Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors
Abstract
1. Introduction
2. Bioprocessing for Microcarrier-Based hMSC Manufacturing
2.1. Seed Train
2.2. Inoculation
2.3. Cell Expansion
2.3.1. Mixing (Agitation and Rocking)
2.3.2. Control System (Temperature and pH and DO)
2.3.3. Medium and Feeding Strategies
2.3.4. Microcarrier Addition
2.4. Harvest
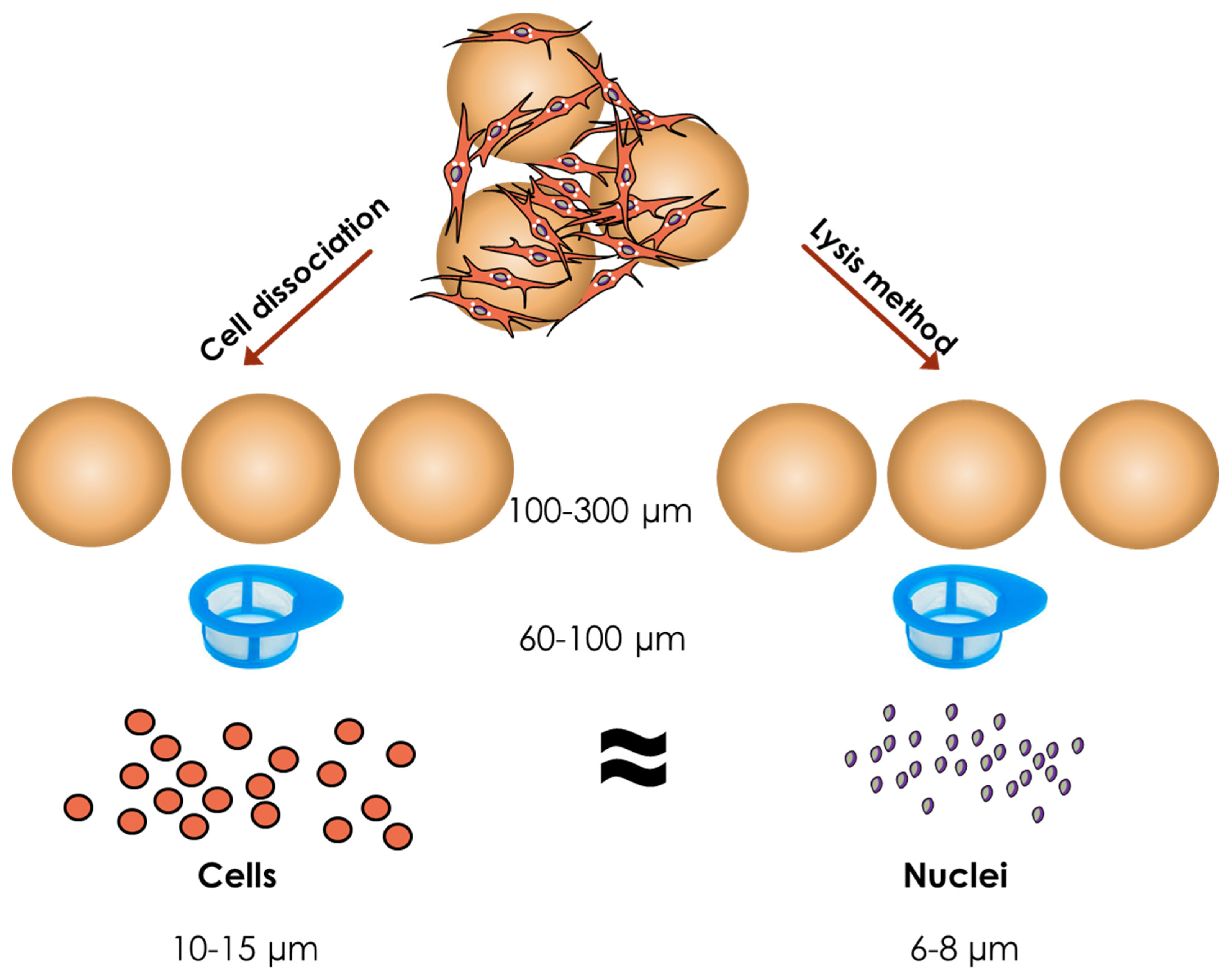
3. Sampling and Cell Counting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2019, 53, e12712. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. Stem. Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal stem cells current clinical applications: A systematic review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004–2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.; Motan, D.; Zhang, Z.; Chen, L.; Ji, H.; Tse, H.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immuno-modulation: Current status and future prospects. Cell Death Dis. 2017, 7, e2062. [Google Scholar] [CrossRef]
- Malik, N. Allogeneic versus autologous stem-cell therapy. BioPharm Int. 2012, 25, 36–40. [Google Scholar]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Jossen, V.; Bos, C.V.D.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef]
- Olsen, T.R.; Ng, K.S.; Lock, L.T.; Ahsan, T.; Rowley, J.A. Peak MSC—Are we there yet? Front. Med. 2018, 5, 178. [Google Scholar] [CrossRef]
- Simaria, A.S.; Hassan, S.; Varadaraju, H.; Rowley, J.; Warren, K.; Vanek, P.; Farid, S.S. Allogeneic cell therapy bioprocess economics and optimization: Single-use cell expansion technologies. Biotechnol. Bioeng. 2014, 111, 69–83. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Csaszar, E.; Dulgar-Tulloch, A. Commercial scale manufacturing of allogeneic cell therapy. Front. Med. 2018, 5, 233. [Google Scholar] [CrossRef]
- Rowley, J.; Campbell, A.; Brandwein, H.; Oh, S. Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess. Int. 2012, 10, 7. [Google Scholar]
- Van Wezel, A.L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nat. Cell Biol. 1967, 216, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Punreddy, S.; Aysola, M.; Kehoe, D.; Murrel, J.; Rook, M.; Niss, K. Growth kinetics of human mesenchymal stem cells in a 3-L single-use, stirred-tank bioreactor. BioPharm Int. 2013, 26, 28–38. [Google Scholar]
- Kehoe, D.; Schnitzler, A.; Simler, J.; DiLeo, A.; Ball, A. Scale-up of human mesenchymal stem cells on microcarriers in suspension in a single-use bioreactor. BioPharm Int. 2012, 25, 28–38. [Google Scholar]
- Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Culture of human mesenchymal stem cells on mi-crocarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett. 2013, 35, 1233–1245. [Google Scholar] [CrossRef]
- Timmins, N.; Kiel, M.; Günther, M.; Heazlewood, C.; Doran, M.; Brooke, G.; Atkinson, K. Closed system isolation and scalable expansion of human placental mesenchymal stem cells. Biotechnol. Bioeng. 2012, 109, 1817–1826. [Google Scholar] [CrossRef]
- Rowley, J.; Anne, M.S. The need for adherent cell manufacturing. BioProcess Int. 2018, 16, 34–40. [Google Scholar]
- Simon, M. Bioreactor design for adherent cell culture: The bolt-on bioreactor project, Part 4—Process economics. BioProcess Int. 2015, 13, 22–29. [Google Scholar]
- McAfee, E.; Abraham, E. Platform solutions for cell therapy manufacturing. BioProcess Int. 2017, 15. Available online: https://bioprocessintl.com/manufacturing/cell-therapies/platform-solutions-cell-therapy-manufacturing/ (accessed on 30 January 2021).
