Effects of Oxygen Transference on Protease Production by Rhodotorula mucilaginosa CBMAI 1528 in a Stirred Tank Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Growth Conditions
2.2. Inoculum and Culture Conditions
2.3. Quantification of Biomass, Glucose, Total Protein, and Proteolytic Activity
2.4. Determination of Volumetric Oxygen Transfer Coefficient (kLa)
2.5. Kinetic Parameters Calculation
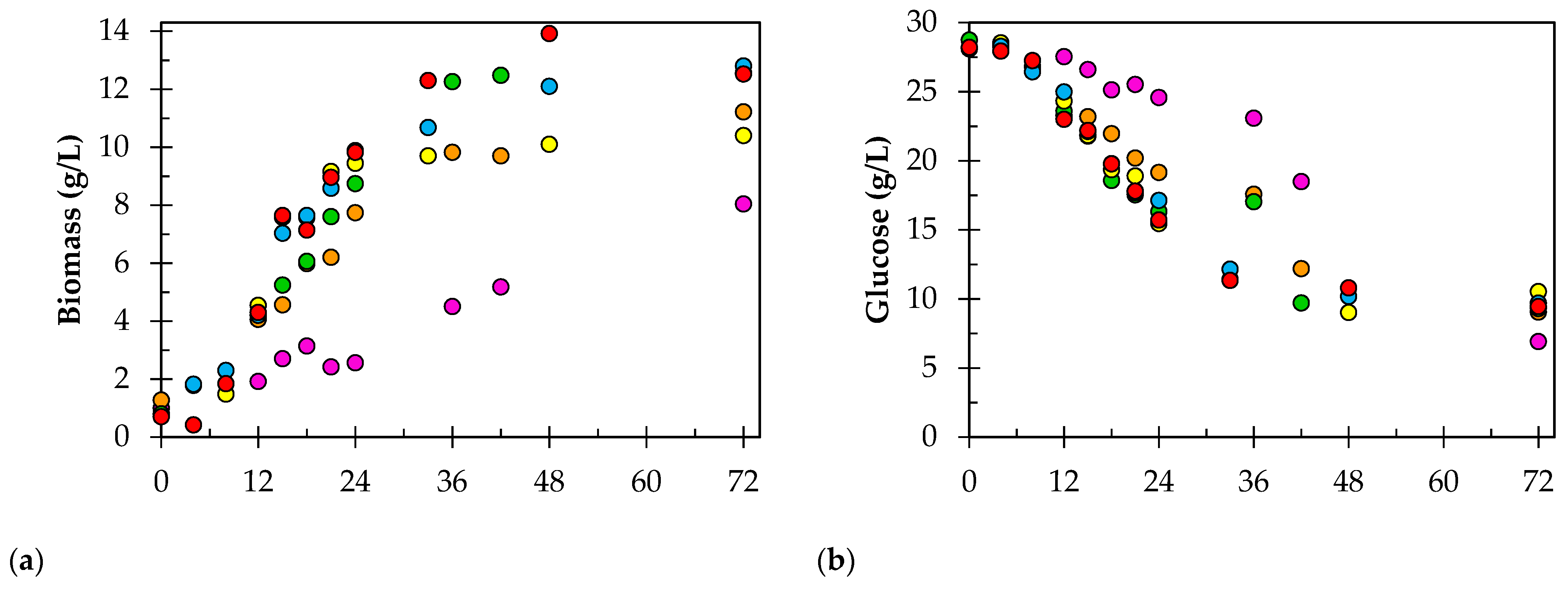
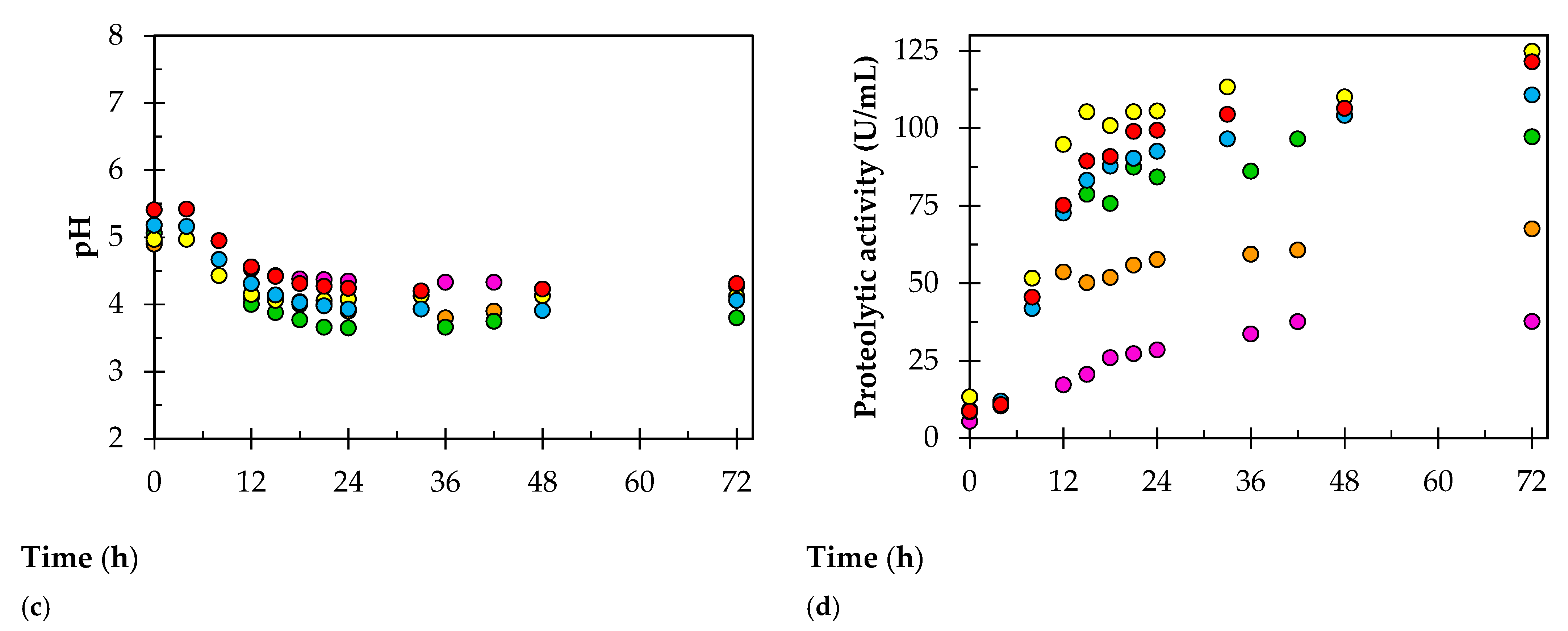
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurumallesh, P.; Alagu, K.; Ramakrishnan, B.; Muthusamy, S. A systematic reconsideration on proteases. Int. J. Biol. Macromol. 2019, 128, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Dadshahi, Z.; Homaei, A.; Zeinali, F.; Sajedi, R.H.; Khajeh, K. Extraction and purification of a highly thermostable alkaline caseinolytic protease from wastes Penaeus vannamei suitable for food and detergent industries. Food Chem. 2016, 202, 110–115. [Google Scholar] [CrossRef]
- Pillaca-Pullo, O.S.; Intiquilla, A.; Santos, J.; Sanchez-Moguel, I.; Brandelli, A.; Zavaleta, A.I. Purification of Pseudomonas sp. proteases through aqueous biphasic systems as an alternative source to obtain bioactive protein hydrolysates. Biotechnol. Prog. 2021, 37, e3003. [Google Scholar] [CrossRef] [PubMed]
- GVR. Enzymes Market Size, Share & Trends Analysis Report by Application (Industrial Enzymes, Specialty Enzymes), by Product (Carbohydrase, Proteases, Lipases), by Source, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/enzymes-industry (accessed on 18 September 2022).
