Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration
Abstract
1. Introduction
Healthy and Pathological Glial Activity
2. Peripheral Nervous System Glia and Electrospun Fibers
2.1. Schwann Cell Response to Electrospun Fibers In Vitro
2.1.1. Schwann Cell Response to Fiber Alignment In Vitro
2.1.2. Schwann Cell Response to Fiber Diameter In Vitro
2.1.3. Schwann Cell Response to Fiber Surface Nanotopography In Vitro
2.1.4. Schwann Cell Response to Fiber Conductivity In Vitro
2.1.5. Schwann Cell Response to Fiber Functionalization In Vitro
2.1.6. Schwann Cell Response to Fiber Therapeutic Delivery In Vitro
2.1.7. Summary of Schwann Cell Response to Electrospun Fibers In Vitro
2.2. Schwann Cell Response to Electrospun Fibers In Vivo
2.2.1. Schwann Cell Response to Fiber Alignment In Vivo
2.2.2. Schwann Cell Response to Fiber Fillers In Vivo
2.2.3. Schwann Cell Response to Fiber Material Selection In Vivo
2.2.4. Schwann Cell Response to Fiber Functionalization and Therapeutic Delivery In Vivo
2.2.5. Summary of Schwann Cell Response to Electrospun Fibers In Vivo
3. Central Nervous System
3.1. Astrocyte Response to Electrospun Fibers In Vitro
3.1.1. Astrocyte Response to Fiber Diameter and Surface Nanotopography
3.1.2. Astrocyte Response to Fiber Functionalization and Therapeutic Delivery
3.1.3. Electrospun Fibers for Generating In Vitro Models to Study Astrocyte Behavior
3.1.4. Summary of Astrocyte Response to Electrospun Fibers In Vitro
3.2. Oligodendroglia Response to Electrospun Fibers In Vitro
3.2.1. Oligodendroglia Response to Fiber Diameter In Vitro
3.2.2. Oligodendroglia Response to Fiber Functionalization and Therapeutic Delivery In Vitro
3.2.3. Summary of Oligodendroglia Response to Electrospun Fibers In Vitro
3.3. Microglia Response to Electrospun Fibers In Vitro
3.4. CNS Glia Response to Electrospun Fibers In Vivo
3.4.1. CNS Glia Response to Fiber Density In Vivo
3.4.2. CNS Glia Response to Therapeutic Delivery from Electrospun Fibers In Vivo
3.4.3. Suggestions for Future Work Investigating CNS Glia Response to Electrospun Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pertici, V.; Martrou, G.; Gigmes, D.; Trimaille, T. Synthetic Polymer-Based Electrospun Fibers: Biofunctionalization Strategies and Recent Advances in Tissue Engineering, Drug Delivery and Diagnostics. Curr. Med. Chem. 2018, 25, 2385–2400. [Google Scholar] [CrossRef]
- Lyu, S.; Huang, C.; Yang, H.; Zhang, X. Electrospun Fibers as a Scaffolding Platform for Bone Tissue Repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 1382–1389. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Sun, S.; Khan, A.R.; Ji, J.; Yang, M.; Zhai, G. Recent Advances in Electrospun for Drug Delivery Purpose. J. Drug Target. 2019, 27, 270–282. [Google Scholar] [CrossRef]
- Seif, S.; Planz, V.; Windbergs, M. Delivery of Therapeutic Proteins Using Electrospun Fibers—Recent Developments and Current Challenges. Arch. Pharm. (Weinheim) 2017, 350, 1700077. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending Instability of Electrically Charged Liquid Jets of Polymer Solutions in Electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Gupta, D.; Venugopal, J.; Prabhakaran, M.P.; Dev, V.R.G.; Low, S.; Choon, A.T.; Ramakrishna, S. Aligned and Random Nanofibrous Substrate for the in Vitro Culture of Schwann Cells for Neural Tissue Engineering. Acta Biomater. 2009, 5, 2560–2569. [Google Scholar] [CrossRef]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of Highly Aligned Electrospun Poly-L-Lactic Acid Fibers for Nerve Regeneration Applications. J. Neural Eng. 2009, 6, 016001. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Liu, W.; Wang, C.-S.; Vaia, R.A. Generation of Electrospun Fibers of Nylon 6 and Nylon 6-Montmorillonite Nanocomposite. Polymer 2002, 43, 775–780. [Google Scholar] [CrossRef]