- António, M.; Fernandes-Platzgummer, A.; da Silva, C.L.; Cabral, J.M. Scalable microcarrier-based manufacturing of mesenchymal stem/stromal cells. J. Biotechnol. 2016, 236, 88–109. [Google Scholar]
- Chen, A.K.-L.; Reuveny, S.; Oh, S.K.W. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnol. Adv. 2013, 31, 1032–1046. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, W.; Wang, J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech. Model. Mechanobiol. 2010, 9, 659–670. [Google Scholar] [CrossRef]
- Lin, Y.M.; Lim, J.F.Y.; Lee, J.; Choolani, M.; Chan, J.K.Y.; Reuveny, S.; Oh, K.W. Expansion in micro-carrier-spinner cultures improves the chondrogenic potential of human early mesenchymal stromal cells. Cytotherapy 2016, 18, 740–753. [Google Scholar] [CrossRef]
- Jossen, V.; Schirmer, C.; Sindi, D.M.; Eibl, R.; Kraume, M.; Pörtner, R.; Eibl, D. Theoretical and practical issues that are relevant when scaling up hmsc microcarrier production processes. Stem Cells Int. 2016, 2016, 4760414. [Google Scholar] [CrossRef]
- Goh, T.K.-P.; Zhang, Z.-Y.; Chen, A.K.-L.; Reuveny, S.; Choolani, M.; Chan, J.K.Y.; Oh, S.K.-W. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. BioResearch Open Access 2013, 2, 84–97. [Google Scholar] [CrossRef]
- Schirmaier, C.; Jossen, V.; Kaiser, S.C.; Jüngerkes, F.; Brill, S.; Safavi-Nab, A.; Siehoff, A.; Bos, C.V.D.; Eibl, D.; Eibl, R. Scale-up of adipose tissue-derived mesenchymal stem cell production in stirred single-use bioreactors under low-serum conditions. Eng. Life Sci. 2014, 14, 292–303. [Google Scholar] [CrossRef]
- Dos Santos, F.; Campbell, A.; Fernandes-Platzgummer, A.; Andrade, P.Z.; Gimble, J.M.; Wen, Y.; Boucher, S.; Vemuri, M.C.; da Silva, C.L.; Cabral, J.M. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol. Bioeng. 2014, 111, 1116–1127. [Google Scholar] [CrossRef]
- Hupfeld, J.; Gorr, I.H.; Schwald, C.; Beaucamp, N.; Wiechmann, K.; Kuentzer, K.; Huss, R.; Rieger, B.; Neubauer, M.; Wegmeyer, H. Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol. Bioeng. 2014, 111, 2290–2302. [Google Scholar] [CrossRef]
- Siddiquee, K.; Sha, M. Billion-cell hypoxic expansion of human mesenchymal stem cells in BioBLU® 5c single-use vessels. Bioprocess. J. 2015, 14, 22–31. [Google Scholar] [CrossRef]
- Chen, A.K.-L.; Chew, K.Y.; Tan, H.Y.; Reuveny, S.; Oh, S.K.W. Increasing efficiency of human mesenchymal stromal cell culture by optimization of microcarrier concentration and design of medium feed. Cytotherapy 2015, 17, 163–173. [Google Scholar] [CrossRef]
- Sousa, M.F.; Silva, M.M.; Giroux, D.; Hashimura, Y.; Wesselschmidt, R.; Lee, B.; Roldão, A.; Carrondo, M.J.; Alves, P.M.; Serra, M. Production of oncolytic adenovirus and human mesenchymal stem cells in a single-use, vertical-wheel bioreactor system: Impact of bioreactor design on performance of microcarrier-based cell culture processes. Biotechnol. Prog. 2015, 31, 1600–1612. [Google Scholar] [CrossRef]
- Cunha, B.; Aguiar, T.; Silva, M.M.; Silva, R.J.; Sousa, M.F.; Pineda, E.; Peixoto, C.; Carrondo, M.J.; Serra, M.; Alves, P.M. Exploring continuous and integrated strategies for the up-and downstream processing of human mesenchymal stem cells. J. Biotechnol. 2015, 213, 97–108. [Google Scholar] [CrossRef]
- Mizukami, A.; Fernandes-Platzgummer, A.; Carmelo, J.G.; Swiech, K.; Covas, D.T.; Cabral, J.M.; da Silva, C.L. Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells. Biotechnol. J. 2016, 11, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Kehoe, D.E.; Schnitzler, A.C.; Rapiejko, P.J.; Der, K.A.; Philbrick, K.; Punreddy, S.; Rigby, S.; Smith, R.; Feng, Q. Process development for expansion of human mesenchymal stromal cells in a 50 L single-use stirred tank bioreactor. Biochem. Eng. J. 2017, 120, 49–62. [Google Scholar] [CrossRef]
- Tozetti, P.A.; Caruso, S.R.; Mizukami, A.; Fernandes, T.R.; da Silva, F.B.; Traina, F.; Covas, D.T.; Orellana, M.D.; Swiech, K. Expansion strategies for human mesenchymal stromal cells culture under xeno-free conditions. Biotechnol. Prog. 2017, 33, 1358–1367. [Google Scholar] [CrossRef]
