1. Introduction
Craniofacial microsomia is a congenital deformity which affects approximately 1 in every 5600 children across the USA. It is characterized by improper growth of skull and face tissues on one or both sides of the face which creates a structural deficiency leading to clinical abnormalities like functional loss in hearing, swallowing, breathing and feeding, and hinders the child’s aesthetic development, such as facial expressions and appearance leading to social issues like acceptance [
1,
2]. The causes of the deformity are unknown, but it is thought to be the result of a genetic abnormality during embryo development [
1]. The treatment involves a combination of four procedures, namely (i) reconstruction of the mandibular ramus, (ii) mandibular distraction and osteogenesis, (iii) bone grafting and (iv) orthognathic surgery, depending on the pathology and anatomy of the defect in the individual patient [
3]. The corrective reconstructive surgery in young patients involves the repairing of deformed jawbones and facial structure followed by filling up the gap with a muscle graft, which has tensile and biological properties similar to healthy native tissue [
3]. Such muscle grafts can be obtained either by removal of tissue from the patient, an autograft, which results in morbidity and scar formation at the donor site, or by tissue engineering, however currently there is no FDA approved tissue engineering treatment in the USA. This latter approach provides a successful therapy to treat the same disease by avoiding the risk of donor site morbidity, but it involves generating viable tissue in the laboratory by culturing cells on a biocompatible and resorbable tissue engineering scaffold [
3,
4]. Previous attempts in tissue engineering for oral and craniofacial reconstruction are mostly associated with bone and cartilage tissue regeneration, with scaffolds fabricated from either metallic biomaterials, such as platinum and other alloys, or synthetic resorbable biomaterials, such as poly(lactide-co-glycolide) and polycaprolactone. There is no FDA approved treatment, as of now, for surgical procedures involving biological grafts for tissue engineering in muscle regeneration [
5,
6,
7].
Facial skeletal muscle enables and controls precise movements of parts of the human anatomy [
8]. Thus, a scaffold used for skeletal muscle regeneration needs to have a higher extension ability and flexibility compared to that used for bone tissue regeneration. A polymeric biomaterial fabricated in a textile-based tissue engineering structure provides several advantages including flexibility and the ability to engineer a structure with the desired physical and mechanical properties [
9]. With the incorporation of biodegradable, bioresorbable polymers, tissue engineering also benefits from avoiding donor site morbidity and the formation of scar tissue, and having no requirement for repeat surgery involving the removal of the biomaterial after complete healing of the tissue [
9,
10]. An earlier approach of designing a scaffold for skeletal muscle regeneration included fabrication of a phosphate glass fiber-collagen composite scaffold to evaluate its ability to provide in vitro assistance for engineering and regeneration of craniofacial skeletal muscle [
9,
11]. However, due to the inherent rigidity of phosphate glass fiber, it is challenging to manipulate the fibers into a curved shape of tissue inside the human body. Furthermore, mechanical stimuli of the sharp end of fibers could cause potential severe body immune reaction.
Textile structures are often used as scaffolds in various tissue engineering applications and medical device designs [
12,
13,
14]. This is because textile structures offer a unique combination of desirable properties including flexibility, thickness, strength and elasticity, and bioresorbability when fabricated from biodegradable polymers [
10]. Knitted textiles in particular are advantageous over other conventional textile structures, such as woven fabrics and nonwovens, as they provide the added benefit of significantly higher total porosity and a higher surface area for cells to attach to, migrate and proliferate, along with higher stretch and elastic recovery obtained from the interlooped yarn structure [
10]. A number of different biodegradable polymers are available, such as polyglycolic acid, polylactic acid, polycaprolactone, polyhydroxyalkanoates and polyurethanes, which can be spun into yarns and then fabricated into textile structures. Each polymer comes with a different set of physical and mechanical characteristics which can provide the unique engineered properties of scaffolds, such as crystallinity, strength, elasticity and resorption rate [
10].
