Culturing of Cardiac Fibroblasts in Engineered Heart Matrix Reduces Myofibroblast Differentiation but Maintains Their Response to Cyclic Stretch and Transforming Growth Factor β1
Abstract
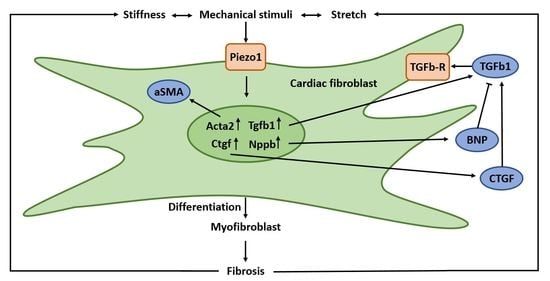
1. Introduction
2. Materials and Methods
2.1. Isolation and Culturing of CF
2.2. Assembly of the Ring Formation Molds
2.3. Collagen Hydrogel
2.4. Engineered Heart Matrix Ring (EHM-Ring) Formation
2.5. Engineered Heart Matrix Fiber (EHM Fiber; Flexcell Tissue Train)
2.6. Measurement of EHM Stiffness
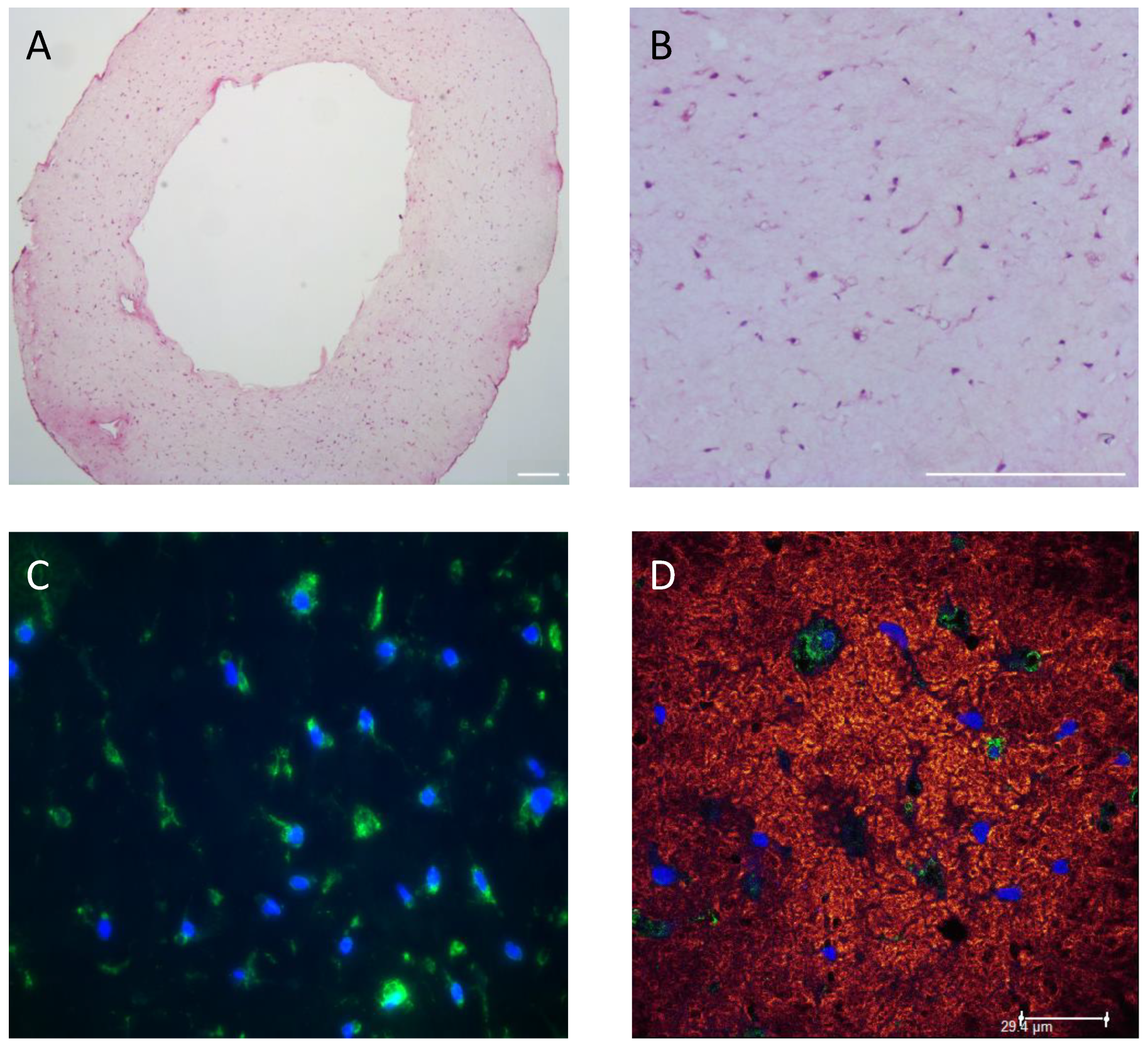
2.7. Histology and Immunohistochemistry (IHC)
2.8. Gene Expression Analysis
2.9. Statistics
3. Results
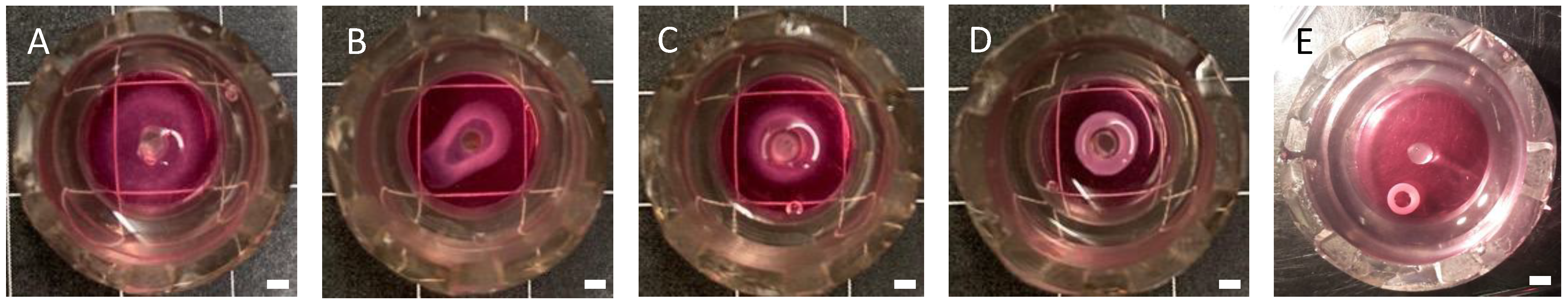
3.1. Optimizing CF Cell Density in EHM Rings
3.2. Optimizing the EHM Initial Hydrogel Collagen Concentration
3.3. EHM Stiffness
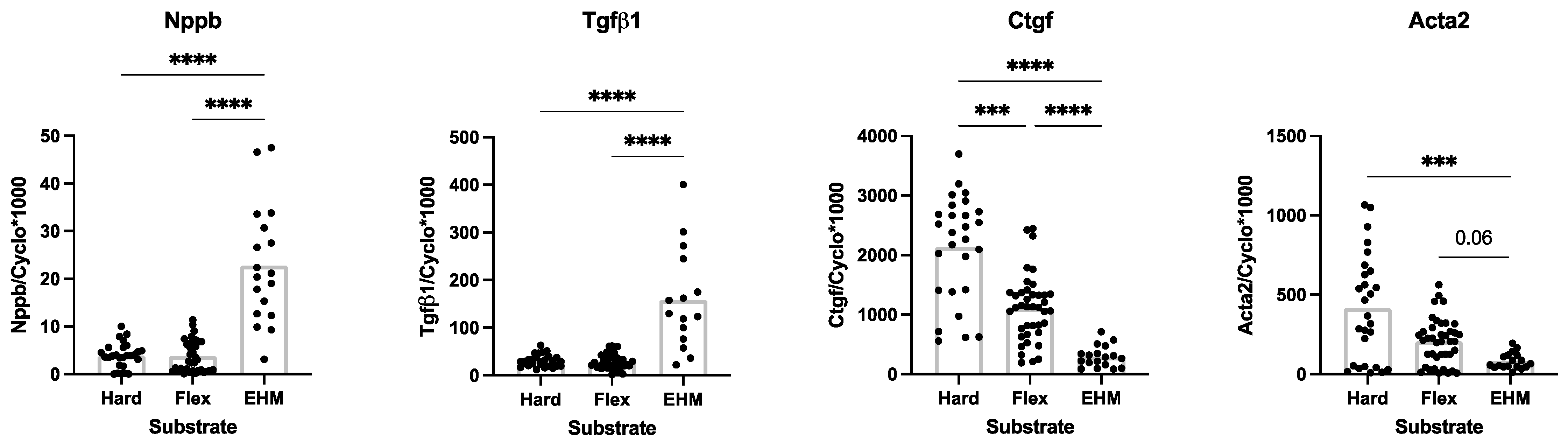
3.4. Baseline CF Gene Expression in 3D (EHM) and 2D (Monolayer) Culturse
3.5. Stretch of EHM Fibers in the Flexcell Tissue Train System
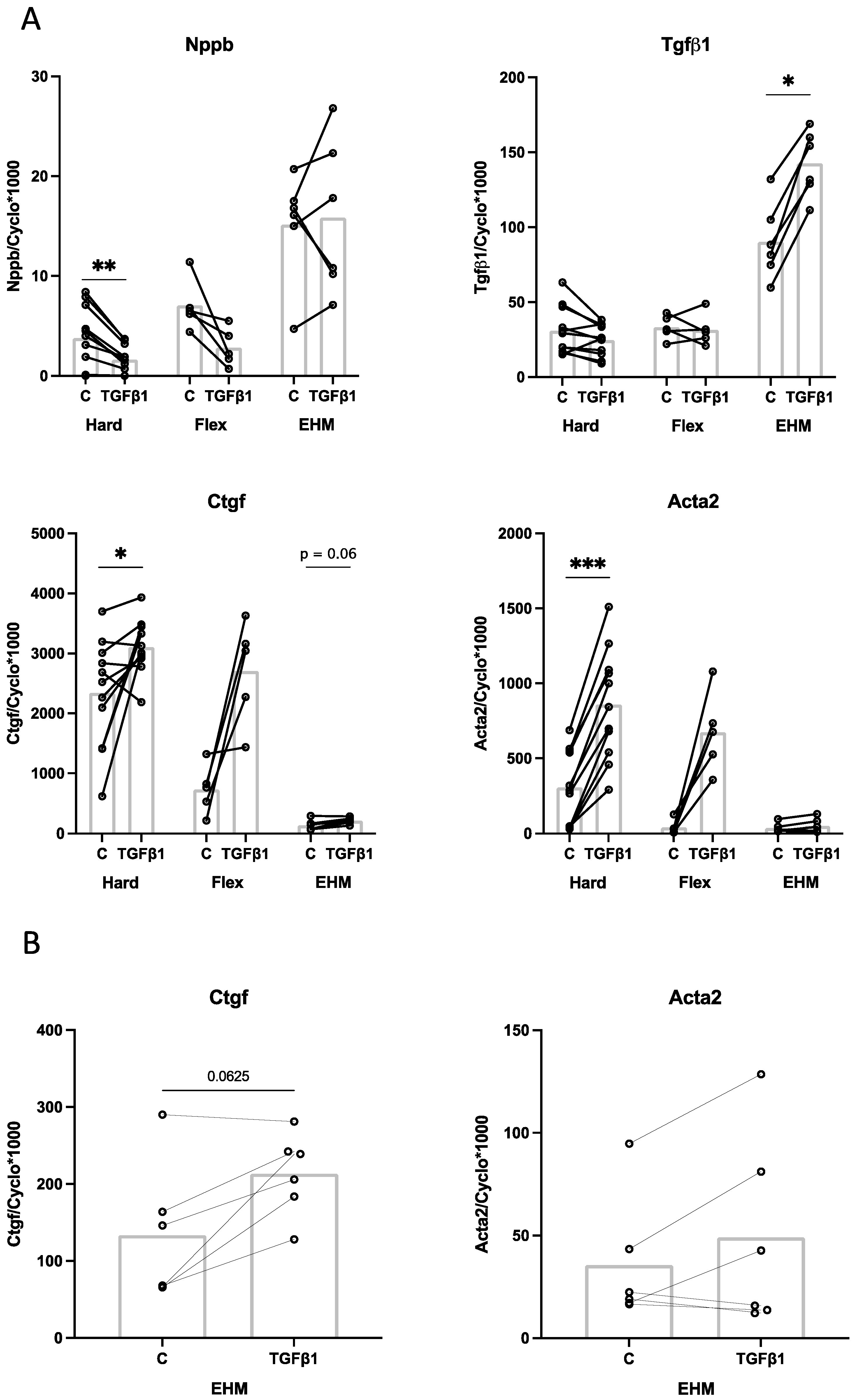
3.6. Effect of TGFβ1 Stimulation on CF Gene Expression in 2D (Monolayer) and 3D (EHM) Cultures
4. Discussion
4.1. Influence of Substrate Stiffness on Baseline CF Gene Expression
4.2. Possible Influence of Culture Medium Differences on Baseline CF Gene Expression
4.3. Effect of Cyclic Stretch and TGFβ1 on CF Gene Expression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariyasinghe, N.R.; Lyra-Leite, D.M.; McCain, M.L. Engineering cardiac microphysiological systems to model pathological extracellular matrix remodeling. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H771–H789. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.A.; Spinale, F.G. The structure and function of the cardiac myocyte: A review of fundamental concepts. J. Thorac. Cardiovasc. Surg. 1999, 118, 375–382. [Google Scholar] [CrossRef]
- Ross, R.S.; Borg, T.K. Integrins and the myocardium. Circ. Res. 2001, 88, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, M. Cardiac Fibroblasts: Function, Regulation of Gene Expression, and Phenotypic Modulation. Basic Res. Cardiol. 1992, 87, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenes. Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef]
