Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Measuring Gelatin-MTG Hydrogel Elastic Modulus and Mass
2.2. Measuring Gelatin-MTG Hydrogel Transparency
2.3. Fabrication of Micromolded Gelatin-MTG Hydrogel Coverslips
2.4. Myoblast and Myotube Culture on Micromolded Hydrogel Coverslips
2.5. Tissue Fixation and Immunostaining
2.6. Image Acquisition and Analysis
2.7. Statistical Analysis
3. Results
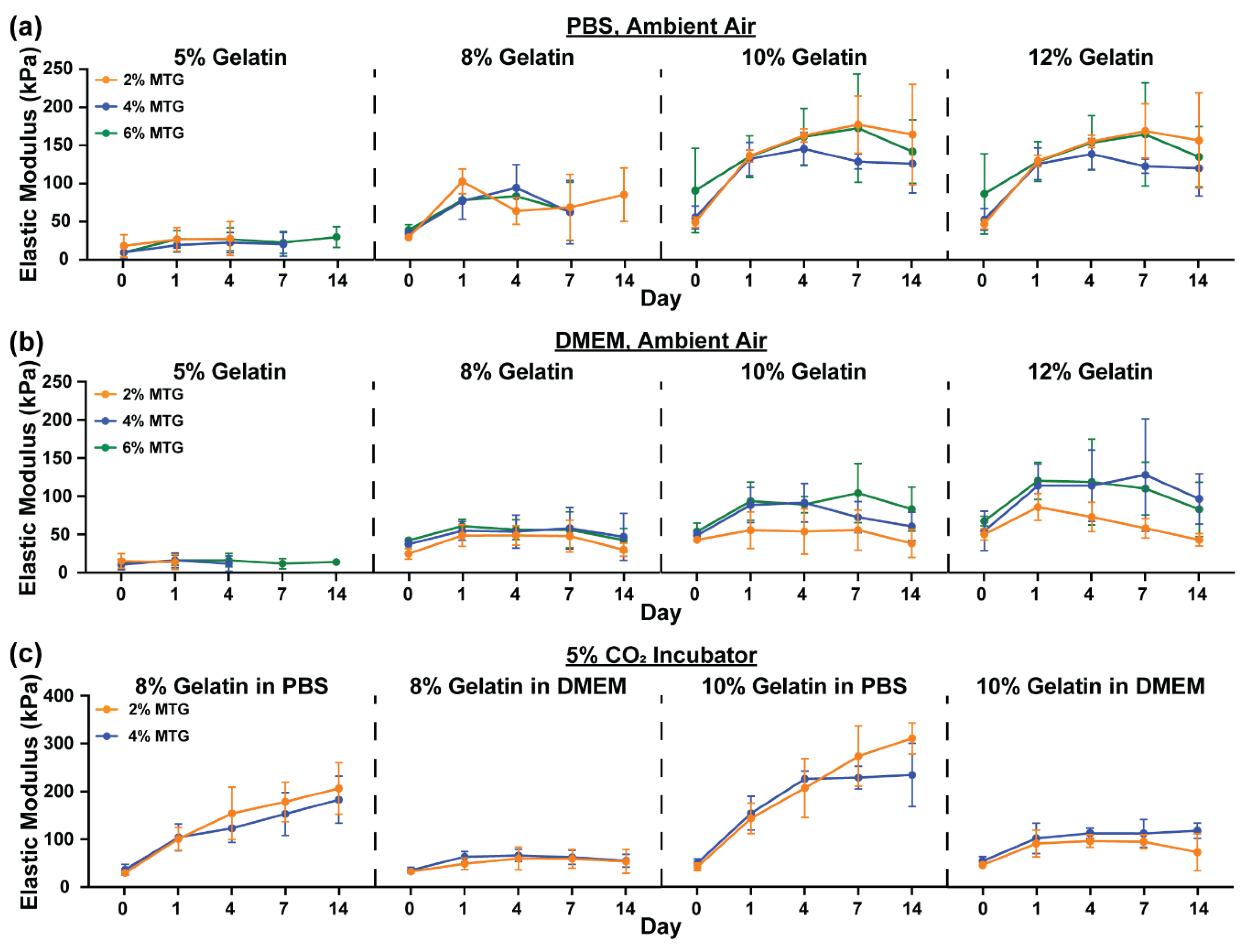
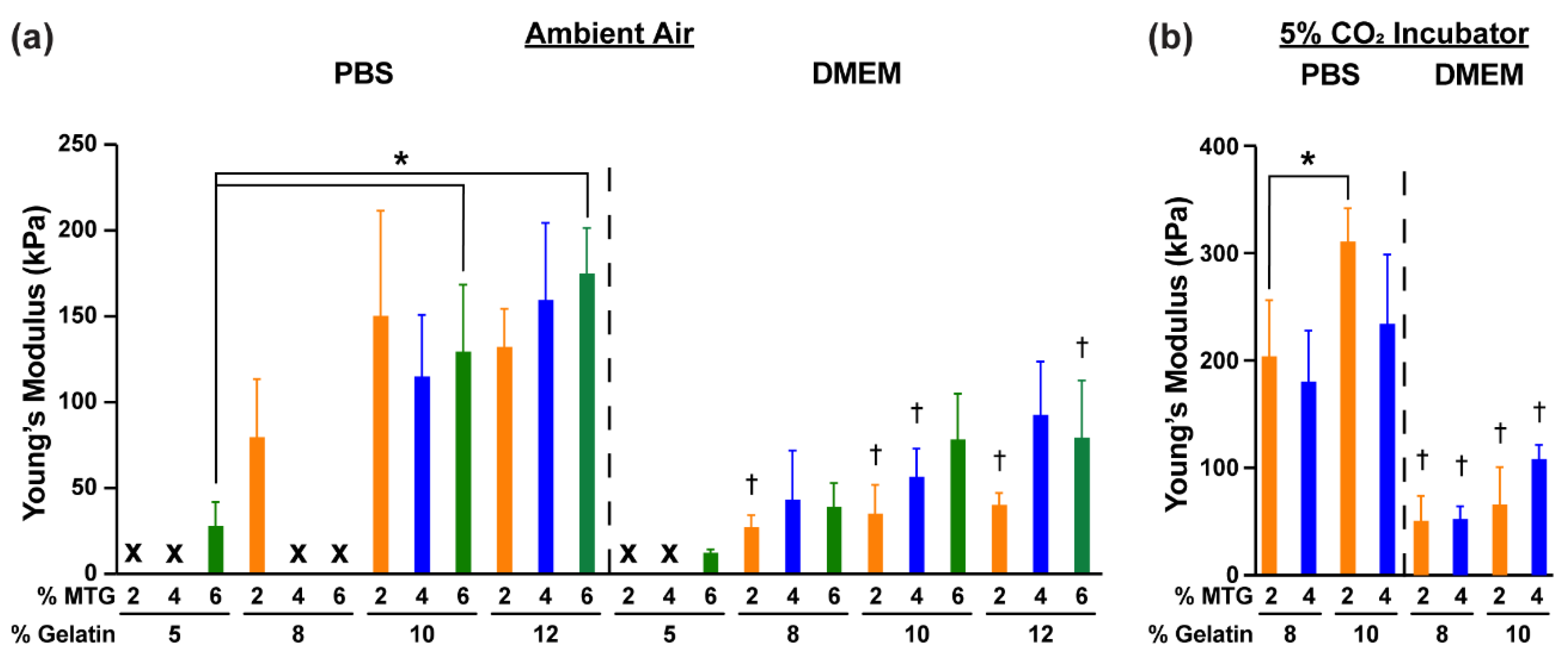
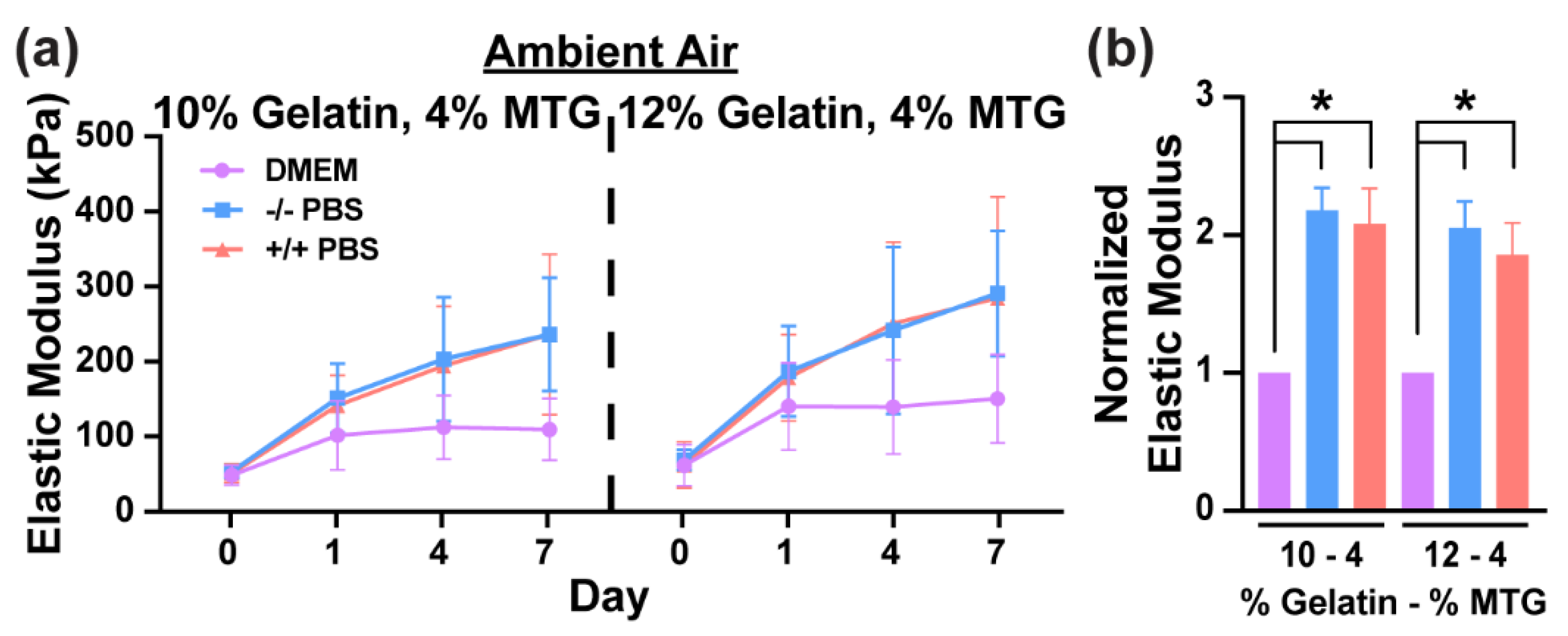
3.1. Characterization of Hydrogel Elastic Modulus and Mass over Time
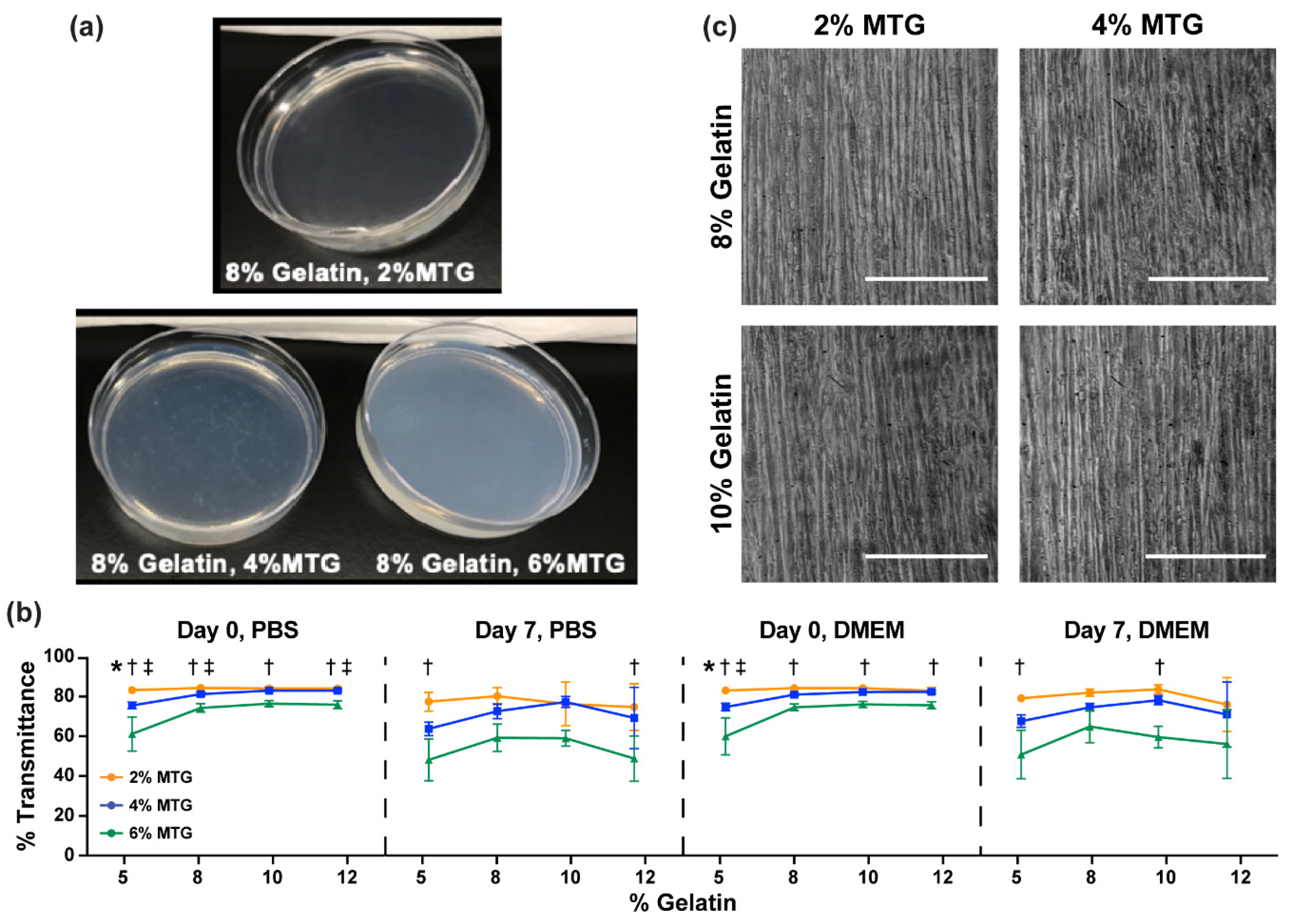
3.2. Characterization of Hydrogel Transparency
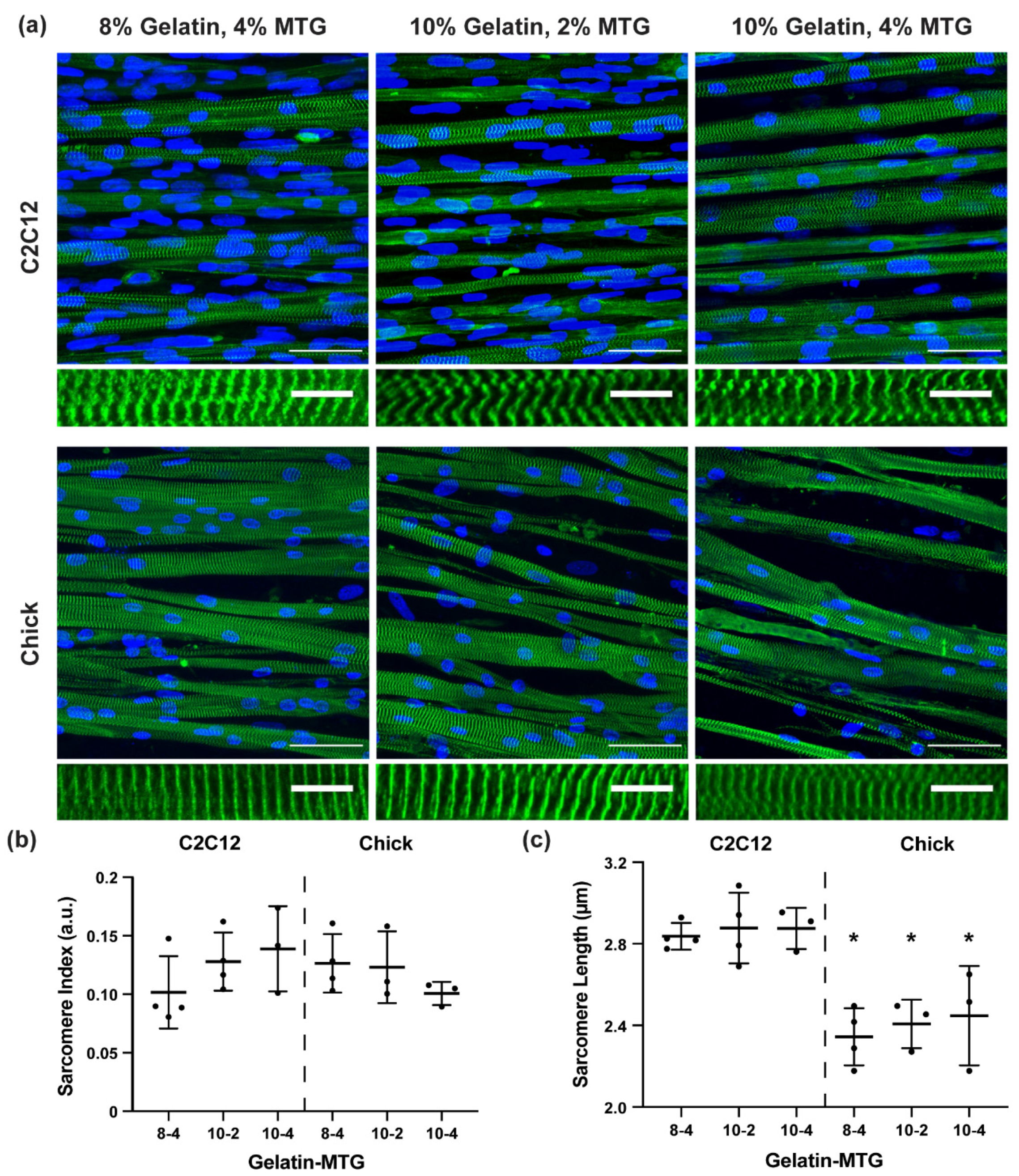
3.3. Characterization of Myotube Maturity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssen, L.; Allard, N.A.E.; Saris, C.G.J.; Keijer, J.; Hopman, M.T.E.; Timmers, S. Muscle Toxicity of Drugs: When Drugs Turn Physiology into Pathophysiology. Physiol. Rev. 2020, 100, 633–672. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Morgan, J.E. Duchenne’s muscular dystrophy: Animal models used to investigate pathogenesis and develop therapeutic strategies. Int. J. Exp. Pathol. 2003, 84, 165–172. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal models of Duchenne muscular dystrophy: From basic mechanisms to gene therapy. Dis. Model. Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef]