- Kakade, M.V.; Givens, S.; Gardner, K.; Lee, K.H.; Chase, D.B.; Rabolt, J.F. Electric Field Induced Orientation of Polymer Chains in Macroscopically Aligned Electrospun Polymer Nanofibers. J. Am. Chem. Soc. 2007, 129, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, E.M.; Wang, Y. Incorporation of Parallel Electrospun Fibers for Improved Topographical Guidance in 3D Nerve Guides. Biofabrication 2013, 5, 035015. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Yao, S.; Mao, H.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt Peripheral Nerve Regeneration Induced by a Hierarchically Aligned Fibrin Nanofiber Hydrogel. Acta Biomater. 2017, 55, 296–309. [Google Scholar] [CrossRef]
- Hurtado, A.; Cregg, J.M.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS Regeneration after Complete Spinal Cord Transection Using Aligned Poly-l-Lactic Acid Microfibers. Biomaterials 2011, 32, 6068–6079. [Google Scholar] [CrossRef]
- Schaub, N.J.; Johnson, C.D.; Cooper, B.; Gilbert, R.J. Electrospun Fibers for Spinal Cord Injury Research and Regeneration. J. Neurotrauma 2016, 33, 1405–1415. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Ziemba, A.M.; Johnson, C.D.L.; Gilbert, R.J. 18-Advances in the use of electrospun fibers for the central nervous system. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices; Guarino, V., Ambrosio, L., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 377–398. ISBN 978-0-08-101745-6. [Google Scholar]
- Frost, H.K.; Andersson, T.; Johansson, S.; Englund-Johansson, U.; Ekström, P.; Dahlin, L.B.; Johansson, F. Electrospun Nerve Guide Conduits Have the Potential to Bridge Peripheral Nerve Injuries in Vivo. Sci. Rep. 2018, 8, 16716. [Google Scholar] [CrossRef]
- Xie, J.; MacEwan, M.R.; Schwartz, A.G.; Xia, Y. Electrospun Nanofibers for Neural Tissue Engineering. Nanoscale 2010, 2, 35–44. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A Biomaterials Approach to Peripheral Nerve Regeneration: Bridging the Peripheral Nerve Gap and Enhancing Functional Recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Ziemba, A.M.; Gilbert, R.J. Biomaterials for Local, Controlled Drug Delivery to the Injured Spinal Cord. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Schaub, N.J.; Britton, T.; Rajachar, R.; Gilbert, R.J. Engineered Nanotopography on Electrospun PLLA Microfibers Modifies RAW 264.7 Cell Response. ACS Appl. Mater. Interfaces 2013, 5, 10173–10184. [Google Scholar] [CrossRef]
- Johnson, C.D.; D’Amato, A.R.; Puhl, D.L.; Wich, D.M.; Vesperman, A.; Gilbert, R.J. Electrospun Fiber Surface Nanotopography Influences Astrocyte-Mediated Neurite Outgrowth. Biomed. Mater. 2018, 13, 054101. [Google Scholar] [CrossRef] [PubMed]
- Omidinia-Anarkoli, A.; Rimal, R.; Chandorkar, Y.; Gehlen, D.B.; Rose, J.C.; Rahimi, K.; Haraszti, T.; De Laporte, L. Solvent-Induced Nanotopographies of Single Microfibers Regulate Cell Mechanotransduction. ACS Appl. Mater. Interfaces 2019, 11, 7671–7685. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.L.; Zuidema, J.M.; Kearns, K.R.; Maguire, A.B.; Desmond, G.P.; Thompson, D.M.; Gilbert, R.J. The Effect of Electrospun Fiber Diameter on Astrocyte-Mediated Neurite Guidance and Protection. ACS Appl. Bio Mater. 2019, 2, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; McCarthy, C.W.; Gilbert, R.J. Varying the Diameter of Aligned Electrospun Fibers Alters Neurite Outgrowth and Schwann Cell Migration. Acta Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Hyzinski-García, M.C.; Vlasselaer, K.V.; Zaccor, N.; Plopper, G.E.; Mongin, A.A.; Gilbert, R.J. Enhanced GLT-1 Mediated Glutamate Uptake and Migration of Primary Astrocytes Directed by Fibronectin-Coated Electrospun Poly-L-Lactic Acid Fibers. Biomaterials 2014, 35, 1439–1449. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, F.; Lu, Y.; Wei, L.; Yuan, W. Progress of Electrospun Fibers as Nerve Conduits for Neural Tissue Repair. Nanomedicine 2014, 9, 1869–1884. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun Conducting Polymer Nanofibers and Electrical Stimulation of Nerve Stem Cells. J. Biosci. Bioeng. 2011, 112, 501–507. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Hao, L.; Wu, C.; Lu, M.; Zhang, L.; Yu, D. Electrospun Cellulose-Based Conductive Polymer Nanofibrous Mats: Composite Scaffolds and Their Influence on Cell Behavior with Electrical Stimulation for Nerve Tissue Engineering. Soft Matter 2020, 16, 6591–6598. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.L.; D’Amato, A.R.; Gilbert, R.J. Electrospun Fibers for Drug Delivery after Spinal Cord Injury and the Effects of Drug Incorporation on Fiber Properties. Cells Tissues Organs 2016, 202, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Repair Schwann Cell and Its Function in Regenerating Nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann Cell Interactions with Axons and Microvessels in Diabetic Neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef]
- Wilson, E.R.; Nunes, G.D.-F.; Weaver, M.R.; Frick, L.R.; Feltri, M.L. Schwann Cell Interactions during the Development of the Peripheral Nervous System. Dev. Neurobiol. 2020, 1–26. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.-P. Migrating Schwann Cells Direct Axon Regeneration within the Peripheral Nerve Bridge. Glia 2020. [Google Scholar] [CrossRef]
- Heredia, D.J.; De Angeli, C.; Fedi, C.; Gould, T.W. Calcium Signaling in Schwann Cells. Neurosci. Lett. 2020, 729, 134959. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Fazal, S.V.; Gomez-Sanchez, J.A.; Wagstaff, L.J.; Musner, N.; Otto, G.; Janz, M.; Mirsky, R.; Jessen, K.R. Graded Elevation of C-Jun in Schwann Cells In Vivo: Gene Dosage Determines Effects on Development, Remyelination, Tumorigenesis, and Hypomyelination. J. Neurosci. 2017, 37, 12297–12313. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Choi, Y.K. The Role of Astrocytes in the Central Nervous System Focused on BK Channel and Heme Oxygenase Metabolites: A Review. Antioxidants 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Barriers to Neurotoxic Inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.-P.; Kothary, R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Abe, Y.; Shibata, S.; Shindo, T.; Fujii, S.; Ikenaka, K.; Tanaka, K.F. Region- and Cell Type-Specific Facilitation of Synaptic Function at Destination Synapses Induced by Oligodendrocyte Depolarization. J. Neurosci. 2019, 39, 4036–4050. [Google Scholar] [CrossRef]
- Moore, S.; Meschkat, M.; Ruhwedel, T.; Tzvetanova, I.D.; Trevisiol, A.; Battefeld, A.; Kusch, K.; Kole, M.; Strenzke, N.; Möbius, W.; et al. A Role of Oligodendrocytes in Information Processing Independent of Conduction Velocity. bioRxiv 2019, 736975. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Hernandez-Ontiveros, D.G.; Tajiri, N.; Acosta, S.; Giunta, B.; Tan, J.; Borlongan, C.V. Microglia Activation as a Biomarker for Traumatic Brain Injury. Front. Neurol. 2013, 4. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of Microglial and Macrophage Responses after Spinal Cord Injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte Scar Formation Aids Central Nervous System Axon Regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional Regeneration Beyond the Glial Scar. Exp. Neurol. 2014, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; Margolis, R.U.; Tuszynski, M.H. The Chondroitin Sulfate Proteoglycans Neurocan, Brevican, Phosphacan, and Versican Are Differentially Regulated Following Spinal Cord Injury. Exp. Neurol. 2003, 182, 399–411. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020, 53. [Google Scholar] [CrossRef]
- Domercq, M.; Vázquez-Villoldo, N.; Matute, C. Neurotransmitter Signaling in the Pathophysiology of Microglia. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Duncan, G.J.; Manesh, S.B.; Hilton, B.J.; Assinck, P.; Plemel, J.R.; Tetzlaff, W. The Fate and Function of Oligodendrocyte Progenitor Cells after Traumatic Spinal Cord Injury. Glia 2020, 68, 227–245. [Google Scholar] [CrossRef]
- Almad, A.; Sahinkaya, F.R.; McTigue, D.M. Oligodendrocyte Fate after Spinal Cord Injury. Neurotherapeutics 2011, 8, 262–273. [Google Scholar] [CrossRef]
