Engineering Spatiotemporal Control in Vascularized Tissues
Abstract
1. Introduction
2. Temporal Biology of Angiogenesis
3. Growth Factors Regulation in Angiogenesis
Combinatorial Regulation Chemical Factors in Engineering Vascularized Tissues
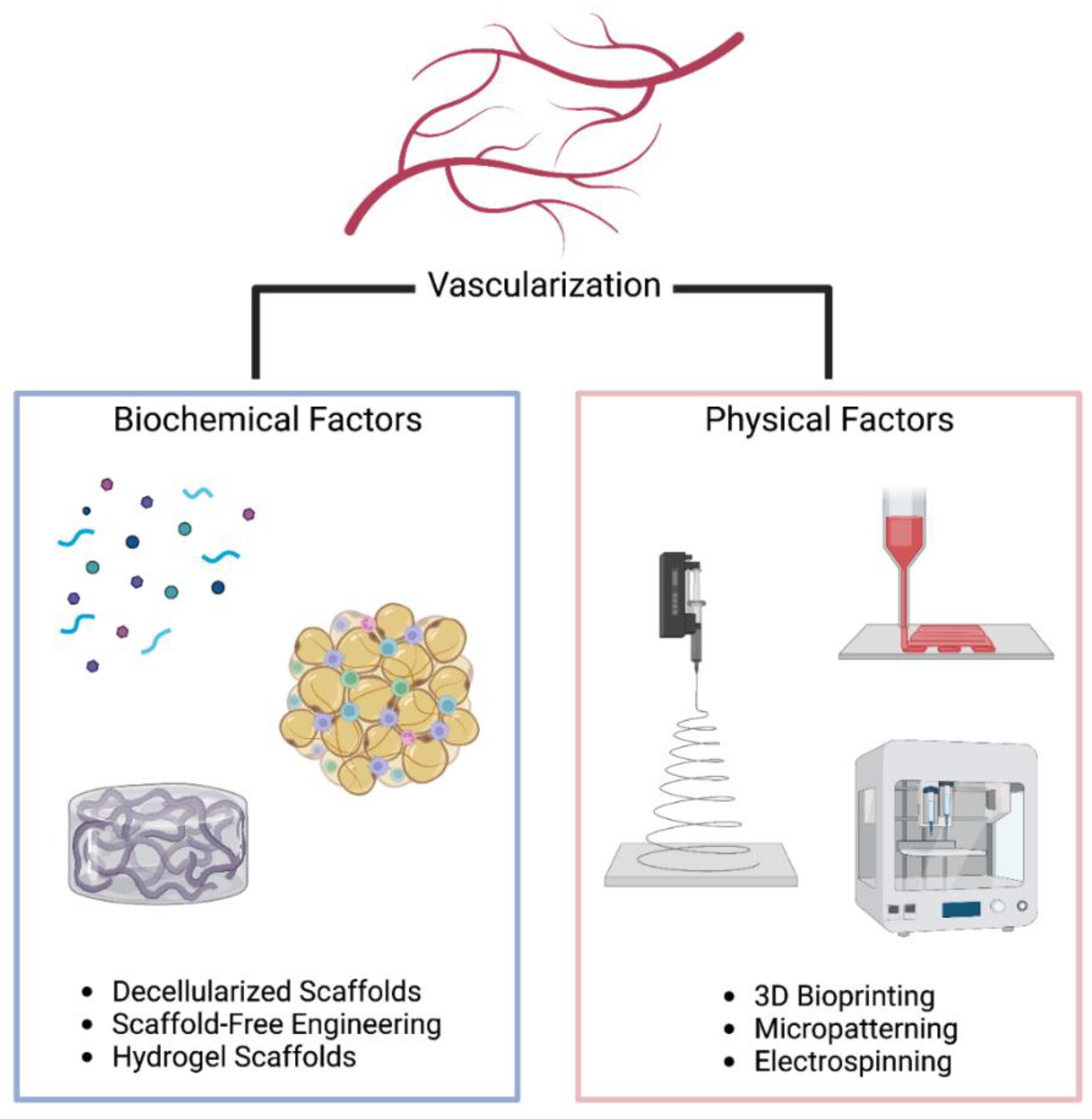
4. Spatial Control in Engineering Vascularized Tissues
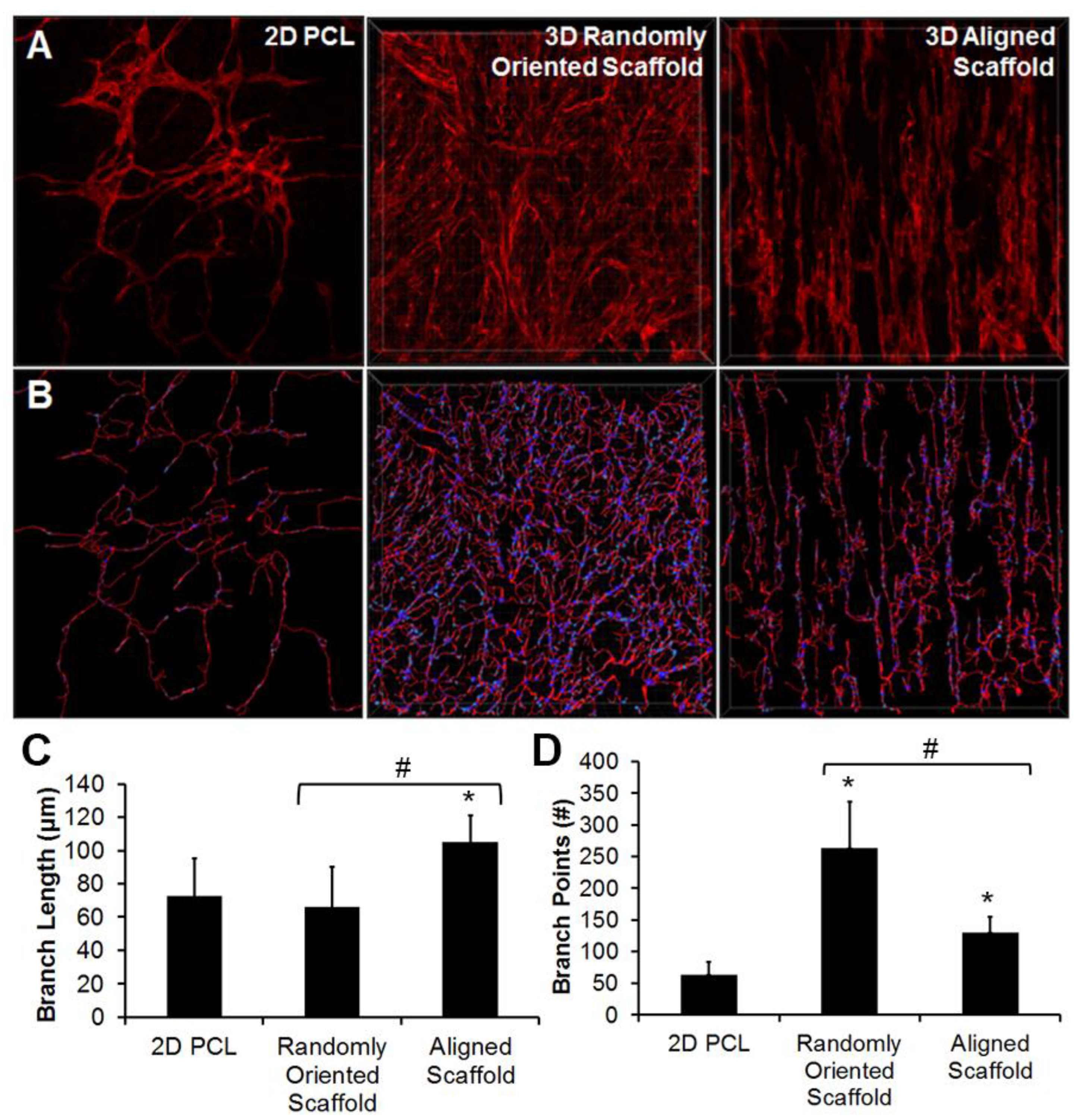
4.1. Three-Dimensiona; Bioprinting
| Bioprinting Technique | Bioprinted Cellular Types | Vascularization Application | Limitations | Ref |
|---|---|---|---|---|
| Inkjet Based Bioprinting | Human umbilical vein endothelial cells (HUVECs) Rat Smooth muscle cells (SMCs) |
|
| [48] |
| Extrusion Based Bioprinting | Human umbilical vein endothelial cells (HUVECs) Human umbilical vein smooth muscle cells (HUVSMCs), human bone marrow derived mesenchymal stem cells (hMSCs) Mouse embryonic fibroblasts (MEF) |
|
| [49] |
|
| [50,51] |
Multi-Material Bioprinting
4.2. Electrospinning
4.3. Patterning of Bioactive Molecules
5. Spatiotemporal Regulation of Engineering Vascularized Cardiac Patches
5.1. Vascularized Cardiac Patch with Temporal Regulation
5.1.1. Engineering Vascularized Patch with Temporal Regulation In Vitro
5.1.2. Engineering Vascularized Patch with Temporal Regulation In Vivo
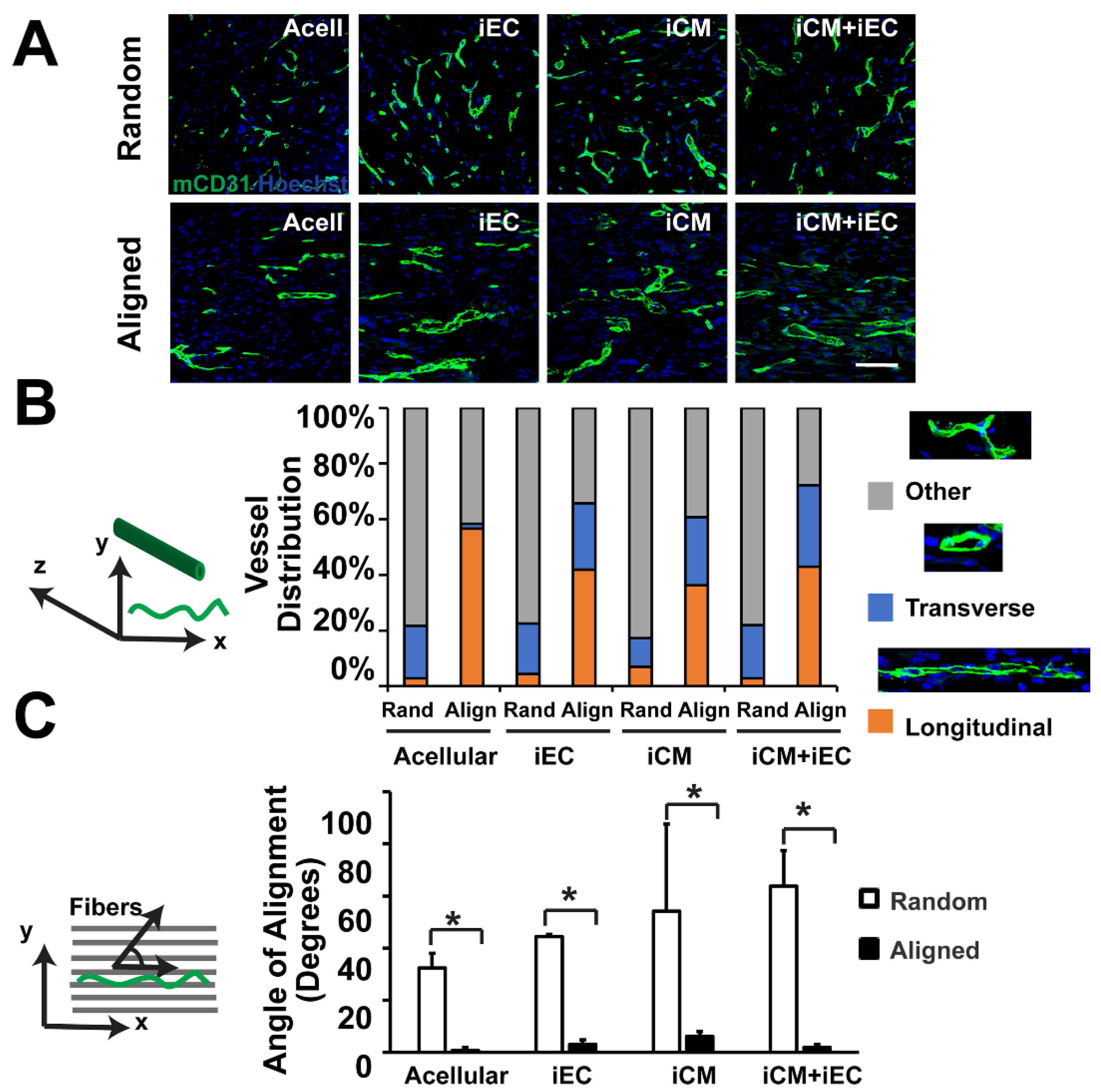
5.2. Engineering Vascularized Patch with Spatial Regulation
5.2.1. Engineering Vascularized Patch with Spatial Regulation In Vitro
5.2.2. Engineering Vascularized Patch with Spatial Regulation In Vivo
6. Challenges and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, X.; Zhan, Z. Metabolic Regulation of Cardiac Regeneration. Front. Cardiovasc. Med. 2022, 9, 933060. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction—From repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A. Fabrication of Human Serum Albumin Film for Enhanced Hemocompatibility and Mitigation of Neointimal Hyperplasia Under Physiologically Relevant Flow Shear Conditions; Clemson University: Clemson, SC, USA, 2017. [Google Scholar]
- Khanna, A.; Luzinov, I.; Burtovvy, R.; Vatansever, F.; Langan, E., III; LaBerge, M. Fabrication of Human Serum Albumin film on expanded polytetrafluoroethylene (e-PTFE) for Enhanced Hemocompatibility and Adhesion Strength. In Proceedings of the Society for Biomaterials, Minneapolis, MN, USA, April 2017; p. 810. [Google Scholar]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Cathery, W.; Faulkner, A.; Maselli, D.; Madeddu, P. Concise Review: The Regenerative Journey of Pericytes Toward Clinical Translation. Stem Cells 2018, 36, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, B.; Rong, W.W.; Goede, A.; Hoffmann, B.; Scarpa, F.; Kuebler, W.M.; Secomb, T.W.; Pries, A.R. Coalescent angiogenesis-evidence for a novel concept of vascular network maturation. Angiogenesis 2022, 25, 35. [Google Scholar] [CrossRef]
- Peluzzo, A.M.; Autieri, M.V. Challenging the Paradigm: Anti-Inflammatory Interleukins and Angiogenesis. Cells 2022, 11, 587. [Google Scholar] [CrossRef]
- Trindade, A.; Duarte, A. Notch Signaling Function in the Angiocrine Regulation of Tumor Development. Cells 2020, 9, 2467. [Google Scholar] [CrossRef]
- Crosby, C.; Zoldan, J. Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen. Biomater. 2019, 6, 61–73. [Google Scholar] [CrossRef]
- Kant, R.J.; Coulombe, K.L. Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomater. 2018, 69, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Wolff, T.; Valente, P.; Di Maggio, N.; Pellegrino, M.; Gürke, L.; Banfi, A.; Gianni-Barrera, R. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss Med. Wkly. 2019, 149, w20011. [Google Scholar] [CrossRef] [PubMed]