- Nesmith, A.P.; Wagner, M.A.; Pasqualini, F.S.; O’Connor, B.B.; Pincus, M.J.; August, P.R.; Parker, K.K. A human in vitro model of Duchenne muscular dystrophy muscle formation and contractility. J. Cell Biol. 2016, 215, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Hicks, M.R.; Ermolova, N.V.; Nakano, H.; Jan, M.; Younesi, S.; Karumbayaram, S.; Kumagai-Cresse, C.; Wang, D.; Zack, J.A.; et al. A Single CRISPR-Cas9 Deletion Strategy that Targets the Majority of DMD Patients Restores Dystrophin Function in hiPSC-Derived Muscle Cells. Cell Stem Cell 2016, 18, 533–540. [Google Scholar] [CrossRef]
- Shahini, A.; Vydiam, K.; Choudhury, D.; Rajabian, N.; Nguyen, T.; Lei, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Skoglund, G.; Lainé, J.; Darabi, R.; Fournier, E.; Perlingeiro, R.; Tabti, N. Physiological and ultrastructural features of human induced pluripotent and embryonic stem cell-derived skeletal myocytes in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 8275–8280. [Google Scholar] [CrossRef]
- Pimentel, M.R.; Falcone, S.; Cadot, B.; Gomes, E.R. In Vitro Differentiation of Mature Myofibers for Live Imaging. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Hosseini, V.; Ahadian, S.; Fujie, T.; Parthiban, S.P.; Ramalingam, M.; Bae, H.; Kaji, H.; Khademhosseini, A. Skeletal muscle tissue engineering: Methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 2014, 20, 403–436. [Google Scholar] [CrossRef]