- van Nieuwenhoven, F.A.; Turner, N.A. The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul. Pharmacol. 2013, 58, 182–188. [Google Scholar] [CrossRef]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B.; Myofibroblasts, I. Paracrine cells important in health and disease. Am. J. Physiol. 1999, 277, C1–C19. [Google Scholar] [CrossRef]
- Tsuruda, T.; Boerrigter, G.; Huntley, B.K.; Noser, J.A.; Cataliotti, A.; Costello-Boerrigter, L.C.; Chen, H.H.; Burnett, J.C., Jr. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ. Res. 2002, 91, 1127–1134. [Google Scholar] [CrossRef]
- van den Borne, S.W.; Diez, J.; Blankesteijn, W.M.; Verjans, J.; Hofstra, L.; Narula, J. Myocardial remodeling after infarction: The role of myofibroblasts. Nat. Rev. Cardiol. 2010, 7, 30–37. [Google Scholar] [CrossRef]
- Brown, R.D.; Ambler, S.K.; Mitchell, M.D.; Long, C.S. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 657–687. [Google Scholar] [CrossRef] [PubMed]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Yekkala, K.; Borg, T.K.; Baudino, T.A. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann. N. Y. Acad. Sci. 2006, 1080, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pedrotty, D.M.; Klinger, R.Y.; Kirkton, R.D.; Bursac, N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc. Res. 2009, 83, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef]
- Jalil, J. Structural vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricle. J. Mol. Cell. Cardiol. 1988, 20, 1179–1187. [Google Scholar] [CrossRef]
- Li, Y.; Asfour, H.; Bursac, N. Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue. Acta Biomater 2017, 55, 120–130. [Google Scholar] [CrossRef]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [CrossRef]