- Campinho, P.; Vilfan, A.; Vermot, J. Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior. Front. Physiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular Mediators of Angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2018, 110, 775–785. [Google Scholar] [CrossRef]
- Pepper, M.S. Manipulating Angiogenesis. Arter. Thromb. Vasc. Biol. 1997, 17, 605–619. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Karaoglu, I.C.; Ozer, O.; Albayrak, C.; Kizilel, S. Neovascularization of engineered tissues for clinical translation: Where we are, where we should be? APL Bioeng. 2021, 5, 021503. [Google Scholar] [CrossRef]
- Jacobo, S.M.P.; Kazlauskas, A. Insulin-like Growth Factor 1 (IGF-1) Stabilizes Nascent Blood Vessels. J. Biol. Chem. 2015, 290, 6349–6360. [Google Scholar] [CrossRef]
- Peters, E.B. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng. Part B Rev. 2018, 24, 1–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zou, T.; Qi, Y.; Yi, B.; Dissanayaka, W.L.; Zhang, C. DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling. Stem Cell Res. Ther. 2021, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Xiong, Y.-Y.; Yang, Y.-J. The Vital Roles of Mesenchymal Stem Cells and the Derived Extracellular Vesicles in Promoting Angiogenesis After Acute Myocardial Infarction. Stem Cells Dev. 2021, 30, 561–577. [Google Scholar] [CrossRef]
- Dierick, F.; Solinc, J.; Bignard, J.; Soubrier, F.; Nadaud, S. Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension. Cells 2021, 10, 1338. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, Y. Therapeutic angiogenesis: Controlled delivery of angiogenic factors. Ther. Deliv. 2012, 3, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 5, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Martino, M.M.; Brkic, S.; Bovo, E.; Burger, M.; Schaefer, D.J.; Wolff, T.; Gürke, L.; Briquez, P.S.; Larsson, H.M.; Gianni-Barrera, R.; et al. Extracellular Matrix and Growth Factor Engineering for Controlled Angiogenesis in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 45. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- Hu, C.; Ayan, B.; Chiang, G.; Chan, A.H.P.; Rando, T.A.; Huang, N.F. Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss. Bioengineering 2022, 9, 37. [Google Scholar] [CrossRef]
- Sedlář, A.; Trávníčková, M.; Matějka, R.; Pražák, Š.; Mészáros, Z.; Bojarová, P.; Bačáková, L.; Křen, V.; Slámová, K. Growth Factors VEGF-A165 and FGF-2 as Multifunctional Biomolecules Governing Cell Adhesion and Proliferation. Int. J. Mol. Sci. 2021, 22, 1843. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, S.; Mathew, D.; Jo, J.-I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.A.; Thiele, J.S.; Stegemann, J.P. Growth factor sequestration and enzyme-mediated release from genipin-crosslinked gelatin microspheres. J. Biomater. Sci. Polym. Ed. 2017, 28, 1826–1846. [Google Scholar] [CrossRef]
- Cuenca, J.P.; Kang, H.-J.; Al Fahad, A.; Park, M.-K.; Choi, M.-J.; Lee, H.-Y.; Lee, B.-T. Physico-mechanical and biological evaluation of heparin/VEGF-loaded electrospun polycaprolactone/decellularized rat aorta extracellular matrix for small-diameter vascular grafts. J. Biomater. Sci. Polym. Ed. 2022, 33, 1664–1684. [Google Scholar] [CrossRef]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-Malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef]
- Sagar, V.; Nair, M. Near-infrared biophotonics-based nanodrug release systems and their potential application for neuro-disorders. Expert Opin. Drug Deliv. 2017, 15, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, L.; Minardi, S.; Taraballi, F.; Liu, X.; Ferrari, M.; Tasciotti, E. Composite microsphere-functionalized scaffold for the controlled release of small molecules in tissue engineering. J. Tissue Eng. 2016, 7, 2041731415624668. [Google Scholar] [CrossRef]