- Duffy, R.M.; Sun, Y.; Feinberg, A.W. Understanding the Role of ECM Protein Composition and Geometric Micropatterning for Engineering Human Skeletal Muscle. Ann. Biomed. Eng. 2016, 44, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.N.; Zhang, L.; Sun, Y.; Feinberg, A.W. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 2012, 7, e51499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duffy, R.; Lee, A.; Feinberg, A.W. Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater. 2013, 9, 7885–7894. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.R.; Tomiya, A.; Regev, G.J.; Thacker, B.E.; Benzl, R.C.; Kim, C.W.; Lieber, R.L. Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. J. Biomech. 2009, 42, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Romanazzo, S.; Forte, G.; Ebara, M.; Uto, K.; Pagliari, S.; Aoyagi, T.; Traversa, E.; Taniguchi, A. Substrate stiffness affects skeletal myoblast differentiation. Sci. Technol. Adv. Mater. 2012, 13, 064211. [Google Scholar] [CrossRef]
- Wang, P.Y.; Thissen, H.; Tsai, W.B. The roles of RGD and grooved topography in the adhesion, morphology, and differentiation of C2C12 skeletal myoblasts. Biotechnol. Bioeng. 2012, 109, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Bettadapur, A.; Suh, G.C.; Geisse, N.A.; Wang, E.R.; Hua, C.; Huber, H.A.; Viscio, A.A.; Kim, J.Y.; Strickland, J.B.; McCain, M.L. Prolonged Culture of Aligned Skeletal Myotubes on Micromolded Gelatin Hydrogels. Sci. Rep. 2016, 6, 28855. [Google Scholar] [CrossRef] [PubMed]
- Ziemkiewicz, N.; Talovic, M.; Madsen, J.; Hill, L.; Scheidt, R.; Patel, A.; Haas, G.; Marcinczyk, M.; Zustiak, S.P.; Garg, K. Laminin-111 functionalized polyethylene glycol hydrogels support myogenic activity in vitro. Biomed. Mater. 2018, 13, 065007. [Google Scholar] [CrossRef]