The Poisson’s ratio of a material refers to the changes in lateral strain compared to longitudinal strain when a tensile load is applied in one direction. Most flexible materials, including conventional textile structures, shrink in the lateral direction when stretched in the longitudinal direction, exhibiting a positive Poisson’s ratio. On the other hand, auxetic structures are characterized by a negative Poisson’s ratio (NPR), as they expand laterally when stretched in the longitudinal direction and collapse laterally when compressed longitudinally [
15]. By demonstrating NPR, a material incorporates some exceptional properties, such as excellent dimensional stability, high shear modulus, and a high capacity to absorb energy [
16]. To capitalize on these properties different NPR textile structures have been developed including fibers, yarns, woven and knitted fabrics from different types of geometries [
15,
16,
17]. Knitting has been found to be a superior fabrication method for NPR fabrics because of the variability in knitted structure and design potential [
16].
The ultimate goal of this project is to fabricate a biocompatible and bioresorbable scaffold for skeletal muscle to be applied in facial muscle reconstruction surgery in order to treat craniofacial microsomia. In order to reach this goal, this study achieved three objectives. The primary objective was to design a highly porous, dimensionally stable textile scaffold that could provide up to 90 percent total porosity. Auxetic elements were utilized in the design of the scaffold structures to achieve the desired dimensional stability. The second objective was to design and fabricate a scaffold with equivalent strength and elasticity to native skeletal muscle tissue. The third objective was to demonstrate the biocompatibility of the scaffold in terms of cell metabolic activity, cell attachment and proliferation, when cultured with neonatal human dermal fibroblasts during seven days of cell culture. The eventual objective will be to use a dynamic bioreactor for mechanical stimulation of the muscle precursor cells and to regenerate muscle tissue in vitro. In the dynamic bioreactor, the scaffold would not be expected to change its lateral dimension when cyclic loading was applied in the longitudinal direction. In this way the cells residing within the scaffold would only