± 0.37 c | 7.94 ± 0.41 b,c | 9.18 ± 0.97 b | 10.60 ± 0.57 a | 7.88 ± 1.42 b,c | 8.99 ± 0.73 b | — | — | 1493 | 1493 |
| Camphor | 76-22-2 | 5.47 ± 0.18 c | 107.67 ± 3.25 a | — | — | 1.85 ± 0.19 c | — | 20.66 ± 1.19 b | 1.90 ± 0.05 c | — | 1.03 ± 0.03 c | 1499 | 1499 |
| 4-Isopropyl-2-cyclohexenone | 500-02-7 | 14.27 ± 2.39 b | — | 7.30 ± 0.75 a | — | — | 5.20 ± 0.43 b | 1.84 ± 0.40 d | 2.76 ± 0.21 c | — | 2.27 ± 0.17 c,d | 1669 | 1669 |
| Piperitone | 89-81-6 | — | — | 21.85 ± 1.37 b | — | — | 35.70 ± 3.85 a | — | — | — | 19.89 ± 1.90 b | 1727 | 1730 |
| Carvone | 99-49-0 | — | — | — | 1.30 ± 0.10 b | — | 6.45 ± 0.51 a | — | — | — | — | 1735 | 1735 |
| Esters | |||||||||||||
| Octyl acetate | 112-14-1 | — | — | — | — | — | 4.82 ± 0.25 a | — | — | — | — | 1482 | 1481 |
| Ethyl nonanoate | 123-29-5 | — | — | — | — | 0.93 ± 0.26 a | — | — | — | — | — | 1537 | 1537 |
| Linalyl acetate | 115-95-7 | — | 380.94 ± 2.56 a | — | 2.35 ± 0.24 d | 1.92 ± 0.33 d | 8.35 ± 0.59 c | — | 37.69 ± 3.79 b | — | — | 1557 | 1555 |
| (−)-Bornyl acetate | 76-49-3 | 3.93 ± 0.17 d | 85.76 ± 0.72 a | — | 0.60 ± 0.05 e | 1.49 ± 0.16 d | 5.07 ± 0.28 b | 1.33 ± 0.12 d | 1.33 ± 0.10 d | 0.67 ± 0.03 e | 2.51 ± 0.15 c | 1578 | 1584 |
| Terpinene 4-acetate | 4821-04-9 | 2.06 ± 0.16 d | 6.73 ± 0.77 a | — | — | — | 4.12 ± 0.12 b | — | — | — | 1.51 ± 0.15 c | 1614 | 1619 |
| Citronellyl acetate | 150-84-5 | — | — | — | — | — | 4.86 ± 0.45 a | — | — | — | 1.61 ± 0.06 b | 1660 | 1657 |
| Geranyl acetate | 105-87-3 | 8.18 ± 1.12 d | 171.98 ± 6.28 a | — | — | — | 29.49 ± 3.23 b | — | — | — | 9.99 ± 0.46 c | 1757 | 1756 |
| Phenethyl acetate | 103-45-7 | 2.70 ± 0.14 d | 4.32 ± 0.39 a | 2.27 ± 0.16 c | — | 1.17 ± 0.23 d | 3.53 ± 0.44 b | — | — | — | — | 1818 | 1825 |
| Ethyl cinnamate | 103-36-6 | — | 2.08 ± 0.20 b | — | 0.80 ± 0.04 c | — | — | 1.17 ± 0.25 c | 2.84 ± 0.65 a | — | — | 2132 | 2132 |
| Cinnamyl acetate | 103-54-8 | 4.28 ± 0.42 b | 66.01 ± 2.73 a | — | — | — | — | — | — | 1.17 ± 0.09 b | — | 2154 | 2156 |
| Phenols | |||||||||||||
| Pinocarveol | 6712-79-4 | 4.20 ± 0.39 d | 8.06 ± 0.60 b | — | 3.03 ± 0.20 c,d | 16.59 ± 2.68 a | 2.47 ± 0.25 c,d | 7.09 ± 1.28 b | 4.49 ± 0.38 c | — | — | 1655 | 1646 |
| Butylated hydroxytoluene | 128-37-0 | — | — | — | — | — | — | 3.21 ± 0.54 a | — | — | — | 1916 | 1919 |
| Methyl eugenol | 93-15-2 | 75.60 ± 9.86 a | 2.33 ± 0.12 d | — | 2.00 ± 0.19 d | — | 14.88 ± 1.44 b | 5.78 ± 1.35 c | 2.01 ± 0.36 d | 0.46 ± 0.05 d | — | 2017 | 2014 |
| 4-Methylphenol | 106-44-5 | 2.40 ± 0.25 a | — | — | — | — | — | — | — | — | — | 2088 | 2090 |