- Li, B.; Lin, M.; Tang, Y.; Wang, B.; Wang, J.H. A novel functional assessment of the differentiation of micropatterned muscle cells. J. Biomech. 2008, 41, 3349–3353. [Google Scholar] [CrossRef]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef]
- Hosseini, V.; Ahadian, S.; Ostrovidov, S.; Camci-Unal, G.; Chen, S.; Kaji, H.; Ramalingam, M.; Khademhosseini, A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng. Part A 2012, 18, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.W.; Wu, L.Q.; Tullman, J.A.; Payne, G.F.; Bentley, W.E.; Barbari, T.A. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J. Biomed. Mater. Res. A 2007, 83, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.C.; Bettadapur, A.; Santoso, J.W.; McCain, M.L. Fabrication of Micromolded Gelatin Hydrogels for Long-Term Culture of Aligned Skeletal Myotubes. Methods Mol. Biol. 2017, 1668, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Denes, L.T.; Riley, L.A.; Mijares, J.R.; Arboleda, J.D.; McKee, K.; Esser, K.A.; Wang, E.T. Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet. Muscle 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Rexius-Hall, M.L.; Khalil, N.N.; Andres, A.M.; McCain, M.L. Mitochondrial division inhibitor 1 (mdivi-1) increases oxidative capacity and contractile stress generated by engineered skeletal muscle. FASEB J. 2020. [Google Scholar] [CrossRef]