- Lai, H.-J.; Kuan, C.-H.; Wu, H.-C.; Tsai, J.-C.; Chen, T.-M.; Hsieh, D.-J.; Wang, T.-W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Jamee, R.; Araf, Y.; Bin Naser, I.; Promon, S.K. The promising rise of bioprinting in revolutionalizing medical science: Advances and possibilities. Regen. Ther. 2021, 18, 133–145. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Huang, Y.; Liu, C.; Yu, Z.-X.; Nowicki, M.; Miao, S.; Cheng, Y.; Zhou, X.; Lee, S.-J.; et al. In vitro and in vivo evaluation of 3D bioprinted small-diameter vasculature with smooth muscle and endothelium. Biofabrication 2019, 12, 015004. [Google Scholar] [CrossRef]
- Millik, S.C.; Dostie, A.M.; Karis, D.G.; Smith, P.T.; McKenna, M.; Chan, N.; Curtis, C.D.; Nance, E.; Theberge, A.B.; Nelson, A. 3D printed coaxial nozzles for the extrusion of hydrogel tubes toward modeling vascular endothelium. Biofabrication 2019, 11, 045009. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; He, Y.; Fu, J.-Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Ko, I.K.; Yoo, J.J. State-of-the-Art Strategies for the Vascularization of Three-Dimensional Engineered Organs. Vasc. Spéc. Int. 2019, 35, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seol, Y.-J.; Ko, I.K.; Kang, H.-W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Ringeisen, B.R. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP). Biofabrication 2010, 2, 014111. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.; Kwon, M.; Burdick, J. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Kérourédan, O.; Bourget, J.-M.; Rémy, M.; Crauste-Manciet, S.; Kalisky, J.; Catros, S.; Thébaud, N.B.; Devillard, R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 28. [Google Scholar] [CrossRef]
- Tan, E.Y.S.; Yeong, W.Y. Concentric Bioprinting Of Alginate-Based Tubular Constructs Using Multi-Nozzle Extrusion-Based Technique. Int. J. Bioprint. 2015, 201, 49–56. [Google Scholar] [CrossRef]
- Campbell, J.; McGuinness, I.; Wirz, H.; Sharon, A.; Sauer-Budge, A.F. Multimaterial and Multiscale Three-Dimensional Bioprinter. J. Nanotechnol. Eng. Med. 2015, 6, 021005. [Google Scholar] [CrossRef]
- Ozler, S.B.; Bakirci, E.; Kucukgul, C.; Koc, B. Three-dimensional direct cell bioprinting for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Kucukgul, C.; Ozler, S.B.; Inci, I.; Karakas, E.; Irmak, S.; Gozuacik, D.; Taralp, A.; Koc, B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol. Bioeng. 2014, 112, 811–821. [Google Scholar] [CrossRef]
- Attalla, R.; Puersten, E.; Jain, N.; Selvaganapathy, P.R. 3D bioprinting of heterogeneous bi- and tri-layered hollow channels within gel scaffolds using scalable multi-axial microfluidic extrusion nozzle. Biofabrication 2018, 11, 015012. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, S.; Tao, X.; Xu, X.-Q.; Hong, Q.; Wu, D.; Wang, Y. Spider-Inspired Multicomponent 3D Printing Technique for Next-Generation Complex Biofabrication. ACS Appl. Bio Mater. 2018, 1, 502–510. [Google Scholar] [CrossRef]
- Feng, F.; He, J.; Li, J.; Mao, M.; Li, D. Multicomponent bioprinting of heterogeneous hydrogel constructs based on microfluidic printheads. Int. J. Bioprin. 2019, 5, 39. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, R. Direct Fabrication of a Hybrid Cell/Hydrogel Construct by a Double-nozzle Assembling Technology. J. Bioact. Compat. Polym. 2009, 24, 249–265. [Google Scholar]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, W.; Nowicki, M.; Zhou, X.; Khademhosseini, A.; Zhang, L.G. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Adv. Healthc. Mater. 2016, 5, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef] [PubMed]