- Landry, N.M.; Rattan, S.G.; Dixon, I.M.C. An Improved Method of Maintaining Primary Murine Cardiac Fibroblasts in Two-Dimensional Cell Culture. Sci. Rep. 2019, 9, 12889. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.J.; Dangerfield, A.L.; Rattan, S.G.; Bathe, K.L.; Cunnington, R.H.; Raizman, J.E.; Bedosky, K.M.; Freed, D.H.; Kardami, E.; Dixon, I.M. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev. Dyn. 2010, 239, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: Implications for the pathogenesis and treatment of fibrosis. Curr. Rheumatol. Rep. 2009, 11, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Galie, P.A.; Westfall, M.V.; Stegemann, J.P. Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovasc. Pathol. 2011, 20, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, W.L.; Sider, K.L.; Yip, C.Y.; Simmons, C.A. beta-catenin mediates mechanically regulated, transforming growth factor-beta1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 590–597. [Google Scholar] [CrossRef]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.L.; Bloomer, S.A.; Chan, E.P.; Gaca, M.D.; Georges, P.C.; Sackey, B.; Uemura, M.; Janmey, P.A.; Wells, R.G. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G110–G118. [Google Scholar] [CrossRef]
- Mayer, D.C.; Leinwand, L.A. Sarcomeric gene expression and contractility in myofibroblasts. J. Cell Biol. 1997, 139, 1477–1484. [Google Scholar] [CrossRef]
- Blaauw, E.; Lorenzen-Schmidt, I.; Babiker, F.A.; Munts, C.; Prinzen, F.W.; Snoeckx, L.H.; van Bilsen, M.; van der Vusse, G.J.; van Nieuwenhoven, F.A. Stretch-induced upregulation of connective tissue growth factor in rabbit cardiomyocytes. J. Cardiovasc Transl Res. 2013, 6, 861–869. [Google Scholar] [CrossRef]
- Tarbit, E.; Singh, I.; Peart, J.N.; Rose’Meyer, R.B. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail. Rev. 2019, 24, 1–15. [Google Scholar] [CrossRef]
- Swaney, J.S.; Roth, D.M.; Olson, E.R.; Naugle, J.E.; Meszaros, J.G.; Insel, P.A. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. USA 2005, 102, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, M.C.; Munts, C.; Prinzen, F.W.; Turner, N.A.; van Bilsen, M.; van Nieuwenhoven, F.A. Piezo1 Mechanosensitive Ion Channel Mediates Stretch-Induced Nppb Expression in Adult Rat Cardiac Fibroblasts. Cells 2021, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Porter, K.E.; Smith, W.H.T.; White, H.L.; Ball, S.G.; Balmforth, A.J. Chronic β2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc. Res. 2003, 57, 784–792. [Google Scholar] [CrossRef]