| 3-Methylphenol | 108-39-4 | 8.39 ± 1.35 b | — | — | — | — | — | — | — | — | — | 2096 | 2091 |
| Eugenol | 97-53-0 | 3.53 ± 0.15 c | 3.92 ± 0.38 c | 2.62 ± 0.47 c | 1.07 ± 0.07 c | 172.74 ± 26.91 b | 274.62 ± 28.03 a | 25.05 ± 2.61 c | 1.37 ± 0.19 c | — | 0.75 ± 0.15 c | 2170 | 2175 |
| Isoeugenol | 97-54-1 | — | — | — | 1.16 ± 10.15 b | 6.19 ± 0.89 a | 1.15 ± 0.32 b | — | — | — | 2260 | 2271 | |
| 2,4-Di-tert-butylphenol | 96-76-4 | — | — | — | — | — | — | 1.20 ± 0.15 a | — | 0.52 ± 0.03 b | 0.46 ± 0.02 b | 2316 | 2321 |
| Total | 3014.66 ± 202.47 b | 6964.08 ± 176.04 a | 1335.85 ± 67.47 e | 1126.97 ± 57.43 e | 2572.14 ± 262.77 c | 2662.65 ± 167.55 c | 1759.03 ± 151.78 d | 2344.11 ± 125.15 c | 367.55 ± 8.55 f | 1140.93 ± 33.96 e |
| No. | Compound | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | B1 | B2 | Olfactory Threshold (μg/kg) 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | N-Pentanal | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 22 |
| 2 | 2-Pinene | 0.31 | 3.82 | 0.14 | 0.24 | 0.62 | 4.61 | 0.00 | 0.55 | 0.63 | 1.17 | 6 |
| 3 | Toluene | 0.03 | 0.09 | 0.05 | 0.05 | 0.08 | 0.09 | 0.00 | 0.07 | 0.00 | 0.02 | 24 |
| 4 | Dimethyl disulfide | 6.57 | 0.00 | 8.77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.3 |
| 5 | N-Hexanal | 0.94 | 0.00 | 0.00 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.18 | 5 |
| 6 | β-pinene | 0.01 | 0.27 | 0.01 | 0.01 | 0.02 | 0.15 | 0.01 | 0.03 | 0.03 | 0.05 | 140 |
| 7 | Sabinen | 0.05 | 0.70 | 0.00 | 0.02 | 0.02 | 1.22 | 0.00 | 0.05 | 0.02 | 0.09 | 37 |
| 8 | 2-Butylfuran | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 | 5 |
| 9 | 3-Carene | 0.01 | 0.11 | 0.01 | 0.00 | 0.03 | 0.07 | 0.00 | 0.01 | 0.03 | 0.03 | 770 |
| 10 | Diallyl sulfide | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.04 | 0.00 | 0.00 | 32.5 |
| 11 | α-Phellandrene | 0.07 | 0.33 | 0.05 | 0.02 | 0.12 | 0.18 | 0.05 | 0.07 | 0.12 | 0.30 | 40 |
| 12 | β-Myrcene | 2.54 | 57.53 | 0.00 | 0.00 | 7.40 | 79.08 | 3.26 | 6.60 | 3.01 | 29.16 | 1.2 |
| 13 | α-Terpilene | 0.02 | 0.05 | 0.01 | 0.00 | 0.00 | 0.07 | 2.84 | 0.02 | 0.04 | 0.14 | 85 |
| 14 | Ethylbenzene | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 1.95 | 0.01 | 0.00 | 0.00 | 200 |
| 15 | Heptanal | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 2.8 |
| 16 | Limonene | 0.23 | 9.24 | 0.24 | 0.36 | 0.68 | 4.59 | 0.07 | 1.08 | 0.59 | 2.31 | 34 |
| 17 | Eucalyptol | 103.70 | 227.14 | 47.45 | 20.84 | 87.45 | 123.29 | 53.04 | 150.30 | 26.16 | 93.71 | 1.1 |
| 18 | β-Phellandrene | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.76 | 0.00 | 0.00 | 0.00 | 0.00 | 36 |