be exposed to strains in the longitudinal direction. To further incorporate more flexibility in terms of bending and torsional properties into the scaffold design, the present study provides an alternative approach for the fabrication and evaluation of a resorbable textile structure that will provide the required physical, mechanical and biological properties for a tissue engineering scaffold that will regenerate craniofacial skeletal muscle.
3. Results and Discussion
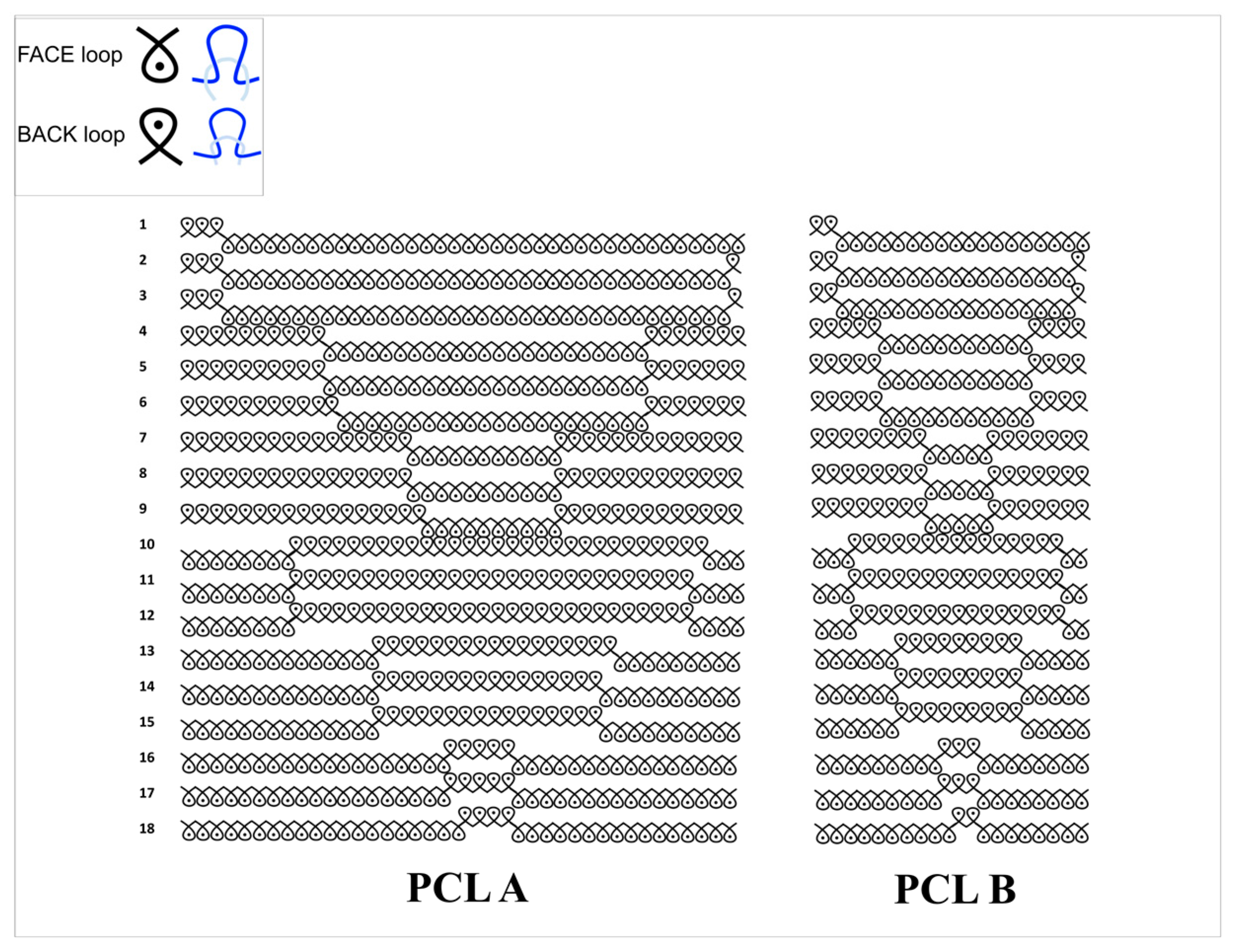
Two scaffold samples, PCL A and PCL B, were fabricated from poly(ε-caprolactone) multifilament yarn (160 denier, 36 filaments, single filament diameter approximately 20 microns) using weft knitting designs, having geometry in two repeat sizes. These samples were evaluated for their suitability in the regeneration of facial skeletal muscle tissue. The evaluation involved physical and mechanical characterization as well as biological tests in order to determine the performance level of both the samples as well as a comparison between the two.
3.1. Characterization of Physical Properties of Knitted Scaffolds
Table 2 includes an overview of the different physical characteristics of both scaffold samples, PCL A and PCL B. PCL A consisted of a 40 × 18 stitch repeat unit, while PCL B consisted of a 20 × 18 repeat unit. The larger repeat unit size, PCL A, reflects a more open knitted structure which results in a lower fabric thickness and weight, and a higher total porosity and average pore size. The tighter knit design of PCL B was observed in its physical properties with higher wales per centimeter and courses per centimeter. Also, PCL B was 1 mm thicker than PCL A, and its fabric weight in grams per square meter was almost twice that of PCL A. Although the pore size range in PCL A was wider than that in PCL B, there was no significant difference in calculated total porosity between the two scaffolds.
The SEM images of both the scaffolds used for typical pore size measurements are shown in
Figure 3. Pore sizes ranging from 48 to 846 microns without a common shape or topology were observed to be randomly distributed throughout the fabric specimens. When the two samples were compared, PCL A was found to have a significantly (
p < 0.05) larger average pore size at 319 microns than PCL B whose average pore size was only 250 microns.