- van Nieuwenhoven, F.A.; Hemmings, K.E.; Porter, K.E.; Turner, N.A. Combined effects of interleukin-1α and transforming growth factor-β1 on modulation of human cardiac fibroblast function. Matrix Biol. 2013, 32, 399–406. [Google Scholar] [CrossRef]
- van Nieuwenhoven, F.A.; Munts, C.; Op’t Veld, R.C.; Gonzalez, A.; Diez, J.; Heymans, S.; Schroen, B.; van Bilsen, M. Cartilage intermediate layer protein 1 (CILP1): A novel mediator of cardiac extracellular matrix remodelling. Sci. Rep. 2017, 7, 16042. [Google Scholar] [CrossRef]
- van Kampen, K.A.; Fernández-Pérez, J.; Baker, M.; Mota, C.; Moroni, L. Fabrication of a mimetic vascular graft using melt spinning with tailorable fiber parameters. Biomater. Adv. 2022, 139, 212972. [Google Scholar] [CrossRef]
- van Kampen, K.A.; Olaret, E.; Stancu, I.C.; Moroni, L.; Mota, C. Controllable four axis extrusion-based additive manufacturing system for the fabrication of tubular scaffolds with tailorable mechanical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111472. [Google Scholar] [CrossRef]
- Geelhoed, W.J.; Lalai, R.A.; Sinnige, J.H.; Jongeleen, P.J.; Storm, C.; Rotmans, J.I. Indirect Burst Pressure Measurements for the Mechanical Assessment of Biological Vessels. Tissue Eng. Part. C Methods 2019, 25, 472–478. [Google Scholar] [CrossRef]
- Shazly, T.; Rachev, A.; Lessner, S.; Argraves, W.S.; Ferdous, J.; Zhou, B.; Moreira, A.M.; Sutton, M. On the Uniaxial Ring Test of Tissue Engineered Constructs. Exp. Mech. 2014, 55, 41–51. [Google Scholar] [CrossRef]
- de Jong, S.; van Middendorp, L.B.; Hermans, R.H.; de Bakker, J.M.; Bierhuizen, M.F.; Prinzen, F.W.; van Rijen, H.V.; Losen, M.; Vos, M.A.; van Zandvoort, M.A. Ex vivo and in vivo administration of fluorescent CNA35 specifically marks cardiac fibrosis. Mol. Imaging 2014, 13, 7290. [Google Scholar] [CrossRef]