- Cunha, B.; Aguiar, T.; Carvalho, S.B.; Silva, M.M.; Gomes, R.A.; Carrondo, M.J.; Gomes-Alves, P.; Peixoto, C.; Serra, M.; Alves, P.M. Bioprocess integration for human mesenchymal stem cells: From up to downstream processing scale-up to cell pro-teome characterization. J. Biotechnol. 2017, 248, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.-L.; Li, J.; Toh, J.P.-W.; Sim, E.J.-H.; Chen, A.K.-L.; Chan, J.K.-Y.; Choolani, M.; Reuveny, S.; Birch, W.; Oh, S.K.-W. Biodegradable poly-ε-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors. Cytotherapy 2017, 19, 419–432. [Google Scholar] [CrossRef]
- Mizukami, A.; Chilima, T.D.P.; Orellana, M.D.; Neto, M.A.; Covas, D.T.; Farid, S.S.; Swiech, K. Technologies for large-scale umbilical cord-derived MSC expansion: Experimental performance and cost of goods analysis. Biochem. Eng. J. 2018, 135, 36–48. [Google Scholar] [CrossRef]
- Gadelorge, M.; Bourdens, M.; Espagnolle, N.; Bardiaux, C.; Murrell, J.; Savary, L.; Ribaud, S.; Chaput, B.; Sensebé, L. Clinical-scale expansion of adipose-derived stromal cells starting from stromal vascular fraction in a single-use bioreactor: Proof of concept for autologous applications. J. Tissue Eng. Regen. Med. 2018, 12, 129–141. [Google Scholar] [CrossRef]
- Heathman, T.R.; Nienow, A.W.; Rafiq, Q.A.; Coopman, K.; Kara, B.; Hewitt, C.J. Agitation and aeration of stirred-bioreactors for the microcarrier culture of human mesenchymal stem cells and potential implications for large-scale bioprocess development. Biochem. Eng. J. 2018, 136, 9–17. [Google Scholar] [CrossRef]
- Mizukami, A.; Thomé, C.H.; Ferreira, G.A.; Lanfredi, G.P.; Covas, D.T.; Pitteri, S.J.; Swiech, K.; Faça, V.M. Proteomic iden-tification and time-course monitoring of secreted proteins during expansion of human mesenchymal stem/stromal in stirred-tank bioreactor. Front. Bioeng. Biotechnol. 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.d.S.; Mizukami, A.; Gil, L.V.G.; de Campos, J.V.; Assis, O.B.; Covas, D.T.; Swiech, K.; Suazo, C.A.T. Improving wave-induced motion bioreactor performance for human mesenchymal stromal cell expansion. Process. Biochem. 2019, 84, 143–152. [Google Scholar] [CrossRef]
- Moreira, F.; Mizukami, A.; de Souza, L.E.B.; Cabral, J.; da Silva, C.L.; Covas, D.T.; Swiech, K. Successful use of human AB serum to support the expansion of adipose tissue-derived mesenchymal stem/stromal cell in a microcarrier-based platform. Front. Bioeng. Biotechnol. 2020, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, K.; Yang, Y.; Deng, D.; Lyu, C.; Xu, H.; Liu, W.; Du, Y. Dispersible and dissolvable porous microcarrier tablets enable efficient large-scale human mesenchymal stem cell expansion. Tissue Eng. Part. C Methods 2020, 26, 263–275. [Google Scholar] [CrossRef]
- Da Silva, J.D.S.; Severino, P.; Wodewotzky, T.I.; Covas, D.T.; Swiech, K.; Marti, L.C.; Suazo, C.A.T. Mesenchymal stromal cells maintain the major quality attributes when expanded in different bioreactor systems. Biochem. Eng. J. 2020, 161, 107693. [Google Scholar] [CrossRef]
- Dosta, P.; Ferber, S.; Zhang, Y.; Wang, K.; Ros, A.; Uth, N.; Levinson, Y.; Abraham, E.; Artzi, N. Scale-up manufacturing of gelatin-based microcarriers for cell therapy. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 2937–2949. [Google Scholar] [CrossRef]
- Kurogi, H.; Takahashi, A.; Isogai, M.; Sakumoto, M.; Takijiri, T.; Hori, A.; Furuno, T.; Koike, T.; Yamada, T.; Nagamura-Inoue, T.; et al. Umbilical cord derived mesenchymal stromal cells in microcarrier based industrial scale sustain the immune regulatory functions. Biotechnol. J. 2021, 2000558. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Orellana, M.D.; Oliveira, M.C.; Covas, D.T.; Swiech, K.; Malmegrim, K.C. Hypoxia priming improves in vitro angiogenic properties of umbilical cord derived-mesenchymal stromal cells expanded in stirred-tank bi-oreactor. Biochem. Eng. J. 2021, 168, 107949. [Google Scholar] [CrossRef]
- FDA. PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. 2004. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pat-framework-innovative-pharmaceutical-development-manufacturing-and-quality-assurance (accessed on 30 January 2021).