| 19 | 2-Pentylfuran | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.09 | 6 |
| 20 | γ-Terpinene | 0.05 | 0.92 | 0.00 | 0.09 | 0.15 | 1.07 | 0.04 | 0.12 | 0.00 | 0.30 | 65 |
| 21 | Styrene | 0.22 | 1.45 | 0.80 | 0.78 | 1.13 | 1.35 | 0.66 | 1.21 | 0.61 | 3.61 | 3.6 |
| 22 | O-Cymene | 0.52 | 6.39 | 0.31 | 0.41 | 0.54 | 5.26 | 0.71 | 0.80 | 0.96 | 2.64 | 5 |
| 23 | Terpinolene | 0.01 | 0.37 | 0.00 | 0.00 | 0.08 | 0.00 | 0.03 | 0.00 | 0.07 | 0.23 | 41 |
| 24 | Octanal | 2.03 | 5.85 | 0.00 | 0.00 | 3.53 | 4.46 | 2.86 | 4.19 | 1.48 | 4.21 | 0.58 |
| 25 | 2-Heptanol | 0.01 | 0.04 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 65 |
| 26 | 6-Methyl-5-hepten-2-one | 0.06 | 0.07 | 0.03 | 0.02 | 0.08 | 0.03 | 0.05 | 0.07 | 0.03 | 0.05 | 68 |
| 27 | Dimethyl trisulfide | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 95.53 | 0.00 | 0.00 | 0.01 |
| 28 | 2-Nonanone | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 40 |
| 29 | 1-Nonanal | 3.45 | 0.00 | 2.84 | 7.33 | 0.00 | 9.05 | 0.00 | 4.19 | 1.63 | 2.49 | 1.1 |
| 30 | (E)-2-Octenal | 0.00 | 0.00 | 0.00 | 0.00 | 2.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 |
| 31 | Linalyl oxide | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100 |
| 32 | Dehydro-P-cymene | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | 0.04 | 85 |
| 33 | 1-Octen-3-ol | 0.00 | 0.00 | 0.00 | 0.58 | 0.00 | 0.00 | 0.74 | 0.00 | 0.00 | 0.00 | 1.5 |
| 34 | Octyl acetate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 47 |
| 35 | Diallyl trisulfide | 0.41 | 3.70 | 0.72 | 0.65 | 0.09 | 8.40 | 0.83 | 2.94 | 0.00 | 0.00 | 30 |
| 36 | 2-Decanone | 1.02 | 3.68 | 2.40 | 2.65 | 3.06 | 3.53 | 2.63 | 3.00 | 0.00 | 0.00 | 3 |
| 37 | Decanal | 0.00 | 0.00 | 0.00 | 5.92 | 0.00 | 12.68 | 9.86 | 2.16 | 1.83 | 1.58 | 0.5 |
| 38 | Camphor | 0.01 | 0.43 | 0.00 | 0.00 | 0.01 | 0.00 | 0.08 | 0.01 | 0.00 | 0.00 | 250 |
| 39 | Benzaldehyde | 0.08 | 0.14 | 0.02 | 0.06 | 0.04 | 0.00 | 0.01 | 0.14 | 0.12 | 0.47 | 300 |
| 40 | Linalool | 202.26 | 886.80 | 51.11 | 163.34 | 267.01 | 66.75 | 270.53 | 306.29 | 7.43 | 32.81 | 1 |
| 41 | Linalyl acetate | 0.00 | 0.38 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 | 0.00 | 1000 |
| 42 | 1-Octanol | 0.01 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.01 | 100 |
| 43 | (−)-Bornyl acetate | 0.02 | 1.14 | 0.00 | 0.01 | 0.02 | 0.07 | 0.02 | 0.02 | 0.01 | 0.03 | 75 |
| 44 | β-Caryophyllene | 1.00 | 12.54 | 10.86 | 2.37 | 7.16 | 7.24 | 1.20 | 2.71 | 0.33 | 0.56 | 64 |
| 45 | Terpinen-4-Ol | 0.39 | 0.58 | 0.35 | 0.14 | 0.34 | 0.56 | 0.44 | 0.30 | 0.05 | 0.21 | 340 |