- Baues, M.; Klinkhammer, B.M.; Ehling, J.; Gremse, F.; van Zandvoort, M.; Reutelingsperger, C.P.M.; Daniel, C.; Amann, K.; Babickova, J.; Kiessling, F.; et al. A collagen-binding protein enables molecular imaging of kidney fibrosis in vivo. Kidney Int. 2020, 97, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Helary, C.; Bataille, I.; Abed, A.; Illoul, C.; Anglo, A.; Louedec, L.; Letourneur, D.; Meddahi-Pelle, A.; Giraud-Guille, M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Carag-Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J. Cell Sci. 2008, 121, 3794–3802. [Google Scholar] [CrossRef] [PubMed]
- Herum, K.M.; Lunde, I.G.; McCulloch, A.D.; Christensen, G. The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart. J. Clin. Med. 2017, 6, 53. [Google Scholar] [CrossRef]
- Herum, K.M.; Choppe, J.; Kumar, A.; Engler, A.J.; McCulloch, A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 2017, 28, 1871–1882. [Google Scholar] [CrossRef]
- Kong, M.; Lee, J.; Yazdi, I.K.; Miri, A.K.; Lin, Y.D.; Seo, J.; Zhang, Y.S.; Khademhosseini, A.; Shin, S.R. Cardiac Fibrotic Remodeling on a Chip with Dynamic Mechanical Stimulation. Adv. Healthc Mater. 2019, 8, e1801146. [Google Scholar] [CrossRef]
- Bracco Gartner, T.C.L.; Deddens, J.C.; Mol, E.A.; Magin Ferrer, M.; van Laake, L.W.; Bouten, C.V.C.; Khademhosseini, A.; Doevendans, P.A.; Suyker, W.J.L.; Sluijter, J.P.G.; et al. Anti-fibrotic Effects of Cardiac Progenitor Cells in a 3D-Model of Human Cardiac Fibrosis. Front. Cardiovasc. Med. 2019, 6, 52. [Google Scholar] [CrossRef]
- Hackett, T.L.; Vriesde, N.; Al-Fouadi, M.; Mostaco-Guidolin, L.; Maftoun, D.; Hsieh, A.; Coxson, N.; Usman, K.; Sin, D.D.; Booth, S.; et al. The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation. Cells 2022, 11, 185. [Google Scholar] [CrossRef]
- Taylor, S.E.; Vaughan-Thomas, A.; Clements, D.N.; Pinchbeck, G.; Macrory, L.C.; Smith, R.K.; Clegg, P.D. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord 2009, 10, 27. [Google Scholar] [CrossRef]