- Jiang, X.; Mi, R.; Hoke, A.; Chew, S.Y. Nanofibrous Nerve Conduit-Enhanced Peripheral Nerve Regeneration. J. Tissue Eng. Regen. Med. 2014, 8, 377–385. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, Z.; Wu, T.; Chen, W.; Li, D.; Zheng, H.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X.; Yu, Y. Development of Nanofiber Sponges-Containing Nerve Guidance Conduit for Peripheral Nerve Regeneration in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 26684–26696. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Haftel, V.K.; Kumar, S.; Bellamkonda, R.V. The Role of Aligned Polymer Fiber-Based Constructs in the Bridging of Long Peripheral Nerve Gaps. Biomaterials 2008, 29, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Meng, H.-Y.; Chang, B.; Liu, G.-B.; Cheng, X.-Q.; Tang, H.; Wang, Y.; Peng, J.; Zhao, Q.; Lu, S.-B. Aligned Fibers Enhance Nerve Guide Conduits When Bridging Peripheral Nerve Defects Focused on Early Repair Stage. Neural Regen. Res. 2019, 14, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Keshel, S.H.; Pouya, M.; Rad, H.; Nava, M.O.; Azarbakhsh, M.; Hooshmand, S. Nanofibrous Nerve Conduits for Repair of 30-Mm-Long Sciatic Nerve Defects. Neural Regen. Res. 2013, 8, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, W.; Zhang, K.; Qiu, S.; Xu, J.; Wang, C.; Chai, Y. Nanofiber Arrangement Regulates Peripheral Nerve Regeneration through Differential Modulation of Macrophage Phenotypes. Acta Biomater. 2019, 83, 291–301. [Google Scholar] [CrossRef]
- Cnops, V.; Chin, J.S.; Milbreta, U.; Chew, S.Y. Biofunctional Scaffolds with High Packing Density of Aligned Electrospun Fibers Support Neural Regeneration. J. Biomed. Mater. Res. A 2020, 108, 2473–2483. [Google Scholar] [CrossRef]
- Chen, C.; Tang, J.; Gu, Y.; Liu, L.; Liu, X.; Deng, L.; Martins, C.; Sarmento, B.; Cui, W.; Chen, L. Bioinspired Hydrogel Electrospun Fibers for Spinal Cord Regeneration. Adv. Funct. Mater. 2019, 29, 1806899. [Google Scholar] [CrossRef]
- Álvarez, Z.; Castaño, O.; Castells, A.A.; Mateos-Timoneda, M.A.; Planell, J.A.; Engel, E.; Alcántara, S. Neurogenesis and Vascularization of the Damaged Brain Using a Lactate-Releasing Biomimetic Scaffold. Biomaterials 2014, 35, 4769–4781. [Google Scholar] [CrossRef]
- Gordon, J.; Amini, S.; White, M.K. General Overview of Neuronal Cell Culture. Methods Mol. Biol. Clifton NJ 2013, 1078, 1–8. [Google Scholar] [CrossRef]
- De Vries, G.H.; Boullerne, A.I. Glial Cell Lines: An Overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Stoyanova, E.I.; Lemiesz, A.E.; Xing, J.; Mash, D.C.; Heintz, N. Species and Cell-Type Properties of Classically Defined Human and Rodent Neurons and Glia. eLife 2018, 7, e37551. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing Injured Peripheral Nerves: Bridging the Gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T. Bridging Long Gap Peripheral Nerve Injury Using Skeletal Muscle-Derived Multipotent Stem Cells. Neural Regen. Res. 2014, 9, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Patel, N.P.; Lyon, K.A.; Huang, J.H. An Update–Tissue Engineered Nerve Grafts for the Repair of Peripheral Nerve Injuries. Neural Regen. Res. 2018, 13, 764–774. [Google Scholar] [CrossRef]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to Peripheral Nerve Repair: Generations of Biomaterial Conduits Yielding to Replacing Autologous Nerve Grafts in Craniomaxillofacial Surgery. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Ray, W.Z.; Mackinnon, S.E. Management of Nerve Gaps: Autografts, Allografts, Nerve Transfers, and End-to-Side Neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef]
- López-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Silva, T.H.; Oliveira, J.M. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater. Sci. Eng. 2017, 3, 3098–3122. [Google Scholar] [CrossRef]