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef]
- Rodarte, M.P.; Dias, D.R.; Vilela, D.M.; Schwan, R.F. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Sci.-Agron. 2011, 33, 457–464. [Google Scholar] [CrossRef]
- Hashmi, S.; Iqbal, S.; Ahmed, I.; Janjua, H.A. Production, Optimization, and partial purification of alkali-thermotolerant proteases from newly isolated Bacillus subtilis S1 and Bacillus amyloliquefaciens KSM12. Processes 2022, 10, 1050. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additives. Appl. Microbiol. Biotechnol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef]
- Ray, M.K.; Devi, K.U.; Kumar, G.S.; Shivaji, S. Extracellular protease from the Antarctic yeast Candida humicola. Appl. Environ. Microbiol. 1992, 58, 1918–1923. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Pazgier, M.; Kalinowska, H.; Bielecki, S. A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 2003, 7, 435–442. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; dos Santos, J.A.; Vianna, M.V.; Vieira, J.M.F.; Mallagutti, V.H.; Inforsato, F.J.; Wentzel, L.C.P.; Lario, L.D.; Rodrigues, A.; Pagnocca, F.C.; et al. Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit. Rev. Biotechnol. 2018, 38, 600–619. [Google Scholar] [CrossRef]
- Arabacı, N.; Arıkan, B. Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep. Biochem. Biotechnol. 2018, 48, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.W.F.; Dayo-Owoyemi, I.; Nobre, F.S.; Pagnocca, F.C.; Chaud, L.C.S.; Pessoa, A.; Felipe, M.G.A.; Sette, L.D. Taxonomic assessment and enzyme production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles 2013, 17, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Chaud, L.C.S.; Lario, L.D.; Bonugli-Santos, R.C.; Sette, L.D.; Pessoa, A.; Felipe, M.D.D. Improvement in extracellular protease production by the marine antarctic yeast Rhodotorula mucilaginosa L7. New Biotechnol. 2016, 33, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Lario, L.D.; Malpiedi, L.P.; Pereira, J.F.B.; Sette, L.D.; Pessoa, A. Liquid-liquid extraction of protease from cold-adapted yeast Rhodotorula mucilaginosa L7 using biocompatible and biodegradable aqueous two-phase systems. Sep. Sci. Technol. 2016, 51, 57–67. [Google Scholar] [CrossRef]
- Lario, L.D.; Chaud, L.; Almeida, M.D.; Converti, A.; Sette, L.D.; Pessoa, A. Production, purification, and characterization of an extracellular acid protease from the marine Antarctic yeast Rhodotorula mucilaginosa L7. Fungal Biol. 2015, 119, 1129–1136. [Google Scholar] [CrossRef]
- Mamo, J.; Assefa, F. The Role of microbial aspartic protease enzyme in food and beverage industries. J. Food Qual. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Nair, I.C.; Jayachandran, K. Aspartic Proteases in Food Industry, Green Bio-Processes: Enzymes in Industrial Food Processing; Springer: Singapore, 2019; pp. 15–30. [Google Scholar]
- Theron, L.W.; Divol, B. Microbial aspartic proteases: Current and potential applications in industry. Appl. Microbiol. Biotechnol. 2014, 98, 8853–8868. [Google Scholar] [CrossRef]