- Pelham, R.J., Jr.; Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Liu, Y.C.; Wang, M.X.; Hu, J.J. Fibroblast-seeded collagen gels in response to dynamic equibiaxial mechanical stimuli: A biomechanical study. J. Biomech. 2018, 78, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nieponice, A.; Maul, T.M.; Cumer, J.M.; Soletti, L.; Vorp, D.A. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J. Biomed. Mater. Res. A 2007, 81, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.S.; Brunner, G.; Krieg, T.; Eckes, B. Cyclic mechanical strain induces TGFbeta1-signalling in dermal fibroblasts embedded in a 3D collagen lattice. Arch. Dermatol. Res. 2015, 307, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Galie, P.A.; Russell, M.W.; Westfall, M.V.; Stegemann, J.P. Interstitial fluid flow and cyclic strain differentially regulate cardiac fibroblast activation via AT1R and TGF-beta1. Exp. Cell Res. 2012, 318, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Lam, A.; Abraham, J.A.; Schreiner, G.F.; Joly, A.H. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: A potential role in heart fibrosis. J. Mol. Cell Cardiol. 2000, 32, 1805–1819. [Google Scholar] [CrossRef]
- Watts, K.L.; Spiteri, M.A. Connective tissue growth factor expression and induction by transforming growth factor-beta is abrogated by simvastatin via a Rho signaling mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L1323–L1332. [Google Scholar] [CrossRef]
- Flanders, K.; Holder, M.G.; Winokur, T.S. Autoinduction of mRNA and protein expression for transforming growth factor-?s in cultured cardiac cells. J. Mol. Cell. Cardiol. 1995, 27, 805–812. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Invesigt. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Phelan, D.; Xu, M.; Collier, P.; Neary, R.; Smolenski, A.; Ledwidge, M.; McDonald, K.; Baugh, J. Mechanical stretch up-regulates the B-type natriuretic peptide system in human cardiac fibroblasts: A possible defense against transforming growth factor-beta mediated fibrosis. Fibrogenes. Tissue Repair 2012, 5, 9. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Alpha-Smooth muscle actin (Acta2) | AAGGCCAACCGGGAGAAAAT | AGTCCAGCACAATACCAGTTGT |