- Shanjani, Y.; Pan, C.C.; Elomaa, L.; Yang, Y. A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication 2015, 7, 045008. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.P.; Toksoy, Z.; Davis, B.A.A.; Geibel, J.P. 3D Bioprinting of Vascularized Tissues for in vitro and in vivo Applications. Front. Bioeng. Biotechnol. 2021, 9, 664188. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Jun, Y.-J.; Kim, D.Y.; Yi, H.-G.; Chae, S.-H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.-S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- Singh, N.K.; Han, W.; Nam, S.A.; Kim, J.W.; Kim, J.Y.; Kim, Y.K.; Cho, D.-W. Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials 2019, 232, 119734. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G. Intestinal Villi Model with Blood Capillaries Fabricated Using Collagen-Based Bioink and Dual-Cell-Printing Process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196. [Google Scholar] [CrossRef]
- Shao, L.; Gao, Q.; Xie, C.; Fu, J.; Xiang, M.; He, Y. Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication 2020, 12, 035014. [Google Scholar] [CrossRef]
- Gaebel, R.; Ma, N.; Liu, J.; Guan, J.; Koch, L.; Klopsch, C.; Gruene, M.; Toelk, A.; Wang, W.; Mark, P.; et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011, 32, 9218–9230. [Google Scholar] [CrossRef]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y.; et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Kim, J.J.; Hou, L.; Yang, G.; Mezak, N.P.; Wanjare, M.; Joubert, L.M.; Huang, N.F. Microfibrous Scaffolds Enhance Endothelial Differentiation and Organization of Induced Pluripotent Stem Cells. Cell. Mol. Bioeng. 2017, 10, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Wanjare, M.; Hou, L.; Nakayama, K.H.; Kim, J.J.; Mezak, N.P.; Abilez, O.J.; Tzatzalos, E.; Wu, J.C.; Huang, N.F. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater. Sci. 2017, 5, 1567–1578. [Google Scholar] [CrossRef]
- Kenar, H.; Ozdogan, C.Y.; Dumlu, C.; Doger, E.; Kose, G.T.; Hasirci, V. Microfibrous scaffolds from poly(l-lactide-co-ε-caprolactone) blended with xeno-free collagen/hyaluronic acid for improvement of vascularization in tissue engineering applications. Mater. Sci. Eng. C 2018, 97, 31–44. [Google Scholar] [CrossRef]
- Cui, L.; Li, J.; Long, Y.; Hu, M.; Li, J.; Lei, Z.; Wang, H.; Huang, R.; Li, X. Vascularization of LBL structured nanofibrous matrices with endothelial cells for tissue regeneration. RSC Adv. 2017, 7, 11462–11477. [Google Scholar] [CrossRef]
- Alsop, A.T.; Pence, J.C.; Weisgerber, D.W.; Harley, B.A.; Bailey, R.C. Photopatterning of vascular endothelial growth factor within collagen-glycosaminoglycan scaffolds can induce a spatially confined response in human umbilical vein endothelial cells. Acta Biomater. 2014, 10, 4715–4722. [Google Scholar] [CrossRef]
- O’Dwyer, J.; Murphy, R.; González-Vázquez, A.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.; Cryan, S. Translational Studies on the Potential of a VEGF Nanoparticle-Loaded Hyaluronic Acid Hydrogel. Pharmaceutics 2021, 13, 779. [Google Scholar] [CrossRef]
- Bai, Y.; Leng, Y.; Yin, G.; Pu, X.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y. Effects of combinations of BMP-2 with FGF-2 and/or VEGF on HUVECs angiogenesis in vitro and CAM angiogenesis in vivo. Cell Tissue Res. 2014, 356, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, Y.; Wang, Y.; Li, L.; Jiang, X.; Tang, J.; Yang, H.; Zhang, J.; Bao, J.; Bu, H. The effect of heparinized decellularized scaffolds on angiogenic capability. J. Biomed. Mater. Res. Part A 2016, 104, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Samorezov, J.E.; Alsberg, E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv. Drug Deliv. Rev. 2014, 84, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Nelson, C.M.; Baranski, J.; Lim, E.; Chen, C. Geometrically Controlled Endothelial Tubulogenesis in Micropatterned Gels. Tissue Eng. Part A 2010, 16, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Baranski, J.D.; Chaturvedi, R.R.; Stevens, K.R.; Eyckmans, J.; Carvalho, B.; Solorzano, R.D.; Yang, M.T.; Miller, J.S.; Bhatia, S.N.; Chen, C.S. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA 2013, 110, 7586–7591. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Taraballi, F.; Pandolfi, L.; Tasciotti, E. Patterning Biomaterials for the Spatiotemporal Delivery of Bioactive Molecules. Front. Bioeng. Biotechnol. 2016, 4, 45. [Google Scholar] [CrossRef]
- Nazarnezhad, S.; Baino, F.; Kim, H.-W.; Webster, T.J.; Kargozar, S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials 2020, 10, 1609. [Google Scholar] [CrossRef]
- Lei, Y.; Zouani, O.F.; Rémy, M.; Ayela, C.; Durrieu, M.-C. Geometrical Microfeature Cues for Directing Tubulogenesis of Endothelial Cells. PLoS ONE 2012, 7, e41163. [Google Scholar] [CrossRef]
- Chow, L.W.; Wang, L.-J.; Kaufman, D.B.; Stupp, S.I. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials 2010, 31, 6154–6161. [Google Scholar] [CrossRef]
- Huang, N.F.; Lai, E.S.; Ribeiro, A.J.; Pan, S.; Pruitt, B.L.; Fuller, G.G.; Cooke, J.P. Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature. Biomaterials 2013, 34, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Ayan, B.; Undieh, A.A.; Yang, Y.P.; Huang, N.F. Advances in three-dimensional bioprinted stem cell-based tissue engineering for cardiovascular regeneration. J. Mol. Cell. Cardiol. 2022, 169, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Khanna, A.; Luzinov, I.; Nagatomi, J.; Harman, M. Surface modification of polypropylene surgical meshes for improving adhesion with poloxamine hydrogel adhesive. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1047–1055. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Jin, X. A Review on the Secondary Surface Morphology of Electrospun Nanofibers: Formation Mechanisms, Characterizations, and Applications. Chem. Sel. 2020, 5, 1335–1348. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised scaffolds: Just a framework? Current knowledge and future directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef]
- White, K.A.; Olabisi, R.M. Spatiotemporal Control Strategies for Bone Formation through Tissue Engineering and Regenerative Medicine Approaches. Adv. Healthc. Mater. 2018, 8, e1801044. [Google Scholar] [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. NPJ Regen. Med. 2021, 6, 68. [Google Scholar] [CrossRef]
- Wanjare, M.; Huang, N.F. Regulation of the microenvironment for cardiac tissue engineering. Regen. Med. 2017, 12, 187–201. [Google Scholar] [CrossRef]
- Huang, N.F.; Lee, R.J.; Li, S. Chemical and Physical Regulation of Stem Cells and Progenitor Cells: Potential for Cardiovascular Tissue Engineering. Tissue Eng. 2007, 13, 1809. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Gugulothu, S.B.; Visweswariah, S.S.; Chatterjee, K. Strategies to Promote Vascularization in 3D Printed Tissue Scaffolds: Trends and Challenges. Biomacromolecules 2022, 23, 2730–2751. [Google Scholar] [CrossRef]
- Shevach, M.; Zax, R.; Abrahamov, A.; Fleischer, S.; Shapira, A.; Dvir, T. Omentum ECM-based hydrogel as a platform for cardiac cell delivery. Biomed. Mater. 2015, 10, 034106. [Google Scholar] [CrossRef]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; Van Helden, R.W.; Garcia, A.K.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862. [Google Scholar] [CrossRef]