- Lovati, A.B.; D’Arrigo, D.; Odella, S.; Tos, P.; Geuna, S.; Raimondo, S. Nerve Repair Using Decellularized Nerve Grafts in Rat Models. A Review of the Literature. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Szynkaruk, M.; Kemp, S.W.P.; Wood, M.D.; Gordon, T.; Borschel, G.H. Experimental and Clinical Evidence for Use of Decellularized Nerve Allografts in Peripheral Nerve Gap Reconstruction. Tissue Eng. Part B Rev. 2013, 19, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve Grafting for Peripheral Nerve Injuries with Extended Defect Sizes. Wien. Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral Nerve Conduits: Technology Update. Med. Devices Auckl. NZ 2014, 7, 405–424. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Williams, D.F. Neural Tissue Engineering Options for Peripheral Nerve Regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Spivey, E.C.; Khaing, Z.Z.; Shear, J.B.; Schmidt, C.E. The Fundamental Role of Subcellular Topography in Peripheral Nerve Repair Therapies. Biomaterials 2012, 33, 4264–4276. [Google Scholar] [CrossRef]
- Quan, Q.; Chang, B.; Meng, H.Y.; Liu, R.X.; Wang, Y.; Lu, S.B.; Peng, J.; Zhao, Q. Use of Electrospinning to Construct Biomaterials for Peripheral Nerve Regeneration. Rev. Neurosci. Berl. 2016, 27, 761–768. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Jiang, X.; Lim, S.H.; Mao, H.-Q.; Chew, S.Y. Current Applications and Future Perspectives of Artificial Nerve Conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef]
- Koh, H.S.; Yong, T.; Teo, W.E.; Chan, C.K.; Puhaindran, M.E.; Tan, T.C.; Lim, A.; Lim, B.H.; Ramakrishna, S. In Vivostudy of Novel Nanofibrous Intra-Luminal Guidance Channels to Promote Nerve Regeneration. J. Neural Eng. 2010, 7, 046003. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhao, W.; Zhu, C.; Zhang, X.; Ye, D.; Zhang, W.; Zhou, Y.; Jiang, X.; Zhang, Z. Sciatic Nerve Regeneration in Rats by a Promising Electrospun Collagen/Poly(ε-Caprolactone) Nerve Conduit with Tailored Degradation Rate. BMC Neurosci. 2011, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, A.; Patel, S.; Kurpinski, K.; Diao, E.; Bao, X.; Kwong, G.; Young, W.L.; Li, S. Engineering Bi-Layer Nanofibrous Conduits for Peripheral Nerve Regeneration. Tissue Eng. Part C Methods 2011, 17, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Kulangara, K.; Leong, K.W. Substrate Topography Shapes Cell Function. Soft Matter 2009, 5, 4072. [Google Scholar] [CrossRef]
- Xie, J.; Willerth, S.M.; Li, X.; Macewan, M.R.; Rader, A.; Sakiyama-Elbert, S.E.; Xia, Y. The Differentiation of Embryonic Stem Cells Seeded on Electrospun Nanofibers into Neural Lineages. Biomaterials 2009, 30, 354–362. [Google Scholar] [CrossRef]
- Bashur, C.A.; Shaffer, R.D.; Dahlgren, L.A.; Guelcher, S.A.; Goldstein, A.S. Effect of Fiber Diameter and Alignment of Electrospun Polyurethane Meshes on Mesenchymal Progenitor Cells. Tissue Eng. Part A 2009, 15, 2435–2445. [Google Scholar] [CrossRef]
- Christopherson, G.T.; Song, H.; Mao, H.-Q. The Influence of Fiber Diameter of Electrospun Substrates on Neural Stem Cell Differentiation and Proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Lim, S.H.; Liu, X.Y.; Song, H.; Yarema, K.J.; Mao, H.-Q. The Effect of Nanofiber-Guided Cell Alignment on the Preferential Differentiation of Neural Stem Cells. Biomaterials 2010, 31, 9031–9039. [Google Scholar] [CrossRef]
- Ren, Y.-J.; Zhang, S.; Mi, R.; Liu, Q.; Zeng, X.; Rao, M.; Hoke, A.; Mao, H.-Q. Enhanced Differentiation of Human Neural Crest Stem Cells towards the Schwann Cell Lineage by Aligned Electrospun Fiber Matrix. Acta Biomater. 2013, 9, 7727–7736. [Google Scholar] [CrossRef]
- Subramony, S.D.; Dargis, B.R.; Castillo, M.; Azeloglu, E.U.; Tracey, M.S.; Su, A.; Lu, H.H. The Guidance of Stem Cell Differentiation by Substrate Alignment and Mechanical Stimulation. Biomaterials 2013, 34, 1942–1953. [Google Scholar] [CrossRef]