- Gelatin Manufacturers Institute of America. Standard Testing Methods for Edible Gelatin. In Official Procedures of the Gelatin Manufacturers Institute of America, Inc.; Gelatin Manufacturers Institute of America, Inc.: New York, NY, USA, 2019; p. 26. [Google Scholar]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- McCain, M.L.; Desplantez, T.; Geisse, N.A.; Rothen-Rutishauser, B.; Oberer, H.; Parker, K.K.; Kleber, A.G. Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: Relation between Cx43 immunofluorescence and intercellular electrical conductance. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H443–H450. [Google Scholar] [CrossRef]
- Son, E.Y.; Ichida, J.K.; Wainger, B.J.; Toma, J.S.; Rafuse, V.F.; Woolf, C.J.; Eggan, K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011, 9, 205–218. [Google Scholar] [CrossRef]
- Pasqualini, F.S.; Sheehy, S.P.; Agarwal, A.; Aratyn-Schaus, Y.; Parker, K.K. Structural phenotyping of stem cell-derived cardiomyocytes. Stem Cell Rep. 2015, 4, 340–347. [Google Scholar] [CrossRef]
- Ajinomoto Food Ingredients LLC. Activia General Information: Transglutaminase Basics. Available online: http://buyersguide.supplysideshow.com/media/54/library/FPDajinomoto5.pdf (accessed on 14 September 2020).
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Uyama, H. Biocompatible Hydrogel Formation of Gelatin from Cold Water Fish via Enzymatic Networking. Polym. J. 2007, 39, 1040–1046. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Devereux, B.; Wallace, D.G. Injectable collagen as a pH-sensitive hydrogel. Biomaterials 1994, 15, 985–995. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Guo, Z.; Timmerman, A.; Grijpma, D.; Poot, A. Enhanced mechanical and cell adhesive properties of photo-crosslinked PEG hydrogels by incorporation of gelatin in the networks. Biomed. Mater. 2019, 14, 024102. [Google Scholar] [CrossRef]
- Hagopian, M. Contraction bands at short sarcomere length in chick muscle. J. Cell Biol. 1970, 47, 790–796. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Mechling, K.; Lee, Y.B. Sarcomere length in normal and dystrophic chick muscles. Exp. Neurol. 1988, 101, 221–227. [Google Scholar] [CrossRef]
- McKenna, N.M.; Johnson, C.S.; Wang, Y.L. Formation and alignment of Z lines in living chick myotubes microinjected with rhodamine-labeled alpha-actinin. J. Cell Biol. 1986, 103, 2163–2171. [Google Scholar] [CrossRef]
- Sanger, J.M.; Mittal, B.; Pochapin, M.B.; Sanger, J.W. Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J. Cell Biol. 1986, 102, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C.; Oskarsson, B.; Jin, L.W. Muscle biopsy evaluation in neuromuscular disorders. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 609–631. [Google Scholar] [CrossRef] [PubMed]
- Salani, S.; Donadoni, C.; Rizzo, F.; Bresolin, N.; Comi, G.P.; Corti, S. Generation of skeletal muscle cells from embryonic and induced pluripotent stem cells as an in vitro model and for therapy of muscular dystrophies. J. Cell Mol. Med. 2012, 16, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gonzalez, M.; Stancescu, M.; Vandenburgh, H.H.; Hickman, J.J. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 2011, 32, 9602–9611. [Google Scholar] [CrossRef]