- Dasgupta, D.; Sharma, T.; Bhatt, A.; Bandhu, S.; Ghosh, D. Cultivation of oleaginous yeast Rhodotorula mucilaginosa IIPL32 in split column airlift reactor and its influence on fuel properties. Biocatal. Agric. Biotechnol. 2017, 10, 308–316. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, T.; Nautiyal, A.K.; Dasgupta, D.; Hazra, S.; Bhaskar, T.; Ghosh, D. Scale-up strategy for yeast single cell oil production for Rhodotorula mucilagenosa IIPL32 from corn cob derived pentosan. Bioresour. Technol. 2020, 309. [Google Scholar] [CrossRef]
- Prabhu, A.A.; Gadela, R.; Bharali, B.; Deshavath, N.N.; Dasu, V.V. Development of high biomass and lipid yielding medium for newly isolated Rhodotorula mucilaginosa. Fuel 2019, 239, 874–885. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; To, R.J.B.; Latta, R.K.; Biely, P.; Schneider, H. Some properties of extracellular acetylxylan esterase produced by yeast Rhodotorula mucilaginosa. Appl. Environ. Microbiol. 1987, 53, 2831–2834. [Google Scholar] [CrossRef] [PubMed]
- Kotik, M.; Brichac, J.; Kyslik, P. Novel microbial epoxide hydrolases for biohydrolysis of glycidyl derivatives. J. Biotechnol. 2005, 120, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Tam, M.F.; Lee, S.S.; Tai, H.Y.; Chang, C.Y.; Chou, C.T.; Shen, H.D. A vacuolar serine protease (Rho m 2) is a major allergen of Rhodotorula mucilaginosa and belongs to a class of highly conserved pan-fungal allergens. Int. Arch. Allergy Immunol. 2005, 138, 134–141. [Google Scholar] [CrossRef]
- Hesham, A.E.L.; Alrumman, S.A.; Al-Dayel, M.A.; Salah, H.A. Screening and genetic identification of acidic and neutral protease-producing yeasts strains by 26S rRNA gene sequencing. Cytol. Genet. 2017, 51, 221–229. [Google Scholar] [CrossRef]
- Zimmer, C.; Platz, T.; Cadez, N.; Giffhorn, F.; Kohring, G.W. A cold active (2R,3R)-(-)-di-O-benzoyl-tartrate hydrolyzing esterase from Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2006, 73, 132–140. [Google Scholar] [CrossRef]
- Potumarthi, R.; Subhakar, C.; Vanajakshi, J.; Jetty, A. Effect of aeration and agitation regimes on lipase production by newly isolated Rhodotorula mucilaginosa-MTCC 8737 in stirred tank reactor using molasses as sole production medium. Appl. Biochem. Biotechnol. 2008, 151, 700–710. [Google Scholar] [CrossRef]
- Chennupati, S.; Potumarthi, R.; Gopal Rao, M.; Manga, P.L.; Sridevi, M.; Jetty, A. Multiple responses optimization and modeling of lipase production by Rhodotorula mucilaginosa MTCC-8737 using response surface methodology. Appl. Biochem. Biotechnol. 2009, 159, 317–329. [Google Scholar] [CrossRef]
- Nuylert, A.; Hongpattarakere, T. Improvement of cell-bound lipase from Rhodotorula mucilaginosa P11I89 for use as a methanol-tolerant, whole-cell biocatalyst for production of palm-oil biodiesel. Ann. Microbiol. 2013, 63, 929–939. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, J.D. Enhancement of phenylalanine ammonia lyase production from Rhodotorula mucilaginosa by optimization of culture conditions in batch and fed-batch. Biotechnol. Biotechnol. Equip. 2012, 26, 3418–3423. [Google Scholar] [CrossRef]