- Olvera, D.; Sathy, B.N.; Carroll, S.F.; Kelly, D.J. Modulating Microfibrillar Alignment and Growth Factor Stimulation to Regulate Mesenchymal Stem Cell Differentiation. Acta Biomater. 2017, 64, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, F.; Chen, H.; Pal, M.; Lai, Y.; Zhao, A.Z.; Tan, L.P. Micropatterning Extracellular Matrix Proteins on Electrospun Fibrous Substrate Promote Human Mesenchymal Stem Cell Differentiation Toward Neurogenic Lineage. ACS Appl. Mater. Interfaces 2016, 8, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; Schoonhoven, J. van The Role of Current Techniques and Concepts in Peripheral Nerve Repair. Plast. Surg. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The Effect of the Alignment of Electrospun Fibrous Scaffolds on Schwann Cell Maturation. Biomaterials 2008, 29, 653–661. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Kuppuswamy, A.A.; Sethuraman, S.; Subramanian, A. Topographic Cue from Electrospun Scaffolds Regulate Myelin-Related Gene Expressions in Schwann Cells. J. Biomed. Nanotechnol. 2015, 11, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, X.; Sun, B.; Wu, T.; Chen, W.; Huang, C.; Ke, Q.; EI-Hamshary, H.A.; Al-Deyab, S.S.; Mo, X. Nerve Conduits Constructed by Electrospun P(LLA-CL) Nanofibers and PLLA Nanofiber Yarns. J. Mater. Chem. B 2015, 3, 8823–8831. [Google Scholar] [CrossRef]
- Valmikinathan, C.M.; Hoffman, J.; Yu, X. Impact of Scaffold Micro and Macro Architecture on Schwann Cell Proliferation under Dynamic Conditions in a Rotating Wall Vessel Bioreactor. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 22–29. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925–12942. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Koenig, B.; Nichterwitz, S.; Oberhoffner, S.; Schlosshauer, B. Strategies for Inducing the Formation of Bands of Büngner in Peripheral Nerve Regeneration. Biomaterials 2009, 30, 5251–5259. [Google Scholar] [CrossRef]
- Santos, D.; Wieringa, P.; Moroni, L.; Navarro, X.; Valle, J.D. PEOT/PBT Guides Enhance Nerve Regeneration in Long Gap Defects. Adv. Healthc. Mater. 2017, 6, 1600298. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, D.; Yan, Z.; Wang, C. Heparin/Collagen Encapsulating Nerve Growth Factor Multilayers Coated Aligned PLLA Nanofibrous Scaffolds for Nerve Tissue Engineering. J. Biomed. Mater. Res. A 2017, 105, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Itoh, S.; Konno, K.; Kikkawa, T.; Ichinose, S.; Sakai, K.; Ohkuma, T.; Watabe, K. Effects of Schwann Cell Alignment along the Oriented Electrospun Chitosan Nanofibers on Nerve Regeneration. J. Biomed. Mater. Res. A 2009, 91A, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Mukhatyar, V.J.; Salmerón-Sánchez, M.; Rudra, S.; Mukhopadaya, S.; Barker, T.H.; García, A.J.; Bellamkonda, R.V. Role of Fibronectin in Topographical Guidance of Neurite Extension on Electrospun Fibers. Biomaterials 2011, 32, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Zhao, B.; Qi, H.; Peng, J.; Zhang, L.; Xu, W.; Hu, P.; Lu, S. Biocompatibility Evaluation of Electrospun Aligned Poly(Propylene Carbonate) Nanofibrous Scaffolds with Peripheral Nerve Tissues and Cellsin Vitro. Chin. Med. J. (Engl.) 2011, 124, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, K.; Tanaka, H.-F.; Takahashi, A.; Awaya, A.; Hashimoto, K. Basic Behavior of Migratory Schwann Cells in Peripheral Nerve Regeneration. Exp. Neurol. 1996, 137, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of Fibronectin Extracellular Matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(l-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Cardwell, R.D.; Dahlgren, L.A.; Goldstein, A.S. Electrospun Fibre Diameter, Not Alignment, Affects Mesenchymal Stem Cell Differentiation into the Tendon/Ligament Lineage. J. Tissue Eng. Regen. Med. 2014, 8, 937–945. [Google Scholar] [CrossRef]
- Xue, J.; Yang, J.; O’Connor, D.M.; Zhu, C.; Huo, D.; Boulis, N.M.; Xia, Y. Differentiation of Bone Marrow Stem Cells into Schwann Cells for the Promotion of Neurite Outgrowth on Electrospun Fibers. ACS Appl. Mater. Interfaces 2017, 9, 12299–12310. [Google Scholar] [CrossRef]