- Steinbeck, J.A.; Jaiswal, M.K.; Calder, E.L.; Kishinevsky, S.; Weishaupt, A.; Toyka, K.V.; Goldstein, P.A.; Studer, L. Functional Connectivity under Optogenetic Control Allows Modeling of Human Neuromuscular Disease. Cell Stem Cell 2016, 18, 134–143. [Google Scholar] [CrossRef]
- Santhanam, N.; Kumanchik, L.; Guo, X.; Sommerhage, F.; Cai, Y.; Jackson, M.; Martin, C.; Saad, G.; McAleer, C.W.; Wang, Y.; et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials 2018, 166, 64–78. [Google Scholar] [CrossRef]
- Happe, C.L.; Tenerelli, K.P.; Gromova, A.K.; Kolb, F.; Engler, A.J. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol. Biol. Cell 2017, 28, 1950–1958. [Google Scholar] [CrossRef]


| Cell Type | |||
|---|---|---|---|
| C2C12 (ATCC, CRL-1772) | Growth Media | ||
| Component | Concentration | Product Information | |
| high glucose DMEM | 4.5 g/L | Invitrogen, 11995-040 | |
| fetal bovine serum | 10% | Hyclone, SH3007103 | |
| penicillin-streptomycin | 1% | Lonza, 17-602E | |
| Differentiation Media | |||
| Component | Concentration | Product Information | |
| high glucose DMEM | 4.5 g/L | Invitrogen, 11995-040 | |
| horse serum | 2% | Hyclone, SH3007103 | |
| penicillin-streptomycin | 1% | Lonza, 17-602E | |
| cytarabine | 10 μM | Sigma, C1768 | |
| ChSKM (AA Lab Eggs) | Growth Media | ||
| Component | Concentration | Product Information | |