- Zamani, M.; Karaca, E.; Huang, N.F. Multicellular Interactions in 3D Engineered Myocardial Tissue. Front. Cardiovasc. Med. 2018, 5, 147. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Huang, N.F.; Jamé, S.; Lee, J.C.; Nguyen, H.N.; Byers, B.; De, A.; Okogbaa, J.; Rollins, M.; Reijo-Pera, R.; et al. Endothelial Cells Derived From Human iPSCS Increase Capillary Density and Improve Perfusion in a Mouse Model of Peripheral Arterial Disease. Arter. Thromb. Vasc. Biol. 2011, 31, e72–e79. [Google Scholar] [CrossRef]
- Huang, N.F.; Dewi, R.E.; Okogbaa, J.; Lee, J.C.; Jalilrufaihah, A.; Heilshorn, S.C.; Cooke, J.P. Chemotaxis of human induced pluripotent stem cell-derived endothelial cells. Am. J. Transl. Res. 2013, 5, 510–520. [Google Scholar]
- Wanjare, M.; Kuo, F.; Gerecht, S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2013, 97, 321. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Ribeiro, A.J.S.; Ang, Y.-S.; Fu, J.-D.; Rivas, R.N.; Mohamed, T.M.A.; Higgs, G.C.; Srivastava, D.; Pruitt, B.L. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 12705–12710. [Google Scholar] [CrossRef]
- Rackov, G.; Garcia-Romero, N.; Esteban-Rubio, S.; Carrión-Navarro, J.; Belda-Iniesta, C.; Ayuso-Sacido, A. Vesicle-Mediated Control of Cell Function: The Role of Extracellular Matrix and Microenvironment. Front. Physiol. 2018, 9, 651. [Google Scholar] [CrossRef]
- Ruvinov, E.; Leor, J.; Cohen, S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011, 32, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Cohen, S. Inducing Endogenous Cardiac Regeneration: Can Biomaterials Connect the Dots? Front. Bioeng. Biotechnol. 2020, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Jeske, R.; Bejoy, J.; Marzano, M.; Li, Y. Human Pluripotent Stem Cell-Derived Extracellular Vesicles: Characteristics and Applications. Tissue Eng. Part B Rev. 2020, 26, 129–144. [Google Scholar] [CrossRef]
- Bae, W.-G.; Kim, J.; Choung, Y.-H.; Chung, Y.; Suh, K.Y.; Pang, C.; Chung, J.H.; Jeong, H.E. Bio-inspired configurable multiscale extracellular matrix-like structures for functional alignment and guided orientation of cells. Biomaterials 2015, 69, 158–164. [Google Scholar] [CrossRef]
- Leijten, J.; Seo, J.; Yue, K.; Santiago, G.T.-D.; Tamayol, A.; Ruiz-Esparza, G.U.; Shin, S.R.; Sharifi, R.; Noshadi, I.; Álvarez, M.M.; et al. Spatially and temporally controlled hydrogels for tissue engineering. Mater. Sci. Eng. R Rep. 2017, 119, 1–35. [Google Scholar] [CrossRef]
- Wang, L.; Serpooshan, V.; Zhang, J. Engineering Human Cardiac Muscle Patch Constructs for Prevention of Post-infarction LV Remodeling. Front. Cardiovasc. Med. 2021, 8, 621781. [Google Scholar] [CrossRef]
- Serbo, J.V.; Gerecht, S. Vascular tissue engineering: Biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res. Ther. 2013, 4, 8. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Liu, J.; Miller, K.; Ma, X.; Dewan, S.; Lawrence, N.; Whang, G.; Chung, P.; McCulloch, A.D.; Chen, S. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials 2020, 256, 120204. [Google Scholar] [CrossRef]
- Aguilar, I.N.; Olivos, D.J.; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.-M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nam, H.; Jang, J. 3D bioprinting of stem cell-laden cardiac patch: A promising alternative for myocardial repair. APL Bioeng. 2021, 5, 031508. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.S.; Kim, B.S.; Choi, Y.J.; Jang, W.B.; Hong, Y.J.; Kwon, S.M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Wanjare, M.; Kawamura, M.; Hu, C.; Alcazar, C.; Wang, H.; Woo, Y.J.; Huang, N.F. Vascularization of Engineered Spatially Patterned Myocardial Tissue Derived From Human Pluripotent Stem Cells in vivo. Front. Bioeng. Biotechnol. 2019, 7, 208. [Google Scholar] [CrossRef]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Reis, L.A.; Chiu, L.L.Y.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Regen. Med. 2014, 10, 11–28. [Google Scholar] [CrossRef]