- Ma, T.; Tsai, A.-C.; Liu, Y. Biomanufacturing of human mesenchymal stem cells in cell therapy: Influence of microenvi-ronment on scalable expansion in bioreactors. Biochem. Eng. J. 2016, 108, 44–50. [Google Scholar] [CrossRef]
- Tsai, A.-C.; Jeske, R.; Chen, X.; Yuan, X.; Li, Y. Influence of microenvironment on mesenchymal stem cell therapeutic potency: From planar culture to microcarriers. Front. Bioeng. Biotechnol. 2020, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.; Oh, S.K.W.; Warkiani, M.E. Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to com-mercialized products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef]
- Loubière, C.; Sion, C.; de Isla, N.; Reppel, L.; Guedon, E.; Chevalot, I.; Olmos, E. Impact of the type of microcarrier and agitation modes on the expansion performances of mesenchymal stem cells derived from um-bilical cord. Biotechnol. Prog. 2019, 35, e2887. [Google Scholar] [CrossRef]
- Rafiq, Q.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol. J. 2016, 11, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Leber, J.; Barekzai, J.; Blumenstock, M.; Pospisil, B.; Salzig, D.; Czermak, P. Microcarrier choice and bead-to-bead transfer for human mesenchymal stem cells in serum-containing and chemically defined media. Process. Biochem. 2017, 59, 255–265. [Google Scholar] [CrossRef]
- Swartz, E. Meeting the needs of the cell-based meat industry. Chem. Eng. Prog. 2019, 115, 41–45. [Google Scholar]
- Yuan, Y.; Kallos, M.S.; Hunter, C.; Sen, A. Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. J. Tissue Eng. Regen. Med. 2014, 8, 210–225. [Google Scholar] [CrossRef]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater. Sci. Eng. C 2019, 103, 109782. [Google Scholar] [CrossRef]
- Hu, W.S.; Meier, J.; Wang, D.I.C. A mechanistic analysis of the inoculum requirement for the cultivation of mammalian cells on microcarriers. Biotechnol. Bioeng. 1985, 27, 585–595. [Google Scholar] [CrossRef]
- Frauenschuh, S.; Reichmann, E.; Ibold, Y.; Goetz, P.M.; Sittinger, M.; Ringe, J. A microcarrier-based cultivation system for expansion of primary mesenchymal stem cells. Biotechnol. Prog. 2007, 23, 187–193. [Google Scholar] [CrossRef]
- Nienow, A.W.; Rafiq, Q.; Coopman, K.; Hewitt, C. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem. Eng. J. 2014, 85, 79–88. [Google Scholar] [CrossRef]
- Tsai, A.-C.; Liu, Y.; Yuan, X.; Chella, R.; Ma, T. Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnol. J. 2017, 12, 1600448. [Google Scholar] [CrossRef]
- Shekaran, A.; Lam, T.L.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.P.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef]
- Dos Santos, F.; Andrade, P.Z.; Abecasis, M.M.; Gimble, J.M.; Chase, L.G.; Campbell, A.M.; Boucher, S.; Vemuri, M.C.; da Silva, C.L.; Cabral, J.M. Toward a Clinical-Grade Expansion of Mesenchymal Stem Cells from Human Sources: A Microcarrier-Based Culture system under xeno-free conditions. Tissue Eng. Part. C Methods 2011, 17, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hashimura, Y.; Pendleton, R.; Harms, J.; Collins, E.; Lee, B. A systematic approach for scale-down model development and characterization of commercial cell culture processes. Biotechnol. Prog. 2006, 22, 696–703. [Google Scholar] [CrossRef]
- Kaiser, S.; Jossen, V.; Schirmaier, C.; Eibl, D.; Brill, S.; van den Bos, C.; Eibl, R. Fluid flow and cell proliferation of mesenchymal adipose-derived stem cells in small-scale, stirred, single-use bioreactors. Chem. Ing. Tech. 2013, 85, 95–102. [Google Scholar] [CrossRef]
- Ibrahim, S.; Nienow, A. Suspension of microcarriers for cell culture with axial flow impellers. Chem. Eng. Res. Des. 2004, 82, 1082–1088. [Google Scholar] [CrossRef]
- Liepe, F.; Sperling, R.; Jembere, S. Rührwerke: Theoretische Grundlagen, Auslegung und Bewertung; Fachhochsch: Köthen, Germany, 1998. [Google Scholar]
- Tsai, A.-C.; Liu, Y.; Ma, T. Expansion of human mesenchymal stem cells in fibrous bed bioreactor. Biochem. Eng. J. 2016, 108, 51–57. [Google Scholar] [CrossRef]
- Nold, P.; Brendel, C.; Neubauer, A.; Bein, G.; Hackstein, H. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem. Biophys. Res. Commun. 2013, 430, 325–330. [Google Scholar] [CrossRef]
- Kalmbach, A.; Bordás, R.; Öncül, A.A.; Thévenin, D.; Genzel, Y.; Reichl, U. Experimental characterization of flow conditions in 2-and 20-l bioreactors with wave-induced motion. Biotechnol. Prog. 2011, 27, 402–409. [Google Scholar] [CrossRef]
- Åkerström, H. Expansion of Adherent Cells for Cell Therapy. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2009. [Google Scholar]