| 46 | (E)-2-Decenal | 0.00 | 31.41 | 0.00 | 3.23 | 0.00 | 5.61 | 4.85 | 6.70 | 0.00 | 4.21 | 0.3 |
| 47 | 1-Nonanol | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 45.5 |
| 48 | Humulene | 0.03 | 0.45 | 0.17 | 0.11 | 0.28 | 0.18 | 0.08 | 0.12 | 0.01 | 0.02 | 120 |
| 49 | Estragole | 1.87 | 27.78 | 0.00 | 4.86 | 8.98 | 6.83 | 1.07 | 0.00 | 0.43 | 0.37 | 6 |
| 50 | (Z)-Citral | 0.08 | 0.64 | 0.08 | 0.00 | 0.00 | 0.04 | 0.11 | 0.28 | 0.00 | 0.00 | 30 |
| 51 | Guaiene | 0.13 | 0.72 | 0.00 | 0.15 | 0.76 | 0.00 | 0.00 | 0.45 | 0.10 | 0.10 | 20 |
| 52 | α-Terpineol | 0.54 | 2.95 | 0.42 | 0.58 | 0.98 | 1.17 | 1.61 | 1.12 | 0.17 | 0.48 | 86 |
| 53 | Borneol | 0.09 | 0.14 | 0.02 | 0.00 | 0.15 | 0.01 | 0.10 | 0.08 | 0.03 | 0.06 | 140 |
| 54 | 1-Dodecanal | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.5 |
| 55 | Piperitone | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.03 | 680 |
| 56 | (E)-Citral | 0.19 | 0.87 | 0.19 | 0.00 | 0.33 | 0.00 | 0.15 | 0.36 | 0.04 | 0.00 | 32 |
| 57 | Carvone | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 27 |
| 58 | Geranyl acetate | 0.08 | 4.78 | 0.00 | 0.00 | 0.00 | 0.82 | 0.00 | 0.00 | 0.00 | 0.28 | 36 |
| 59 | 4-Isopropylbenzaldehyde | 0.09 | 0.09 | 0.05 | 0.01 | 0.05 | 0.07 | 0.00 | 0.12 | 0.04 | 0.09 | 60 |
| 60 | Nerol | 0.00 | 0.07 | 0.00 | 0.01 | 0.02 | 0.00 | 0.05 | 0.02 | 0.00 | 0.00 | 290 |
| 61 | Phenethyl acetate | 0.05 | 0.22 | 0.11 | 0.00 | 0.06 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 | 20 |
| 62 | Anethole | 0.17 | 7.29 | 0.10 | 0.24 | 0.23 | 0.81 | 0.26 | 0.55 | 0.19 | 0.47 | 50 |
| 63 | Geraniol | 5.31 | 65.49 | 6.75 | 10.33 | 23.52 | 2.86 | 33.05 | 37.12 | 1.26 | 7.53 | 1 |
| 64 | 1-Methylnaphthalene | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 75 |
| 65 | 2-Phenylethanol | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 60 |
| 66 | O-Anisaldehyde | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.01 | 0.01 | 0.02 | 174 |
| 67 | Caryophyllene oxide | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 200 |
| 68 | Methyl eugenol | 0.37 | 0.03 | 0.00 | 0.03 | 0.00 | 0.22 | 0.08 | 0.03 | 0.01 | 0.00 | 68 |
| 69 | 4-Methoxybenzaldehyde | 0.07 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.07 | 0.04 | 0.02 | 0.00 | 27 |
| 70 | Cinnamaldehyde | 0.00 | 0.17 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.10 | 0.01 | 0.03 | 6000 |
| 71 | 4-Methylphenol | 0.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.9 |
| 72 | 3-Methylphenol | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 15 |