- Xue, J.; Li, H.; Xia, Y. Nanofiber-Based Multi-Tubular Conduits with a Honeycomb Structure for Potential Application in Peripheral Nerve Repair. Macromol. Biosci. 2018, 18, 1800090. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Song, Z.-B.; Qin, J.-Q. The Possible Influence of Varying Diameter of Aligned Electrospun Fibers on Schwann Cells Maturation in Culture. Med. Hypotheses 2013, 81, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.L.; Brophy, P.J. Mechanisms of Axon Ensheathment and Myelin Growth. Nat. Rev. Neurosci. 2005, 6, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Schwann Cell Myelination. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The Influence of Electrospun Fibre Size on Schwann Cell Behaviour and Axonal Outgrowth. Mater. Sci. Eng. C 2015, 48, 620–631. [Google Scholar] [CrossRef]
- Daud, M.F.B.; Pawar, K.C.; Claeyssens, F.; Ryan, A.J.; Haycock, J.W. An Aligned 3D Neuronal-Glial Co-Culture Model for Peripheral Nerve Studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef]
- Schaub, N.J.; D’Amato, A.R.; Mason, A.; Corr, D.T.; Harmon, E.Y.; Lennartz, M.R.; Gilbert, R.J. The Effect of Engineered Nanotopography of Electrospun Microfibers on Fiber Rigidity and Macrophage Cytokine Production. J. Biomater. Sci. Polym. Ed. 2017, 28, 1303–1323. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Puhl, D.L.; Ziemba, A.M.; Johnson, C.D.L.; Doedee, J.; Bao, J.; Gilbert, R.J. Exploring the Effects of Electrospun Fiber Surface Nanotopography on Neurite Outgrowth and Branching in Neuron Cultures. PLoS ONE 2019, 14, e0211731. [Google Scholar] [CrossRef]
- Ziemba, A.M.; Fink, T.D.; Crochiere, M.C.; Puhl, D.L.; Sapkota, S.; Gilbert, R.J.; Zha, R.H. Coating Topologically Complex Electrospun Fibers with Nanothin Silk Fibroin Enhances Neurite Outgrowth in Vitro. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The Influence of Surface Nanoroughness of Electrospun PLGA Nanofibrous Scaffold on Nerve Cell Adhesion and Proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Leong, M.F.; Chian, K.S.; Mhaisalkar, P.S.; Ong, W.F.; Ratner, B.D. Effect of Electrospun Poly(D,L-Lactide) Fibrous Scaffold with Nanoporous Surface on Attachment of Porcine Esophageal Epithelial Cells and Protein Adsorption. J. Biomed. Mater. Res. A 2009, 89A, 1040–1048. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Y.; Liu, X.; Sutti, A.; Ke, Q.; Mo, X.; Wang, X.; Morsi, Y.; Lin, T. Electrospinning of Nanofibres with Parallel Line Surface Texture for Improvement of Nerve Cell Growth. Soft Matter 2011, 7, 10812–10817. [Google Scholar] [CrossRef]
- Wu, T.; Xue, J.; Xia, Y. Engraving the Surface of Electrospun Microfibers with Nanoscale Grooves Promotes the Outgrowth of Neurites and the Migration of Schwann Cells. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Huang, J.; Lu, L.; Zhang, J.; Hu, X.; Zhang, Y.; Liang, W.; Wu, S.; Luo, Z. Electrical Stimulation to Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat Model of Large Nerve Defect. PLoS ONE 2012, 7, e39526. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef]
- Song, S.; George, P.M. Conductive Polymer Scaffolds to Improve Neural Recovery. Neural Regen. Res. 2017, 12, 1976–1978. [Google Scholar] [CrossRef]
- Anderson, M.; Shelke, N.B.; Manoukian, O.S.; Yu, X.; McCullough, L.D.; Kumbar, S.G. Peripheral Nerve Regeneration Strategies: Electrically Stimulating Polymer Based Nerve Growth Conduits. Crit. Rev. Biomed. Eng. 2015, 43, 131–159. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Xu, Y.; Li, L.; Liu, J.; He, Y.; Zou, Y.; Yu, L.; Qiu, X.; Guo, J. Electric Conductivity on Aligned Nanofibers Facilitates the Transdifferentiation of Mesenchymal Stem Cells into Schwann Cells and Regeneration of Injured Peripheral Nerve. Adv. Healthc. Mater. 2020, 9, 1901570. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Niu, C.; Wei, Z.; Shi, J.; Li, G.; Yang, Y.; Wang, H. A New Electrospun Graphene-Silk Fibroin Composite Scaffolds for Guiding Schwann Cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 2171–2185. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Niu, C.-M.; Shi, J.-Q.; Wang, Y.-Y.; Yang, Y.-M.; Wang, H.-B. Novel Conductive Polypyrrole/Silk Fibroin Scaffold for Neural Tissue Repair. Neural Regen. Res. 2018, 13, 1455. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA Nanofibrous Scaffolds Coated with Graphene Oxide for Promoting Neural Cell Growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef]