- Vaz, A.B.M.; Rosa, L.H.; Vieira, M.L.A.; de Garcia, V.; Brandao, L.R.; Teixeira, L.C.R.S.; Moline, M.; Libkind, D.; van Broock, M.; Rosa, C.A. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz. J. Microbiol. 2011, 42, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Hamid, B.; Singh, P.; Ranjan, K.; Chauhan, D.; Rana, R.S.; Chaurse, V.K. Evaluation of pectinolytic activities for oenological uses from psychrotrophic yeasts. Lett. Appl. Microbiol. 2013, 57, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Ran, Q.Q.; Zhang, X. Screening and identification of a cutinase-producing Rhodotorula mucilaginosa and properties of the cutinase. Appl. Biochem. Biotechnol. 2015, 175, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, X.T.; Liu, J.W. Purification and characterization of a novel cold-adapted phytase from Rhodotorula mucilaginosa strain JMUY14 isolated from Antarctic. J. Basic Microbiol. 2015, 55, 1029–1039. [Google Scholar] [CrossRef]
- Hu, K.; Zhu, X.L.; Mu, H.; Ma, Y.; Ullah, N.; Tao, Y.S. A novel extracellular glycosidase activity from Rhodotorula mucilaginosa: Its application potential in wine aroma enhancement. Lett. Appl. Microbiol. 2016, 62, 169–176. [Google Scholar] [CrossRef]
- Li, N.; Cui, R.; Zhang, F.; Meng, X.H.; Liu, B.J. A novel enzyme from Rhodotorula mucilaginosa aldolase: Isolation, identification and degradation for patulin in apple juice. Process Biochem. 2022, 116, 148–156. [Google Scholar] [CrossRef]
- Ali, H.; Zhu, S.; Solsvik, J. Effects of geometric parameters on volumetric mass transfer coefficient of non-Newtonian fluids in stirred tanks. Int. J. Chem. React. Eng. 2021, 20, 697–711. [Google Scholar] [CrossRef]
- Pillaca-Pullo, O.; Vieira, L.D.; Takagi, M. Scale-up of capsular polysaccharide production process by Haemophilus influenzae type b using kLa criterion. Bioengineering 2022, 9, 415. [Google Scholar] [CrossRef]
- Mahler, N.; Tschirren, S.; Pflügl, S.; Herwig, C. Optimized bioreactor setup for scale-up studies of extreme halophilic cultures. Biochem. Eng. J. 2018, 130, 39–46. [Google Scholar] [CrossRef]
- Michelin, M.; Mota, A.M.D.O.; Polizeli, M.D.L.T.D.M.; da Silva, D.P.; Vicente, A.A.; Teixeira, J.A. Influence of volumetric oxygen transfer coefficient (kLa) on xylanases batch production by Aspergillus niger van Tieghem in stirred tank and internal-loop airlift bioreactors. Biochem. Eng. J. 2013, 80, 19–26. [Google Scholar] [CrossRef]
- Lario, L.D.; Pillaca-Pullo, O.S.; Durães Sette, L.; Converti, A.; Casati, P.; Spampinato, C.; Pessoa, A. Optimization of protease production and sequence analysis of the purified enzyme from the cold adapted yeast. Biotechnol. Rep. 2020, 28, e00546. [Google Scholar] [CrossRef] [PubMed]
- PIRT, S.J. Oxygen Demand and Supply—Principles of Microbe and Cell Cultivation; John Wiley: New York, NY, USA, 1975; pp. 81–94. [Google Scholar]
- Mainardi, P.H.; Feitosa, V.A.; de Paiva, L.B.B.; Bonugli-Santos, R.C.; Squina, F.M.; Pessoa, A.; Sette, L.D. Laccase production in bioreactor scale under saline condition by the marine-derived basidiomycete Peniophora sp CBMAI 1063. Fungal Biol. 2018, 122, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Pillaca-Pullo, O.; Rodrigues, D.; Sanchez-Moguel, I.; Lopes, A.; Pimenta, M.; Basi, T.; Feitosa, V.; Zavaleta, A.I.; Monteiro, G.; Pessoa, A.; et al. Recombinantl-asparaginase production using Pichia pastoris(MUT(s)strain): Establishment of conditions for growth and induction phases. J. Chem. Technol. Biotechnol. 2021, 96, 283–292. [Google Scholar] [CrossRef]