| 73 | Ethyl cinnamate | 0.00 | 0.12 | 0.00 | 0.05 | 0.00 | 0.00 | 0.07 | 0.17 | 0.00 | 0.00 | 17 |
| 74 | Cinnamyl acetate | 0.01 | 0.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 150 |
| 75 | Eugenol | 0.47 | 1.57 | 1.05 | 0.43 | 69.10 | 109.85 | 10.02 | 0.55 | 0.00 | 0.30 | 2.5 |
| 76 | Isoeugenol | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.06 | 0.01 | 0.00 | 0.00 | 0.00 | 100 |
| 77 | Myristicin | 0.35 | 0.06 | 0.00 | 0.05 | 0.00 | 0.34 | 0.08 | 0.03 | 0.04 | 0.00 | 30 |
| 78 | Cinnamyl alcohol | 0.00 | 0.03 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 | 77 |
| Sensor Name | 1 A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | B1 | B2 |
|---|---|---|---|---|---|---|---|---|---|---|
| W1C | 0.93 ± 0.00 d | 0.92 ± 0.00 e | 0.93 ± 0.00 d | 0.95 ± 0.00 b | 0.93 ± 0.00 d | 0.94 ± 0.00 b,c | 0.92 ± 0.00 e | 0.94 ± 0.00 c | 0.95 ± 0.00 a | 0.96 ± 0.00 a |
| W5S | 17.64 ± 1.27 c,d | 52.42 ± 10.77 a | 18.17 ± 1.32 c,d | 16.05 ± 0.44 d,e | 25.99 ± 1.28 b | 19.97 ± 0.61 c,d | 16.74 ± 1.76 c,d e | 22.19 ± 0.98 b,c | 5.55 ± 0.16 f | 11.52 ± 1.27 e |
| W3C | 0.95 ± 0.00 b | 0.94 ± 0.00 c | 0.95 ± 0.00 b | 0.96 ± 0.00 a | 0.95 ± 0.00 b | 0.96 ± 0.00 a | 0.94 ± 0.00 c | 0.96 ± 0.00 a | 0.95 ± 0.00 b | 0.96 ± 0.00 a |
| W6S | 1.29 ± 0.01 a | 1.32 ± 0.01 a | 1.29 ± 0.07 a | 1.20 ± 0.00 b | 1.23 ± 0.00 b | 1.21 ± 0.00 b | 1.32 ± 0.04 a | 1.19 ± 0.00 b | 1.33 ± 0.01 a | 1.19 ± 0.00 b |
| W5C | 0.94 ± 0.00 c,d | 0.93 ± 0.00 e,f | 0.94 ± 0.00 c | 0.95 ± 0.00 b | 0.94 ± 0.00 b | 0.95 ± 0.00 b | 0.93 ± 0.01 f | 0.95 ± 0.00 b | 0.93 ± 0.00 d,e | 0.96 ± 0.00 a |
| W1S | 2.00 ± 0.05 b | 1.95 ± 0.06 b,c | 1.92 ± 0.05 c | 1.72 ± 0.01 e | 1.85 ± 0.08 d | 1.71 ± 0.04 e,f | 2.19 ± 0.05 a | 1.84 ± 0.02 d | 1.65 ± 0.05 f | 1.51 ± 0.03 g |
| W1W | 13.66 ± 1.02 c | 33.62 ± 4.48 a | 14.31 ± 0.91 c | 12.88 ± 0.26 c | 19.39 ± 1.88 b | 16.89 ± 0.47 b | 13.09 ± 1.73 c | 18.11 ± 0.77 b | 3.61 ± 0.16 e | 10.27 ± 1.18 d |
| W2S | 2.86 ± 0.09 c | 2.94 ± 0.06 c | 2.60 ± 0.10 d | 2.17 ± 0.02 f | 2.37 ± 0.09 e | 2.19 ± 0.05 f | 3.19 ± 0.09 a | 2.29 ± 0.03 e | 3.05 ± 0.05 b | 1.98 ± 0.04 g |
| W2W | 3.94 ± 0.16 e,f | 6.44 ± 0.34 a | 3.98 ± 0.17 d | 3.67 ± 0.00 f | 4.77 ± 0.20 b | 4.35 ± 0.10 c | 3.89 ± 0.27 e,f | 4.27 ± 0.07 c | 2.43 ± 0.15 f | 3.65 ± 0.23 f |
| W3S | 1.84 ± 0.03 b | 1.96 ± 0.03 a | 1.76 ± 0.03 c | 1.56 ± 0.01 e,f | 1.63 ± 0.02 d | 1.59 ± 0.01 d,e | 1.92 ± 0.05 a | 1.56 ± 0.00 e,f | 1.93 ± 0.04 a | 1.54 ± 0.01 g |