| Connective tissue growth factor (Ctgf) | CACAGAGTGGAGCGCCTGTTC | GATGCACTTTTTGCCCTTCTTAATG |
| Transforming growth factor, beta 1 (Tgfβ1) | GCACCATCCATGACATGAAC | GCTGAAGCAGTAGTTGGTATC |
| Brain Natriuretic Peptide (Nppb) | AGACAGCTCTCAAAGGACCA | CTATCTTCTGCCCAAAGCAG |
| Cyclophilin-A (Cyclo) | CAAATGCTGGACCAAACACAA | TTCACCTTCCCAAAGACCACAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ploeg, M.C.; Munts, C.; Seddiqi, T.; ten Brink, T.J.L.; Breemhaar, J.; Moroni, L.; Prinzen, F.W.; van Nieuwenhoven, F.A. Culturing of Cardiac Fibroblasts in Engineered Heart Matrix Reduces Myofibroblast Differentiation but Maintains Their Response to Cyclic Stretch and Transforming Growth Factor β1. Bioengineering 2022, 9, 551. https://doi.org/10.3390/bioengineering9100551
Ploeg MC, Munts C, Seddiqi T, ten Brink TJL, Breemhaar J, Moroni L, Prinzen FW, van Nieuwenhoven FA. Culturing of Cardiac Fibroblasts in Engineered Heart Matrix Reduces Myofibroblast Differentiation but Maintains Their Response to Cyclic Stretch and Transforming Growth Factor β1. Bioengineering. 2022; 9(10):551. https://doi.org/10.3390/bioengineering9100551
Chicago/Turabian StylePloeg, Meike C., Chantal Munts, Tayeba Seddiqi, Tim J. L. ten Brink, Jonathan Breemhaar, Lorenzo Moroni, Frits. W. Prinzen, and Frans. A. van Nieuwenhoven. 2022. "Culturing of Cardiac Fibroblasts in Engineered Heart Matrix Reduces Myofibroblast Differentiation but Maintains Their Response to Cyclic Stretch and Transforming Growth Factor β1" Bioengineering 9, no. 10: 551. https://doi.org/10.3390/bioengineering9100551
APA StylePloeg, M. C., Munts, C., Seddiqi, T., ten Brink, T. J. L., Breemhaar, J., Moroni, L., Prinzen, F. W., & van Nieuwenhoven, F. A. (2022). Culturing of Cardiac Fibroblasts in Engineered Heart Matrix Reduces Myofibroblast Differentiation but Maintains Their Response to Cyclic Stretch and Transforming Growth Factor β1. Bioengineering, 9(10), 551. https://doi.org/10.3390/bioengineering9100551