- Heathman, T.R.; Nienow, A.W.; Rafiq, Q.A.; Coopman, K.; Kara, B.; Hewitt, C.J. Development of a pro-cess control strategy for the serum-free microcarrier expansion of human mesenchymal stem cells towards cost-effective and commercially viable manufacturing. Biochem. Eng. J. 2019, 141, 200–209. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Jossen, V.; Pörtner, R.; Kaiser, S.C.; Kraume, M.; Eibl, D.; Eibl, R. Mass production of mesenchymal stem cells—Impact of bioreactor design and flow conditions on proliferation and differentiation. Cells Biomater. Regen. Med. 2014. [Google Scholar] [CrossRef]
- Zhao, F.; Pathi, P.; Grayson, W.; Xing, Q.; Locke, B.R.; Ma, T. Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: Experiments and mathematical model. Biotechnol. Prog. 2005, 21, 1269–1280. [Google Scholar] [CrossRef]
- Kane, J. Measuring kLa for better bioreactor performance. BioProcess Int. 2012, 10, 46–49. [Google Scholar]
- Shah, R.; Park, H.-S. Proposed design model of single use bioreactor for mesenchymal stem cells proliferation. Procedia CIRP 2016, 41, 382–386. [Google Scholar] [CrossRef][Green Version]
- Higuera, G.; Schop, D.; Janssen, F.; van Dijkhuizen-Radersma, R.; van Boxtel, T.; van Blitterswijk, C.A. Quantifying in vitro growth and metabolism kinetics of human mesenchymal stem cells using a mathematical model. Tissue Eng. Part A 2009, 15, 2653–2663. [Google Scholar] [CrossRef]
- Jung, S.; Panchalingam, K.M.; Wuerth, R.D.; Rosenberg, L.; Behie, L.A. Large-scale production of hu-man mesenchymal stem cells for clinical applications. Biotechnol. Appl. Biochem. 2012, 59, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Lembong, J.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T. Bioreactor parameters for microcarrier-based human MSC expansion under xeno-free conditions in a vertical-wheel system. Bioengineering 2020, 7, 73. [Google Scholar] [CrossRef]
- Rafiq, Q.A.; Ruck, S.; Hanga, M.P.; Heathman, T.R.; Coopman, K.; Nienow, A.W.; Williams, D.J.; Hewitt, C.J. Qualitative and quantitative demonstration of bead-to-bead transfer with bone marrow-derived human mesenchymal stem cells on microcarriers: Utilising the phenomenon to improve culture performance. Biochem. Eng. J. 2018, 135, 11–21. [Google Scholar] [CrossRef]
- Nienow, A.W.; Hewitt, C.; Heathman, T.R.; Glyn, V.A.; Fonte, G.N.; Hanga, M.P.; Coopman, K.; Rafiq, Q. Agitation conditions for the culture and detachment of hMSCs from microcarriers in multiple bioreactor platforms. Biochem. Eng. J. 2016, 108, 24–29. [Google Scholar] [CrossRef]
- Scientific, T.F. Scalability of Microcarrier Bead Separation Using the Harvestainer Systems. 2018. Available online: https://assets.thermofisher.com/TFS-Assets/BPD/Application-Notes/scalability-harvestainer-app-note.pdf (accessed on 30 January 2021).
- Yuan, X.; Tsai, A.-C.; Farrance, I.; Rowley, J.A.; Ma, T. Aggregation of culture expanded human mesenchymal stem cells in microcarrier-based bioreactor. Biochem. Eng. J. 2018, 131, 39–46. [Google Scholar] [CrossRef]
- Song, K.; Yang, Y.; Wu, S.; Zhang, Y.; Feng, S.; Wang, H.; Wang, Y.; Wang, L.; Liu, T. In vitro culture and harvest of BMMSCs on the surface of a novel thermosensitive glass microcarrier. Mater. Sci. Eng. C 2016, 58, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Storm, E. Single-use tangential flow filtration in bioprocessing. BioProcess Int. 2011, 9, 38–47. [Google Scholar]
- Pattasseril, J.; Varadaraju, H.; Lock, L.; Rowley, J.A. Downstream technology landscape for large-scale therapeutic cell processing. Bioprocess. Int. 2013, 11, 38–47. [Google Scholar]
- Tsai, A.-C.; Ma, T. Expansion of human mesenchymal stem cells in a microcarrier bioreactor. In Methods in Molecular Biology; Springer Science and Business Media LLC & Humana Press: New York, NY, USA, 2016; pp. 77–86. [Google Scholar]
- Radel, D.; Madde, P.; Dietz, A. Comparison of two automated cell counters for enumeration and viability of mesenchymal stem cells for clinical cellular therapy trials. Cytotherapy 2018, 20, S69–S70. [Google Scholar] [CrossRef]
| Cell Type | Seed Train | Vessel Type | Vessel Volume (L) | Working Volume (L) | MC Type | MC Concentration (g/L) | Cell-to-Bead Ratio | Cell Inoculation Concentration (Cells/mL) | Cell Seeding Density (Cells/cm2; Cells/mL) | Strategy for Cell Attachment | Action Time for Attachment (h) | Counting Method | Attachment Efficiency | Colonization Efficiency | pH | Gas Input | Mixing | Power Ratio (W/m3) | Feeding Method and Regime | Period (day) | Recovery Mesh Size (µm) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hPL-MSC | Planar culture | CultiBag or Cellbag | 2 | 0.5 | CultiSpher-S | - | 5 | - | - | Cell were inoculated at minimum volume of medium. Gentle rocking to distribute the cells, maintaining static culture for overnight prior to addition of medium to working volume. | 18 | CyQUANT cell quantification assay kit for unattached cells. | 90% | - | - | 5% O2 5% CO2 Air | - | - | - | 7 | - | [18] |