3.2. Characterization of Mechanical Properties of Scaffolds
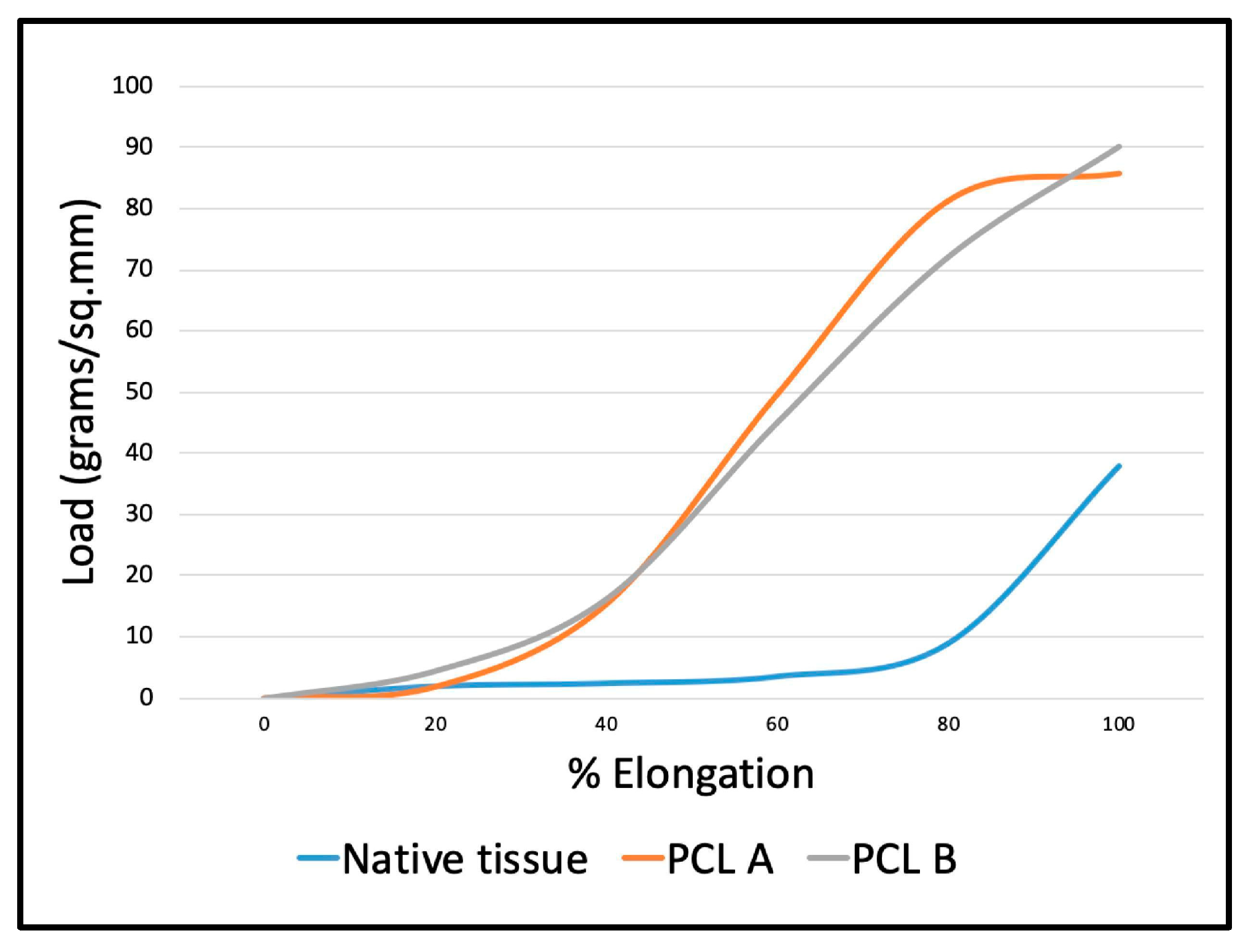
In order to be used as a scaffold for tissue regeneration, the structure needs to be strong enough to sustain a marginally higher load than the original tissue it is replacing. Hence, it was important to evaluate the mechanical properties of the scaffolds, such as the ultimate bursting strength and the elongation at break.