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Maldonade, I.R.; Rodriguez-Amaya, D.B.; Scamparini, A.R.P. Statistical optimisation of cell growth and carotenoid production by Rhodotorula mucilaginosa. Braz. J. Microbiol. 2012, 43, 109–115. [Google Scholar] [CrossRef]
- Rendon-Castrillon, L.; Ramirez-Carmona, M.; Ocampo-Lopez, C.; Gomez-Arroyave, L. Mathematical model for scaling up bioprocesses using experiment design combined with Buckingham Pi theorem. Appl. Sci. 2021, 11, 11338. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Guimaraes, A.A.C.; Rocha, L.V.F.; Winterburn, J.; Santos-Ebinuma, V.D.; Pereira, J.F.B. Improvement of carotenoids production from Rhodotorula glutinis CCT-2186. Biochem. Eng. J. 2021, 165, 107827. [Google Scholar] [CrossRef]
- Fenice, M.; Barghini, P.; Selbmann, L.; Federici, F. Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003. Microb. Cell Factories 2012, 11, 12. [Google Scholar] [CrossRef]
- Teruasmaki, P.; Latua-Kokko, M.; Taskila, S.; Tanskanen, J. Effect of oxygen transfer on yeast growth-growth kinetic and reactor model to estimate scale-up effects in bioreactors. Food Bioprod. Process. 2018, 111, 129–140. [Google Scholar] [CrossRef]
- Pessoa, A.; Vitolo, M.; Hustedt, H. Use of KLa as a criterion for scaling up the inulinase fermentation process. Appl. Biochem. Biotechnol. 1996, 57–58, 699–709. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.R.; He, H.W.; Sang, B.; Yu, D.L.; Feng, J.T.; Zhang, X. Effects of agitation, aeration and temperature on production of a novel glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santisteban, B.O.Y.; Maugeri, F. Agitation, aeration and shear stress as key factors in inulinase production by Kluyveromyces marxianus. Enzym. Microb. Technol. 2005, 36, 717–724. [Google Scholar] [CrossRef]
- Kim Gail, C. The oxygen transfer rate and overall volumetric oxygen transfer coefficient. In Bioprocess Engineering; Kim Gail, C., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 147–170. [Google Scholar]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.; Bárzana, E. Single Cell Protein: Yeasts and Bacteria. In Encyclopedia of Food Microbiology, 2nd ed.; Carl, B., Ed.; Academic Press: Oxford, UK, 2014; pp. 431–438. [Google Scholar]
- Kante, R.K.; Somavarapu, S.; Vemula, S.; Kethineni, C.; Mallu, M.R.; Ronda, S.R. Production of recombinant human asparaginase from Escherichia coli under optimized fermentation conditions: Effect of Physicochemical properties on enzyme activity. Biotechnol. Bioprocess Eng. 2019, 24, 824–832. [Google Scholar] [CrossRef]
- Abdella, A.; Segato, F.; Wilkins, M.R. Optimization of process parameters and fermentation strategy for xylanase production in a stirred tank reactor using a mutant. Biotechnol. Rep. 2020, 26, e00457. [Google Scholar] [CrossRef]
| Experiment | Agitation (rpm) | Aeration (vvm) | kLa (h−1) |
|---|---|---|---|
| 1 | 100 | 1.0 | 18 |
| 2 | 300 | 1.0 | 49 |
| 3 | 500 | 1.0 | 99 |
| 4 | 500 | 1.5 | 121 |
| 5 | 500 | 2.0 | 135 |
| 6 | 500 | 2.5 | 102 |
| Parameter | Experiment (kLa) | |||||
|---|---|---|---|---|---|---|
| 1 (18 h−1) | 2 (49 h−1) | 3 (99 h−1) | 4 (121 h−1) | 5 (135 h−1) | 6 (102 h−1) | |
| Agitation (rpm) | 100 | 300 | 500 | 500 | 500 | 500 |