| hBM-MSC | Directly transfer from spinner flask MC culture | Mobius CellReady 3L | 3 | 2 | SoloHill collagen-coated | - | 4.5 α | 30,000 | 5000 (seeding before transferring) | 200 mL MCs with attached cells were directly transferred from spinners into the bioreactor containing 800 mL of media with fresh MCs. 25 rpm at low volume (1 L) and then increased to 40 rpm at the larger volume (2 L) on Day 3. | - | Using NC-100 after lysing the cells off the MCs. | - | - | - | - | 25 rpm to 40 rpm (after Day 3) | - | 50% addition | 7 | 100 | [16] |
| Hf-MSC | Planar culture | Biostat B-DCU | 1 | 1 | Cytodex 3 | 8.34 | 3 or 4 α | 100,000 | 4440 α | 50% working volume at 30 rpm (lower agitation) for 24 h. Then, add to full working volume at 50 rpm. | 24 | Attached cells on MCs were counted by nuclei count NC-100; Unattached cells were counted by trypan blue. | - | - | NaOH and CO2 gas 7.2–7.3 | O2 CO2 Air | 50 rpm | - | 50% medium change every 2 day | 8 | 70 | [27] |
| hBM-MSC | Planar culture | Biostat B Plus | 5 | 2.5 | Plastic P-102L | 27.8 α | 5 | 24,000 | 6000 | Static for 18 h. | 18 | Attached cells on MCs were counted by NC-100 with propidium iodide. | - | - | no control (7.2 to 6.7 and 6.9) | - | 75 rpm | - | 50% medium change every 2 day after Day 3 | 9 | 60 | [17] |
| hBM-MSC | Planar culture or Fresh Thawed | Mobius CellReady | 3 | ] | - | 15 | - | 5000 | - | Low agitation at 25–35 rpm. | 24 | Samples were spun down at 200 rpm for 5 min and cell numbers were measured using NC-100. | 60% | - | - | 5% CO2 | - | - | Feeding twice (Day 6 and Day 10) | 12 | - | [15] |
| hAD-MSC | Fresh thawed | UniVessel® SU | 2 | 2 | ProNectin® F-COATED | 7.5 | 2.5 α | 7560 α | 2800 | The 4-h attachment phase was realized in 0.7 L culture volume without stirring before the medium was filled up to 2 L. | 4 | Cell densities were determined using NC-100. | - | - | 7.2 | 0.1 vvm | 100–140 rpm | 1.54 at 100 rpm; 2.87 at 125 rpm; 3.94 at 140 rpm | 50% medium change on Day 4 | 7 | 63 | [28] |
| CultiBag STR | 50 | 35 | ProNectin® F-COATED | 7.14 | 7197 α | After inoculation into CultiBag RM 20-L which was placed in an incubator at 37 °C, 80% humidity and 5% CO2, and kept stationary for 4 h. Afterwards, the MC-cell suspension was transferred into the CultiBag STR 50-L. | 7.2–7.3 | 0.03 vvm | 50 rpm | 0.63 | 9 | |||||||||||
| hBM-MSC hAD-MSC | Directly transfer from spinner flask MC culture | Bioflo® 110 | 1.3 | 0.8 | SoloHill plastic | 5 (in spinner) | 3.1 (in spinner) α | 50,000 (in spinner) | 3472 (in spinner) α | 25 rpm for 18 h followed by a non-agitation period of 6 h (in spinner). | 24 (in spinner) | Samples were washed with PBS and incubated with TrypLE Express at 37 °C for 5–7 min at 650 rpm using Thermomixer confort. for detaching cells. Single cell suspension were then counted by Trypan Blue. | - | - | 7.2 | CO2 sparging; Air 5 ccm | 60 rpm | - | 25% medium daily | 7 | - | [29] |
| hBM-MSC | Planar culture | DASGIP (cellferm-pro) | - | 0.4 | SoloHill plastic | 20 | 3.4 α | 27,000 | 3750 α | 40 rpm for 2 min followed by a non-agitation period of 2 h. | 24 | MC-cell pellet was instantly frozen at -80 ntil cell counting using CyQUANT1 Cell Proliferation Assay (Life Technologies) based onDNA. | - | - | 7 | - | 40 rpm | - | Perfusion at 25% working volume (100 mL/day) after 3 days | 11 | - | |
| hUC-MSC | Fresh thawed | B-DCU1 2 L univesselQuad version | 2 | 1.5 | Cytode× 1 | - | 1.2 (in spinner) α | - | 1200 | 450 mL in static culture for orvernight. Then, medium addition to 1500 mL at 50 rpm. | 24 | - | 50% | - | 7.35 | 2 | 50 rpm | - | 50% medium change twice per week | 8–13 (various donors) | 80 | [30] |
| hAD-MSC | Directly transfer from shaking flask MC culture | BioBLU 5c | 3.75 | 3.75 | SoloHill collagen-coated | 17 | 2.7 (in flask) α | 17,500 | 3000 (in flask) | 3.5 L MCs with attached cells were transferred into the bioreactor at 25 rpm. After 1 h, addition of 0.25 L medium was added to reach 3.75 L working volume. | - | Cells on MCs were counted by NC-100. | - | - | 7 | Air, CO2, N2, O2 N2 sparging at 0.01 SLPM after Day 6 | 25–35 rpm after Day 6 | - | 50% medium change on Day 4, 8, 12 and addition of 0.5 g/L glucose on Day 15 | 18 (peak on Day 16) | - | [31] |
| hfMSC | Harvest from spinner flask MC culture | Biostat B-DCU | 2 | 0.8 to 1.9 | Cytode× 3 | 8 α | 4 | 100,000 | 4629 α | - | - | Total and non-viable cell concentrations in the MC culture were determined by NC-3000. | - | - | 7.2–7.3 | O2 CO2 Air | 60–80 rpm | - | Periodic feeding concentrated medium every 1.5 h | 6 | - | [32] |
| hBM-MSC | Planar culture | PBS-VW | - | 2.2 | Synthemax® II | 16 to 48 after Day 6 | 3.9 α | 25,000 | 4340 α | 0–6 h: 17 rpm, 1 min; off, 20 min 6 h–day 6: 17 rpm Day 6–10: 17 rpm, 5 min; off, 1 h Day 10–14: 17 rpm. | 12 | Total cells were briefly disrupted using 0.1 M citric acid with 1% TritonX-100 at 37 °C overnight and the nuclei were stained with 0.1% crystal violet for counting by hemocytometer. | 95% | 68% | 7.2 | - | 17 rpm | 0.3 | 50% medium change every 2.5 day after Day 5 | 14 | - | [33] |