The maximum breaking load and maximum extension at break were determined using the compression cage assembly on the Instron mechanical tester. The scaffold specimen was pre-tensioned, so the crimps were removed, and the fabric was in a flat condition prior to placing it between the horizontal clamping plates. This enabled the relative bursting strengths of the two scaffold samples to be compared with each other, and with the natural skeletal muscle tissue of mammals. According to the geometry, the area under load was taken to be 113.04 mm
2 and the mean bursting strength values in kPa were calculated from the average maximum load at break for each of the two samples. The bursting strength of a tissue engineering scaffold is expected to be marginally higher than that of the original tissue it will replace. The bursting strength of mammal skeletal muscle tissue is approximately 1075 kPa, while the biaxial elongation at break is approximately 65% [
19]. The two PCL fabric scaffold samples gave comparable values for average bursting strength, and PCL A was found to have significantly higher strength than PCL B, as shown in
Table 3. (
p < 0.05).
The percent elongation at break values were compared to that of a mammal skeletal muscle tissue, which has been reported to be 65% under biaxial loading [
19]. The elongation at break values for both the scaffolds were found to exceed this reference value. The difference between the percent elongation at break for the two samples was not statistically significant (
p > 0.05).
Figure 4 shows the load-elongation curves obtained from the two scaffold samples during bursting strength testing compared to that of the abdominal skeletal muscle of a mammal. The initial shape of the curves up to 20 percent elongation at low stress are similar for both the reference tissue and the scaffolds.