| low glucose DMEM | 1.0 g/L | Invitrogen, 11885-084 | |
| horse serum | 10% | Hyclone, SH3007103 | |
| chicken serum | 5% | Gibco, 16110082 | |
| vitamin B12 | 4 μg/mL | Sigma, V-2876 | |
| penicillin | 500 units/mL | Sigma, P-4687 | |
| calcium chloride | 3 μM | Sigma, 449709 | |
| Differentiation Media | |||
| Component | Concentration | Product Information | |
| DMEM/F12 | 50% | Gibco, 11320-033 | |
| Neurobasal | 50% | Gibco, 21103-049 | |
| N-2 Supplement | 0.5× | Gibco, 17502-048 | |
| B-27 Supplement | 0.5× | Gibco, 17504-044 | |
| vitamin C | 0.1 mM | Sigma, A92902 | |
| Glutamax | 1× | ||
| vitamin B12 | 4 μg/mL | Sigma, V-2876 | |
| penicillin | 500 units/mL | Sigma, P-4687 | |
| cytarabine | 10 μM | Sigma, C1768 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, D.; Santoso, J.W.; McCain, M.L. Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates. Bioengineering 2021, 8, 6. https://doi.org/10.3390/bioengineering8010006
Gupta D, Santoso JW, McCain ML. Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates. Bioengineering. 2021; 8(1):6. https://doi.org/10.3390/bioengineering8010006
Chicago/Turabian StyleGupta, Divya, Jeffrey W. Santoso, and Megan L. McCain. 2021. "Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates" Bioengineering 8, no. 1: 6. https://doi.org/10.3390/bioengineering8010006
APA StyleGupta, D., Santoso, J. W., & McCain, M. L. (2021). Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates. Bioengineering, 8(1), 6. https://doi.org/10.3390/bioengineering8010006