| Biostat Qplus ST | - | 0.25 | 0–6 h: 40 rpm, 1 min; off, 20 min 6 h–day 6: 40 rpm Day 6–10: 40 rpm, 5 min; off, 1 h Day 10–14: 45 rpm. | 48% | - | 40–45 rpm | Avg. 0.1–0.2 Max. 0.6–0.8 | |||||||||||||||
| hBM-MSC | - | Biostat Qplus stirred tank | - | 0.4 | Synthemax®II | 16 to 48 after Day 6 | 3.9 α | 25,000 | 4340 α | For the first 6 h, 200 mL at 60 rpm for 1 min and 0 rpm for 20 min. Then, another 200 mL medium was added to reach 400 mL working volume at 40–60 rpm. | 6 | Trypan blue; Fluorescein diacetate-propidium iodide staining; LDH assay. | 7.2 | 0.1 vvm | 40–60 rpm (from Day 6 to 9, an intermittent agitation was set at 15 rpm for 5 min and 0 rpm for 55 min) | - | 50% medium change every 2.5 day after Day 5 or perfusion rate at 20% daily after Day 5 | 14 | 75 | [34] | ||
| hAD-MSC | Fresh thawed | CultiBag | 2 | 1.5 | MC-2 | 13.63 α | 5.8 α | 31,902 α | 6500 | MCs were incubated at 37 °C overnight before cell inoculation into 1 L shake flasks. After a 20-h static attachment, MCs with cells were transferred into the culture bag prior to addition of medium to working volume. | 20 | Cells were dissociated from MCs by TrypLE Select for 30 min at 37 °C and counted by NC-200. | - | - | 7.3 | 0.05 vvm | 4° and 31 rpm | Avg. 8.92 Max. 17.69 | 50% medium change on Day 5 after settling down MCs for 15 min | 9 | - | [26] |
| hUCM-MSC | Planar culture | Celligen 310 | 2.5 | 0.8 | Cultispher®S | 1 | 31.25 α | 25,000 | - | 0–24 h: 30 rpm 24–72 h: 40 rpm after 72 h: 50 rpm. | 24 | Samples were trypsinized to recover bead-free cell suspension (i.e., no filtration step needed). | 75 | - | 7.3 (NaHCO3 and H2SO4) | N2 O2 Air | 50 rpm | - | no medium change | 6 (peak on Day 4) | - | [35] |
| hBM-MSC | Planar culture | Mobius®3 L | 3 | 2.4 | SoloHill collagen-coated | 15 | 2.5 α | 15,000 | 2777 α | 1 L for cell attachment. | - | - | - | - | 7.4–7.6 | - | - | - | - | 13 | - | [36] |
| Mobius®50 L | 50 | 50 | 20 L at 64 rpm for 4 h. | 4 | Total cells were counted by nuclei using NC-100; unattached cells were counted after filtration with 100 µm sieve; attached cells were counted after unattached cell removal and PBS wash. | 13% at 4h and >100% after 24 h | - | 7.45 (NaHCO3) | 1 lpmAir CO2 N2 O2 instead of Air after Day 4 | 75 rpm - 85 rpm after Day 7 - 95 rpm after Day 9 -100 rpm at final | - | Day 3 and Day 7 | 11 | - | ||||||||
| hUCM-MSC | Directly transfer from spinner flask MC culture | Celligen 310 | 2.5 | 0.8 | Plastic P102L | 10 (in spinner) | 10 (in spinner) α | 40,000 (in spinner) | 11,000 (in spinner) | Intermittent stirring was set for 3 days: 2 min agitating at 30 rpm followed by 15 min static (in spinner). | 72 (in spinner) | MCs with attached cells were treated with TrypLE and Collagenase for 7 min. Detached cells were counted by Trypan Blue exclusion method. | - | - | 7.3 | - | 30 rpm | - | 25% medium change daily after Day 5 | 7 | 100 | [37] |
| hBM-MSC hAD-MSC | Planar culture | 2 L UniVessel®SU | 2 | 2 | Synthemax®II | 32 to 16 after 5 h | 3.9 α | 25,000 | 4340 α | 50% working volume for 5 h (On:100 rpm for 1 min; Off: 0 rpm for 20 min). Addition of medium to full working volume at 100 rpm. | 5 | - | 84% | 85 | 7.2 | 0.1 vvm | 100 rpm | 1.54 | 50% medium change on Day 5 | 7 | - | [38] |
| hWJ-MSC | Planar culture | B-DCU | 1 | 0.3 to 0.48 | Low density Polycaprolactone | 31.3 α | 4 | 120,000 | 7330 α | - | - | Viable cells were counted with NC-3000. | - | - | 7.2 | Air CO2 N2 O2 | 80 rpm | - | Medium addition to maintain glucose concentration at 0.2 g/L after Day 3 | 7 (peak on Day 5) | - | [39] |
| hUCM-MSC | Planar culture | Celligen® 310 | 2.5 | 0.8 | CultiSpher-S® | 2 | - | - | 5000 | a stirring period (50 rpm for 1 min) followed by 30 min of nostirring. After 6 h of cell adhesion, a continuous stirring was set at 50 rpm. | 6 | Cell number was quantified using MTT assay. | - | - | 7.3 (NaHCO3 and H2SO4) | N2 O2 Air | 50 rpm | - | 50% medium change daily after Day 4 | 7 | 100 | [40] |
| hAD-SC | Planar culture | Mobius® 3-L | 3 | 2 | Corning® Enhanced Attachment | 15 | 3.6 α | 21,625 α | 4000 | 0.5% PL instead of 2% and supplemented with 0.1% Pluronic® F68 for 24. Then, PL was added to reach 5% PL at 800 mL working volume. | 24 | Cells were harvested and counted using Trypan blue exclusion. | - | - | 7.5 | Air (27 mL/min) CO2 | 35 rpm | - | - | 12 | - | [41] |
| hBM-MSC | Planar culture | DASGIP DASbox | 0.25 | 0.1 | Plastic P-102L | 13.8 α | 5 | 30,000 α | 6000 | The culture was static for one hour and then start to agitate. | 24 | Total and non-viable cell concentrations in the MC culture were determined by NC-3000. | 30% at sparging and 60% at overlay after 24 h | - | 7.4 | Air sparging 0.1VVM | 115 rpm | 2.56α | 50% medium change every other day | 6 | - | [42] |