Figure 4 also shows that the two PCL fabrics are initially able to mechanically support and reinforce the native muscle tissue because of their superior mechanical strength. Though the native tissue is less rigid and more compliant than the scaffolds, the initial curve up to 20% extension is comparable.
3.3. Dimensional Stability Determination
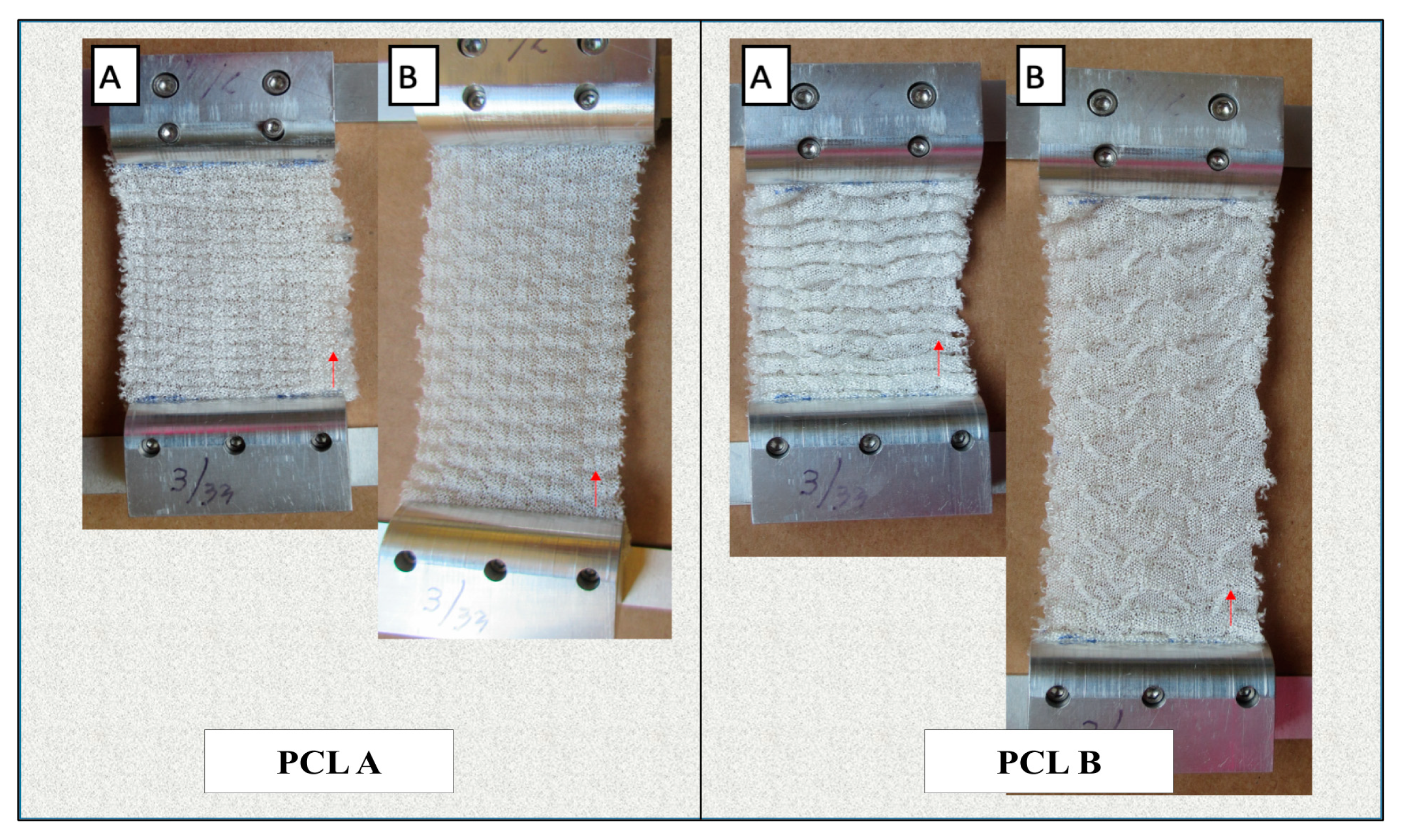
Figure 5 shows the changes in dimensions of the scaffolds during manual lengthwise extension.
Table 4 compares the change in dimensions and volume of the two scaffolds before and after extension in order to determine their dimensional stability in the lengthwise direction. We observed that both scaffolds showed a high extension in length before any crosswise shrinkage was observed in the width. Also, PCL A showed a 61.8% increase in volume at 76% extension, while PCL B showed an increase of 8.4% in volume at more than 150% extension. This confirms that both scaffolds had a negative Poisson’s ratio during more than 50% extension, which ensures that neither scaffold experienced lateral shrinking during initial extension, which is different from most conventional fabric structures.
Both scaffolds were also evaluated for their tendency to ravel during 100% extension in both the warp and weft directions. Conventional weft knitted structures are easily raveled from the open end when under extension, but auxetic designed fabrics do not have a tendency to ravel, which is an important property for any scaffold that is exposed to a dynamic bioreactor with cyclic loading and unloading.
3.4. Cell Metabolic Activity and Biocompatibility
The biocompatibility of the scaffolds was determined using two in vitro cell culture assays; the alamarBlue® assay for cell metabolic activity, and the live/dead staining assay using confocal microscopy to assess cell proliferation and attachment at Day 3 and Day 7.