| Aeration (vvm) | 1.0 | 1.0 | 1.0 | 1.5 | 2.0 | 2.5 |
| Biomass (g.L−1) ± sd | 8.0 ± 0.3 | 11.2 ± 0.5 | 12.6 ± 0.2 | 10.4 ± 0.6 | 12.8 ± 0.4 | 12.5 ± 0.8 |
| pH | 4.3 | 3.8 | 3.8 | 4.1 | 4.0 | 4.3 |
| Proteolytic activity (U.mL−1) ± sd | 37.7 ± 7.1 | 67.6 ± 5.3 | 97.2 ± 1.3 | 124.9 ± 5.1 | 110.8 ± 3.4 | 121.5 ± 2.5 |
| Glucose (g.L−1) ± sd | 6.9 ± 0.3 | 9.0 ± 0.8 | 9.3 ± 0.5 | 10.5 ± 1.2 | 9.7 ± 0.7 | 9.5 ± 1.1 |
| Total protein (g.L−1) ± sd | 3.8 ± 0.1 | 3.6 ± 0.3 | 3.4 ± 0.1 | 4.0 ± 0.2 | 4.6 ± 0.4 | 4.2 ± 0.2 |
| Time (h) | Experiment (kLa) / Productivity (U/L.h−1) | |||||
|---|---|---|---|---|---|---|
| 1 (18 h−1) | 2 (49 h−1) | 3 (99 h−1) | 4 (121 h−1) | 5 (135 h−1) | 6 (102 h−1) | |
| 4 | nc | nc | nc | nc | 0.813 ± 0.033 | 0.519 ± 0.031 |
| 8 | nc | nc | nc | 4.784 ± 0.012 | 4.144 ± 0.010 | 4.598 ± 0.014 |
| 12 | 0.977 ± 0.023 | 3.764 ± 0.031 | 5.488 ± 0.012 | 6.784 ± 0.023 | 5.327 ± 0.010 | 5.535 ± 0.019 |
| 15 | 1.009 ± 0.020 | 2.781 ± 0.019 | 4.639 ± 0.014 | 6.132 ± 0.019 | 4.967 ± 0.021 | 5.378 ± 0.010 |
| 18 | 1.139 ± 0.011 | 2.411 ± 0.020 | 3.699 ± 0.023 | 4.861 ± 0.010 | 4.394 ± 0.024 | 4.563 ± 0.022 |
| 21 | 1.039 ± 0.019 | 2.255 ± 0.022 | 3.728 ± 0.021 | 4.377 ± 0.010 | 3.884 ± 0.011 | 4.299 ± 0.020 |
| 24 | 0.960 ± 0.038 | 2.049 ± 0.012 | 3.131 ± 0.011 | 3.841 ± 0.034 | 3.494 ± 0.023 | 3.776 ± 0.011 |
| 33 | nc | nc | nc | 3.030 ± 0.024 | 2.663 ± 0.012 | 2.902 ± 0.010 |
| 36 | 0.782 ± 0.021 | 1.414 ± 0.011 | 2.138 ± 0.028 | nc | nc | nc |
| 42 | 0.765 ± 0.014 | 1.244 ± 0.028 | 2.081 ± 0.024 | nc | nc | nc |
| 48 | nc | nc | nc | 2.016 ± 0.011 | 1.989 ± 0.026 | 2.035 ± 0.012 |
| 72 | 0.447 ± 0.030 | 0.820 ± 0.019 | 1.223 ± 0.014 | 1.549 ± 0.010 | 1.417 ± 0.020 | 1.565 ± 0.023 |
| Parameter | Experiment (kLa) | |||||
|---|---|---|---|---|---|---|
| 1 (18 h−1) | 2 (49 h−1) | 3 (99 h−1) | 4 (121 h−1) | 5 (135 h−1) | 6 (102 h−1) | |
| µmáx (h−1) | 0.06 | 0.08 | 0.12 | 0.14 | 0.12 | 0.13 |
| Yx/s (g.g−1) | 0.29 | 0.52 | 0.61 | 0.48 | 0.54 | 0.63 |
| Enzyme | Strain | Maximum Activity | Time (h) | rpm | T (°C) | Main Nutrients | X (g.L−1) | Initial pH | Final pH | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetylxylan esterase | NRC 211003 | 2.1 µmol/mL.h | 120 | 200 | 30 | (NH4)2SO4, glycerol | nr | 5.5 | nr | [24] |
| Epoxide hydrolase | M002 | nr | nr | nr | nr | nr | nr | nr | nr | [25] |
| Serine protease | nr | nr | 24 | nr | 28 | Dextrose, peptone, (NH4)2SO4, | nr | 5.5 | nr | [26] |
| Aspartic protease | CBMAI 1528 | 11.1 U/mL | 120 | 150 | 25 | Dextrose, animal peptone, casein peptone | nr | 5.5 | nr | [13] |
| Aspartic protease | CBMAI 1528 | ~65 U/mL | 48 | 150 | 25 | Dextrose, peptone | ~70 Log CFU/mL | 5.6 | ~3.6 | [16] |
| Aspartic protease | CBMAI 1528 | 33.4 U/mL | 120 | 150 | 25 | Dextrose, peptone | 3 × 108 cells/mL | 5.5 | nr | [14] |
| Neutral protease | KKU-M12c | 140.3 U/mL | 48 | 120 | 30 | Yeast extract, peptone, dextrose, casein | nr | nr | nr | [27] |
| Acid protease | KKU-M12c | 175 U/mL | 48 | 120 | 30 | Yeast Extract, peptone, dextrose, casein | nr | nr | nr | [27] |
| Lipase | MTCC-8737 | 29.9 U/L | 120 | 150 | 28 | Dextrose, malt extract, yeast extract, peptone | 0.14 | nr | nr | [30] |
| Lipase | P11I89 | 272.7 U/L | 60 | 200 | 30 | Palm oil, yeast extract, NH4NO3 | 11.2 | nr | nr | [31] |