| hUCM-MSC | Planar culture | Celligen®310 | 2.5 | 0.8 | CultiSpher-S® | 2 | - | - | 5000 | A stirring period (50 rpm for 1 min) followed by 30 min of no stirring. After 6 h of cell adhesion, a continuous stirring was set at 50 rpm. | 6 | Cell number was quantified using MTT assay. | - | - | 7.3 (NaHCO3 and H2SO4) | N2 O2 Air | 50 rpm | - | 50% medium change daily after Day 4 | 7 | - | [43] |
| hUCM-MSC | Directly transfer from shaking flask MC culture | Xuri™ 2/10Cellbag | 2 | 0.6 | CultiSpher-S® | 2.1 (in spinner) | 30 | 50,000 (in spinner) | - | 0–1 h: 200 mL at 50 rpm for 1 min every 15 min 0–3 h: 200 mL at 50 rpm for 1 min every 30 min 3–9 h: 200 mL static 9–24 h: – After 24 h, the culture was transferred into cellbag and medium was added to reach 600 mL at 24 rpm 4° | 24 | Cells adhered on MCs was indirectly measured by MTT assay. | 94.80% | - | 6.8–7.4 | 5–10% CO2 Air 0.02 to 0.04 lpm after 24 h | 24–48 h: 15 rpm 7° 48–168 h: 24 rpm 4° 168–216 h: 27 rpm 3° 216–240 h: 33 rpm 2° | - | - | 10 | - | [44] |
| Planar culture | 8.3 to 0.7 after 24 h | 200,000 | - | 0–24 h: 50 mL at Static After 24 h, medium addition to reach 600 mL at 24 rpm 4°. | 24 | 60.5%–77.8% | - | 24 rpm 4° | - | - | ||||||||||||
| hAD-MSC | Planar culture | Applikon mini | 500 | 250 | SoloHill plastic | 20 to 12 after Day 3 | 10.4 α | 83,333 α | 11,574 α | 0–24 h: 150 mL at 85 rpm 24–48 h: 150 mL at 95 rpm 48–72 h: 250 mL at 95 rpm. | 24 | Samples were washed with PBS and incubated with TrypLE Express at 37 °C for 7 min at 650 rpm using Thermomixer. Then, after quenching enzymatic activity, the cell/MC suspension was filtered using a 100 mm cell strainer for counting cells by Trypan Blue exclusion method. | 22 | - | 7.3 | N2 O2 Air | 85 rpm - 95 rpm after Day 2 - 105 rpm after Day 5 | - | 25% medium change daily after Day 4 | 9 (peak on Day 7) | - | [45] |
| hAD-MSC | Directly transfer from spinner flask MC culture | 3D FloTrix vivaSPIN | 1 | 1 | 3D TableTrix | 3.3 (in spinner) | 2 (in spinner) | 33,333 (in spinner) α | 1111 (in spinner) α | 58 cycles at 60 rpm for 5 min and 0 rpm for 20 min. Then, the culture was maintained at 60 rpm (in spinner). | 24 (in spinner) | MCs attached with cells were dissolved for counting. | 98 (in spinner) | - | - | 5% CO2 95% Air | 60 rpm | - | 50% medium change every other day | 7 | - | [46] |
| hUC-MSC | Planar culture | Xuri™ 2/10 Cellbag | 2 | 0.6 | CultiSpher-S® | 8.3 to 0.7 after 24 h | 16 30 | 204,000 to 17,000 after 24 h (high seeding) 108,000 to 9000 after 24 h (low seeding) | - | 50 mL at Static for 24 h. Then, medium addition to reach 600 mL at 24 rpm 4°. | 24 | Cells on MCs were measured by MTT assay. | >73% | - | 7–7.4 | 5–10% CO2 Air 0.02 to0.04 lpm after 24 h | 24 rpm 4° | - | no medium change | 7 (high seeding) 11 (low seeding) | - | [47] |
| hMSC | Planar culture | BioBLU 3 L | 3 | 1 | redox-sensitive beads (RS beads) regular gelatin-based beads (Reg beads) | 4 | - | - | - | The first 6.8 h: 0 RPM for 60 min, then 50 RPM for 10 min. | 6.8 | MCs with attached cells were washed with PBS and incubated with dissolution reagent in a 1:1 volume. Once dissolved, the sample was measured by NC-200. | - | - | 7.2 | Air CO2 N2 O2 | 55 to 100 rpm | - | Perfusion at 100% working volume per day | 7 | - | [48] |
| hUC-MSC | Directly transfer from spinner flask MC culture | Middle Scale Bioreactor BCP | 0.5 | 0.5 | Corning® CellBIND® | - | 2.7 to 4 (in spinner) α | - | 3000 to 4500 | 15 to 30 rpm after inoculation and 50 rpm on Day 2. | 72 | - | - | - | - | 190 cc/h Air 10 cc/h CO2 | 10 to 15 rpm after transferring and 25 rpm on Day 3 | - | 50% medium change on Day 7 and Day 11 | 11 | - | [49] |
| hUC-MSC | Planar culture | MiniBio | 0.5 | 0.2 | SphereCol® | 5 | 17 | 40,000 | 22,222 α | For the first 4 h, 80 rpm for 30 s, followed by 0 rpm for 30 min. | 4 | Cell number and viability were determined by Trypan Blue (0.4%)exclusion method after sample cell harvesting. | 63% | 7.2–7.4(NaHCO3 and H2SO4) | CO2 N2O2 | 80 to 120 rpm | - | - | 5 | 100 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, A.-C.; Pacak, C.A. Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors. Bioengineering 2021, 8, 96. https://doi.org/10.3390/bioengineering8070096
Tsai A-C, Pacak CA. Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors. Bioengineering. 2021; 8(7):96. https://doi.org/10.3390/bioengineering8070096
Chicago/Turabian StyleTsai, Ang-Chen, and Christina A. Pacak. 2021. "Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors" Bioengineering 8, no. 7: 96. https://doi.org/10.3390/bioengineering8070096
APA StyleTsai, A.-C., & Pacak, C. A. (2021). Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors. Bioengineering, 8(7), 96. https://doi.org/10.3390/bioengineering8070096