3.4.1. AlamarBlue® Assay
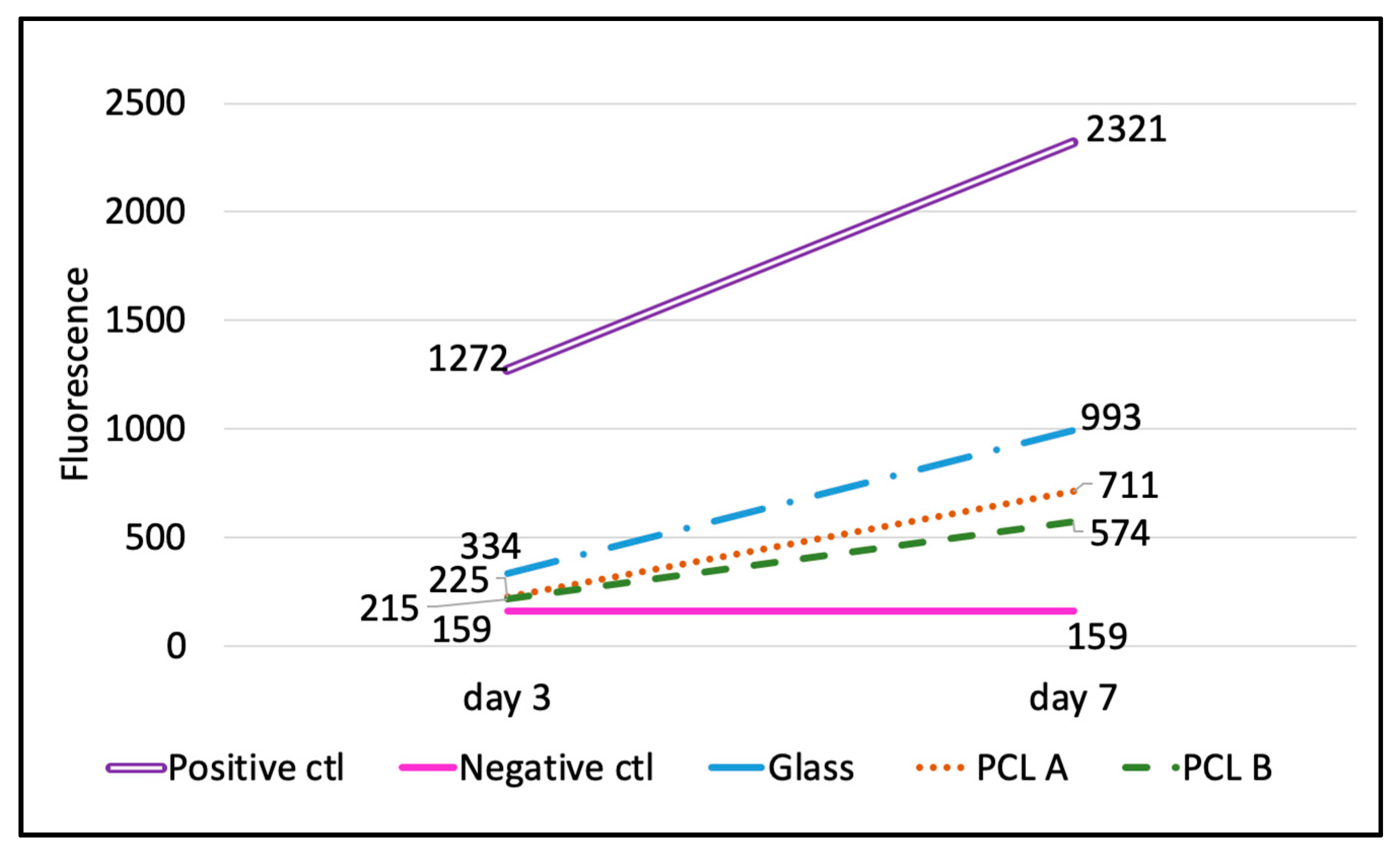
The results obtained from the alamarBlue
® assay plate reading on Day 3 and Day 7 are shown in
Table 5 and
Figure 6. The percent reduction of alamarBlue
® dye for each of the three biomaterials, including two scaffolds and the glass coverslip for reference, was calculated using the Equation (3), given in
Section 2.4.4.
The percent reduction is the quantitative measure of the cell metabolic activity on the scaffolds compared to the positive control, as it is directly proportional to the number of live cells in the well.
Table 5 summarizes the percent reduction in the scaffold samples PCL A and PCL B and glass coverslip at Day 3 and Day 7. It also compares the percent increase in the cell metabolic activity from Day 3 to Day 7, which shows that although the initial cell attachment on the scaffolds was less compared to the glass coverslip, the increase in cell numbers from Day 3 to Day 7 was more rapid in both scaffold samples compared to the glass coverslip. This indicates that the scaffold samples do promote cell proliferation, but the scaffold surface needs to be improved so as to encourage greater initial cell attachment.