- Barrs, R.; Jia, J.; Silver, S.E.; Yost, M.; Mei, Y. Biomaterials for Bioprinting Microvasculature. Chem. Rev. 2020, 120, 10887–10949. [Google Scholar] [CrossRef]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef]
- Zhao, P.; Huo, S.; Fan, J.; Chen, J.; Kiessling, F.; Boersma, A.J.; Göstl, R.; Herrmann, A. Activation of the Catalytic Activity of Thrombin for Fibrin Formation by Ultrasound. Angew. Chem. Int. Ed. 2021, 60, 14707–14714. [Google Scholar] [CrossRef]
- Song, H.H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Van Laake, L.W.; Botker, H.E.; Davidson, S.M.; De Caterina, R.; Engel, F.; Eschenhagen, T.; Fernandez-Aviles, F.; Hausenloy, D.J.; Hulot, J.-S.; et al. ESC Working Group on Cellular Biology of the Heart: Position paper for Cardiovascular Research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 2019, 115, 488–500. [Google Scholar] [CrossRef] [PubMed]
| Bioactive Molecules | Angiogenic Effects | Ref |
|---|---|---|
| VEGF | Facilitates EC migration and proliferation Regulates EC proliferation, migration, and survival; allows mobilization of BM-derived cells such as HSCs, and recruit SMCs for stabilization of vessel. | [20] |
| FGF | FGF-2 Enhances EC proliferation. bFGF facilitates the activation, proliferation, and migration of EPC; regulate vasculogenesis and the formation of immature primary vascular networks. FGF-2 Interacts with ECM molecules such as heparin, heparan sulfate proteoglycans (HSPGs); promotes EC response and neovascularization process. FGF-2 facilitates proliferation of ECs, SMCs; endothelial capillary formation | [20] |
| IGF-1 | Facilitates formation of neovasculature from the endothelium of pre-existing vessels andInduces endothelial cell migration for vascularization Induces the activation of the PI3-kinase/Akt signaling pathway and expression of growth factors | [21] |
| PDGF | Promotes vessel maturation by recruitment of MSCs, pericytes, and SMCs. Facilitates remodeling by inducing collagenases secretion by fibroblasts. Increases VEGF production and promote angiogenesis Regulates the production of ECM molecules for basement membrane and blood vessel stabilization | [22] |
| TGF-β | Promotes EC migration, proliferation, and differentiation. Increases VEGF secretion by ECs; and PGF and bFGF expression by SMCs. Enhances angiogenesis. Facilitates vessel stabilization and maturation Stimulates ECM deposition | [23] |
| HGF | Induces VEGF secretion Promotes angiogenesis by ECs expression of VEGF. | [24] |
| TNF-α | Inhibits proliferation of endothelial cells; promotes angiogenesis | [23] |
| Angiopoietin | Facilitates TGF-β-induced differentiation of MSCs. Promotes vessel maturation Inhibits VEGF activity and facilitates EC-SMC interactions Enhnaces type IV collagen deposition Promotes EC proliferation Induces VEGF mediated angiogenic sprouting. | [22] |
| SDF-1 | Facilitates vessel stabilization by recruitment of progenitors of SMCs Initiate vascular remodeling; upregulate metalloproteinases and downregulate angiostatin | [25] |
| Bioprinting Approach | Targeted Vascularized Tissue | Bioprinter Used | Bioink | Vascularization Impact | Ref |
|---|---|---|---|---|---|
| Multi-material bioprinting | Vascularized liver | Double nozzle printing system | ADSC-laden gelatin/alginate/fibrinogen Hepatocytes-laden gelatin/alginate/chitosan | Functional hepatocytes were formed with endothelial like structures in tissue construct. | [60] |
| Vascularized bone | 3D-bioprinter with two controllable printheads | hMSCs laden gelatin-fibrinogen HUVEC laden gelatin-fibrinogen hydrogel | Osteogenic differentiation factors perfusion through vascular network resulted in osteogenic tissue formation. | [61] | |