| Attributes | 1 A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | B1 | B2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall acceptability | 8.88 ± 0.74 a | 6.18 ± 0.55 f | 7.02 ± 0.71 e | 6.14 ± 0.72 f | 7.18 ± 0.62 e | 7.58 ± 0.67 d | 8.46 ± 0.85 b | 7.78 ± 0.70 c,d | 6.22 ± 0.54 d | 7.96 ± 0.77 c |
| Beef like | 3.60 ± 0.80 b,c | 4.70 ± 0.90 a | 3.60 ± 0.66 b,c | 2.60 ± 0.66 d | 4.00 ± 1.00 a,b,c | 4.30 ± 1.00 a,b | 3.70 ± 0.78 b,c | 3.40 ± 0.80 c | 2.60 ± 0.49 d | 3.70 ± 0.64 b,c |
| Fat like | 2.20 ± 0.60 a | 1.40 ± 0.49 b | 2.30 ± 0.46 a | 1.60 ± 0.49 b | 1.40 ± 0.49 b | 1.30 ± 0.46 b | 1.50 ± 0.50 b | 1.30 ± 0.46 b | 1.30 ± 0.46 b | 1.70 ± 0.46 b |
| Spice like | 6.90 ± 0.70 c,d | 9.20 ± 0.75 a | 5.30 ± 0.64 e | 6.30 ± 0.64 d | 7.10 ± 0.54 c | 5.50 ± 0.67 e | 7.00 ± 0.63 c | 8.00 ± 0.63 b | 5.30 ± 0.78 e | 7.10 ± 0.70 c |
| Salty taste | 5.60 ± 0.80 c,d | 6.30 ± 0.64 a,b | 5.80 ± 0.60 b,c | 6.60 ± 0.49 a | 4.90 ± 0.70 e | 5.00 ± 0.63 d,e | 6.10 ± 0.70 a,b,c | 6.10 ± 0.70 a,b,c | 4.00 ± 0.77 f | 6.70 ± 0.46 a |
| Astringency taste | 2.30 ± 0.46 a,b | 2.00 ± 0.63 a,b,c | 2.40 ± 0.49 a,b | 1.90 ± 0.54 a,b,c,d | 1.50 ± 0.50 c,d | 2.20 ± 0.60 a,b | 2.00 ± 0.45 a,b,c | 1.80 ± 0.60 b,c,d | 1.40 ± 0.49 d | 1.40 ± 0.49 d |
| Sour taste | 1.70 ± 0.64 a | 1.20 ± 0.40 b,c | 1.50 ± 0.50 a,b,c | 1.60 ± 0.49 a,b | 1.90 ± 0.30 a | 1.80 ± 0.60 a | 1.50 ± 0.50 a,b,c | 1.50 ± 0.50 a,b,c | 1.80 ± 0.40 a | 1.10 ± 0.30 c |
| Bitter taste | 2.20 ± 0.40 d | 3.10 ± 0.70 a,b,c | 2.70 ± 0.46 b,c,d | 3.60 ± 0.66 a | 2.50 ± 0.50 d | 3.20 ± 0.40 b,c | 2.60 ± 0.49 c,d | 2.50 ± 0.50 d | 2.40 ± 0.49 d | 2.30 ± 0.46 d |
| Umami taste | 7.90 ± 0.83 a | 5.90 ± 0.70 e | 6.00 ± 0.63 d,e | 6.30 ± 0.64 d,e | 6.70 ± 0.78 d,e | 7.10 ± 0.70 b,c | 7.80 ± 0.75 a,b | 7.10 ± 0.70 b,c | 6.10 ± 0.70 d,e | 7.20 ± 0.75 a,b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Jiang, J.; Zang, M.; Zhang, K.; Li, D.; Li, X. Flavor Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue. Bioengineering 2022, 9, 582. https://doi.org/10.3390/bioengineering9100582
Zhang Z, Jiang J, Zang M, Zhang K, Li D, Li X. Flavor Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue. Bioengineering. 2022; 9(10):582. https://doi.org/10.3390/bioengineering9100582
Chicago/Turabian StyleZhang, Zheqi, Jiaolong Jiang, Mingwu Zang, Kaihua Zhang, Dan Li, and Xiaoman Li. 2022. "Flavor Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue" Bioengineering 9, no. 10: 582. https://doi.org/10.3390/bioengineering9100582
APA StyleZhang, Z., Jiang, J., Zang, M., Zhang, K., Li, D., & Li, X. (2022). Flavor Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue. Bioengineering, 9(10), 582. https://doi.org/10.3390/bioengineering9100582