Figure 6 compares the increasing rate of cell metabolic activity in terms of the fluorescence values for all three biomaterials with that of the positive and negative controls. The image supports the claim that the scaffolds encouraged cell proliferation between Day 3 and Day 7, but when compared to the positive control and to the glass coverslips, the scaffolds held a lot of scope for improvement in cell adhesion.
3.4.2. Confocal Microscopy with Live/Dead Staining Assay
Figure 7 describes the comparison of cell attachment on and within the three-dimensional PCL. We attempted to visualize the proportion of viable cells to dead cells and the migration of cells through the scaffolds between Day 3 and Day 7. It can be seen from
Figure 7a that the cells attached to the individual filament surfaces favored residing in the space between the filaments and not in the pores between yarns.
Figure 7b shows that the cells penetrated up to a distance of 450 microns deep into the three-dimensional scaffold, both at Day 3 and Day 7. This shows that the morphology and structure of the scaffolds helped the cells to be distributed up to 450 microns into the scaffolds, but no deeper. The depth of cell penetration could have been influenced by the initial cell seeding density or by differences in the scaffold structure that affects cell seeding or migration.
Figure 7c,d visually compares the proportion of live and dead cells on the scaffold sample PCL A between Day 3 and Day 7. The live cells were observed to be well attached and elongated on Day 7, but there was no difference between Day 3 and Day 7 dead cells, which supported the results obtained from the alamarBlue
® assay.
Similarly,
Figure 8 describes the morphology and compares the proportion and distribution of live and dead cells on scaffold sample PCL B. Please note that there was no noticeable difference observed between the proportion of live and dead cells at Day 3 and Day 7 during the fibroblast cell culture on this sample. However, from the morphology of the live cells, on Day 7 the cells were observed to be healthy compared to Day 3, in terms of the number of elongated cells and the average length of cells. The effect of scaffold pore size may have influenced the appearance and orientation of the cells attached to the filament surface. It was noted that the cells on the PCL A scaffold with its larger average pore size, which would have provided better fluid transfer, were observed on Day 7 to be more intimately attached compared to those on the PCL B scaffold with smaller pores. When viewing the three-dimensional z-stack image of the scaffold, the depth of cell distribution was observed to be about 450 microns on Day 3 and about 400 microns on Day 7. The significance of the cell penetration results would have been easier to understand if the culture study had been extended further up to 14 and 28 days.
4. Conclusions and Future Work
The poly(ε-caprolactone) multifilament yarn was successfully fabricated into two knitted textile scaffolds, PCL A and PCL B. By selecting an open weft knit structure for both samples, total porosities in excess of 90 percent were achieved. While neither sample showed a tendency to ravel, PCL A showed superior dimensional stability compared to PCL B. The results of bursting strength and biaxial elongation at break were compared with reference values obtained from skeletal muscle tissue reported in the literature. The load-elongation curves and initial stiffness values of both scaffold samples were compared to those of the reference skeletal muscle tissue, and the ultimate strength of the scaffolds exceeded that of the native tissue, thereby ensuring mechanical support and reinforcement during the initial period after implantation. The biological performance of the scaffolds was measured in terms of alamarBlue® and live/dead assays using confocal microscopy. This has enabled an evaluation of the metabolic activity, attachment and distribution of neonatal human dermal fibroblast cells on both scaffolds. The PCL Fabric A was found to support cell growth better than PCL B in terms of cell metabolic activity after 7 days of cell culture.
Since the scaffolds are intended for the regeneration of facial skeletal muscle, the next step will be to evaluate the biocompatibility with muscle precursor cells in vitro and to determine the ability to regenerate muscle tissue in vivo. The resorption rate of the scaffolds needs to be evaluated to compare it with the time period for complete healing and growth of the original tissue in situ. The capacity of the knitted structures to provide dimensional stability during mechanical stimulation of the scaffold must also be evaluated by culturing the skeletal muscle cells on the scaffolds in a dynamic bioreactor.