| Vascularized cardiac patch | Multi-head extrusion-based 3D bioprinting | ECs within sacrificial gelatin CMs laden ECM bioink | Heart structure with mechanically stable and robust perfusable vessels | [62] | |
| Vascularized tissue model | 3D bioprinter with more than two controllable printheads | Fibroblast-cell laden GelMA EC injection through microchannels | Fabrication of vascularized tissue constructs. | [63] | |
| Dual 3D bioprinting | SLA-based and extrusion-based bioprinting | Vascularized bone | ECs and hMSCs laden VEGF modified Gel MA- based bioink | Spatial controlled localization of growth factors and perfusion lead to interconnected vascularized bone construct. | [64] |
| Extrusion and inkjet bioprinting | Vascularized skin | Adipose-derived dECM and fibrinogen bioink encapsulated human adipocytes Fibroblast cells laden skin dECM and fibrinogen | Formation of vascularized channels between dermis and hypodermis leads to maturation of epidermis with human like structure. | [65] | |
| Extrusion-based and SLA-based bioprinting platform | Multiphasic hybrid construct vascular conduit model | Cells encapsulated within PEGDA | Diffusion of media into cells resulted in a thick construct | [66] | |
| Co-axial and extrusion bioprinting platform | Vascular model | Human coronary artery SMCs laden modified Gel MA | Bioprinted vascular construct with biomechanics, perfusion ablility and permeability. | [67] | |
| Co-axial Bioprinting | Coaxial nozzle bioprinting | Vascularized muscle | Endothelial cell-laden vascular dECM | Formation of pre-vascularized muscle with integration into the host tissue and functional recovery. | [68] |
| Coaxial nozzle bioprinting | Perfusable renal tissue | Hybrid hydrogel bioink incorporated with kidney dECM and alginate | Renal proximal tube integrated into the host tissues in vivo | [69] | |
| Coaxial nozzle bioprinting | Vascularized intestinal villi | HUVEC extruded from core region of coaxial nozzle | Human intestine regeneration and organ-on-a-chip system | [70] | |
| Coaxial bioprinting platform | Vascularized tissue > 1 cm | Cell-laden GelMA Endothelial cell laden gelatin | Generation of tissue models | [71] | |
| Light-based bioprinting | LIFT-Based bioprinting | Vascularized cardiac patch | Deposition of MSCs on a cardiac patch within ECs mesh structure | Pre-vascularized patches with enhanced angiogenesis | [72] |
| DLP based-bioprinting | Vascularized thick tissue | Photopolymerizable glycidyl methacrylate- hyaluronic acid and GelMA | Fabrication of vascularized tissue constructs with high resolution. | [73] |
| Technique/Structures | Application | Limitations | Ref |
|---|---|---|---|
| 3D Bioprinting |
|
| [94] |
| Micropatterning |
|
| [95] |
| Hydrogel |
|
| [96] |
| Electrospinning |
|
| [97] |
| Decellularized Scaffolds |
|
| [98] |
| Tissue Engineered Heart |
|
| [99] |
| Scaffold-free Engineering |
|
| [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, A.; Oropeza, B.P.; Huang, N.F. Engineering Spatiotemporal Control in Vascularized Tissues. Bioengineering 2022, 9, 555. https://doi.org/10.3390/bioengineering9100555
Khanna A, Oropeza BP, Huang NF. Engineering Spatiotemporal Control in Vascularized Tissues. Bioengineering. 2022; 9(10):555. https://doi.org/10.3390/bioengineering9100555
Chicago/Turabian StyleKhanna, Astha, Beu P. Oropeza, and Ngan F. Huang. 2022. "Engineering Spatiotemporal Control in Vascularized Tissues" Bioengineering 9, no. 10: 555. https://doi.org/10.3390/bioengineering9100555
APA StyleKhanna, A., Oropeza, B. P., & Huang, N. F. (2022). Engineering Spatiotemporal Control in Vascularized Tissues. Bioengineering, 9(10), 555. https://doi.org/10.3390/bioengineering9100555