| Pectinase | CRUB138 | nr | nr | na | nr | Dextrose, pectin, yeast extract, peptone, agar | nr | 7.0 | nr | [33] |
| Pectinase | PT1 | 400 U/L | nr | 150 | 12 | Malt extract, peptone, pectin, K2HPO4, citrate | nr | 5.0 | nr | [34] |
| Cutinase | Pink | 9.5 U/mL | 96-120 | 160 | 30 | Lactose, yeast extract, | nr | 6.5 | nr | [35] |
| Phytase | JMUY14 | 205.5 U/mL | 168 | 150 | 15 | Dextrose, peptone, (NH4)2SO4 | nr | 5.5 | nr | [36] |
| Glycosidase | nr | 0.42 U/mL | 72 | nr | nr | nr | nr | nr | nr | [37] |
| Biomolecules | Strain | Maximum Production | Time (h) | Bioreactor | rpm | vvm | kLa (h−1) | T (°C) | Main Nutrients | X (g.L−1) | Initial pH | Final pH | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspartic protease | CBMAI 1528 | 124.9 U/mL | 72 | STR | 500 | 1.5 | 135 | 25 | Glucose, animal peptone, casein peptone | 10.4 | 5.5 | 4.1 | This study |
| Aspartic protease | CBMAI 1528 | 111.2 U/mL | nr | STR | 500 | 2.0 | 92 | 20 | Glucose, casein tryptone | 6.7 | 5.6 | nr | [43] |
| Esterase | saar1 | 19.5 U/mg | 20 | nr | 300 | 2.5 | nr | nr | Dibenzoyl-tartrate, yeast extract, KNO3, (NH4)2SO4, NH4Cl | nr | 7.4 | 7.7 | [28] |
| Lipase | MTCC 8737 | 72 U/mL | 96 | STR | 200 | 2.0 | nr | 30 | Dextrose, malt extract, yeast extract, peptone | 6.6 | 7.0 | 7.0 | [29] |
| Phenylalanine ammonia-lyase | nr | 41 U/g | 50 | STR | 200 | 1.0 | nr | 30 | Dextrose, peptone, yeast extract, (NH4)2SO4 | 3.4 | 6.0–7.0 | 6.0–7.0 | [32] |
| Lipids | IIPL32 | 8.6% w/w | 12 | Split column airlift | nr | 1.5 | 0.894 | 32 | Sugarcane bagasse | 11.6 | 4.5 | 4.5 | [20] |
| Lipids | IIPL32 | 1.83 g/L | nr | STR | 180 | nr | nr | 32 | Xylose rich corn cob hydrolysate | nr | 5.5 | 5.5 | [21] |
| Lipids | nr | 0.25 g/g | 50 | STR | 300 | 1.0 | nr | 28 | Glucose, malt extract, peptone | 15.0 | 6.0 | 6.0 | [22] |
| Carotenoids | MTCC-1403 | 819.23 µg/g | 84 | STR | 120 | 1.0 | nr | 26 | Onion peel, mung bean husk | nr | 6.2 | 6.2 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, S.; Feitosa, V.; Pillaca-Pullo, O.; Lario, L.; Sette, L.; Pessoa, A., Jr.; Alves, H. Effects of Oxygen Transference on Protease Production by Rhodotorula mucilaginosa CBMAI 1528 in a Stirred Tank Bioreactor. Bioengineering 2022, 9, 694. https://doi.org/10.3390/bioengineering9110694
Machado S, Feitosa V, Pillaca-Pullo O, Lario L, Sette L, Pessoa A Jr., Alves H. Effects of Oxygen Transference on Protease Production by Rhodotorula mucilaginosa CBMAI 1528 in a Stirred Tank Bioreactor. Bioengineering. 2022; 9(11):694. https://doi.org/10.3390/bioengineering9110694
Chicago/Turabian StyleMachado, Suellen, Valker Feitosa, Omar Pillaca-Pullo, Luciana Lario, Lara Sette, Adalberto Pessoa, Jr., and Harley Alves. 2022. "Effects of Oxygen Transference on Protease Production by Rhodotorula mucilaginosa CBMAI 1528 in a Stirred Tank Bioreactor" Bioengineering 9, no. 11: 694. https://doi.org/10.3390/bioengineering9110694
APA StyleMachado, S., Feitosa, V., Pillaca-Pullo, O., Lario, L., Sette, L., Pessoa, A., Jr., & Alves, H. (2022). Effects of Oxygen Transference on Protease Production by Rhodotorula mucilaginosa CBMAI 1528 in a Stirred Tank Bioreactor. Bioengineering, 9(11), 694. https://doi.org/10.3390/bioengineering9110694