- Shueibi, O.; Zhou, Z.; Wang, X.; Yi, B.; He, X.; Zhang, Y. Effects of GO and RGO Incorporated Nanofibrous Scaffolds on the Proliferation of Schwann Cells. Biomed. Phys. Eng. Express 2019, 5, 025002. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, K.; Sun, B.; Fang, J.; Zhang, K.; EI-Hamshary, H.; S. Al-Deyab, S.; Mo, X. The Aligned Core–Sheath Nanofibers with Electrical Conductivity for Neural Tissue Engineering. J. Mater. Chem. B 2014, 2, 7945–7954. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-Functionalized Electrospun Nanofibers for Tissue Engineering and Drug Delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Cembran, A.; Bruggeman, K.F.; Williams, R.J.; Parish, C.L.; Nisbet, D.R. Biomimetic Materials and Their Utility in Modeling the 3-Dimensional Neural Environment. iScience 2020, 23, 100788. [Google Scholar] [CrossRef]
- Zheng, J.; Kontoveros, D.; Lin, F.; Hua, G.; Reneker, D.H.; Becker, M.L.; Willits, R.K. Enhanced Schwann Cell Attachment and Alignment Using One-Pot “Dual Click” GRGDS and YIGSR Derivatized Nanofibers. Biomacromolecules 2015, 16, 357–363. [Google Scholar] [CrossRef]
- Rajabi, M.; Firouzi, M.; Hassannejad, Z.; Haririan, I.; Zahedi, P. Fabrication and Characterization of Electrospun Laminin-Functionalized Silk Fibroin/Poly(Ethylene Oxide) Nanofibrous Scaffolds for Peripheral Nerve Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1595–1604. [Google Scholar] [CrossRef]
- Wu, T.; Li, D.; Wang, Y.; Sun, B.; Li, D.; Morsi, Y.; El-Hamshary, H.; S. Al-Deyab, S.; Mo, X. Laminin-Coated Nerve Guidance Conduits Based on Poly( l -Lactide- Co -Glycolide) Fibers and Yarns for Promoting Schwann Cells’ Proliferation and Migration. J. Mater. Chem. B 2017, 5, 3186–3194. [Google Scholar] [CrossRef]
- Bockelmann, J.; Klinkhammer, K.; von Holst, A.; Seiler, N.; Faissner, A.; Brook, G.A.; Klee, D.; Mey, J. Functionalization of Electrospun Poly(ε-Caprolactone) Fibers with the Extracellular Matrix-Derived Peptide GRGDS Improves Guidance of Schwann Cell Migration and Axonal Growth. Tissue Eng. Part A 2011, 17, 475–486. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Zou, J.; Qiu, S.; Rao, Z.; Liu, S.; Sun, X.; Xu, Y.; Zhu, Q.; Liu, X.; et al. Promoting Neurite Growth and Schwann Cell Migration by the Harnessing Decellularized Nerve Matrix onto Nanofibrous Guidance. ACS Appl. Mater. Interfaces 2019, 11, 17167–17176. [Google Scholar] [CrossRef]
- Nune, M.; Krishnan, U.M.; Sethuraman, S. PLGA Nanofibers Blended with Designer Self-Assembling Peptides for Peripheral Neural Regeneration. Mater. Sci. Eng. C 2016, 62, 329–337. [Google Scholar] [CrossRef]
- Feng, S.; Yan, Z.; Guo, C.; Chen, Z.; Zhang, K.; Mo, X.; Gu, Y. Effects of an Avidin-Biotin Binding System on Schwann Cells Attachment, Proliferation, and Gene Expressions onto Electrospun Scaffolds. J. Biomed. Mater. Res. A 2011, 97A, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, D.; Tebyanian, H.; Soufdoost, R.S.; Emamgholi, A.; Barkhordari, A.; Herfedoost, G.R.; Kaka, G.R.; Rashidiani, J. Modified PLGA Nanofibers as a Nerve Regenerator with Schwann Cells. Cell. Mol. Biol. 2018, 64, 66. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of Polymeric Nanofibers for Drug Delivery Applications. J. Controlled Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, M.A.; Wu, T.; Sun, B.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Fabrication and Characterization of Vitamin B5 Loaded Poly (l-Lactide-Co-Caprolactone)/Silk Fiber Aligned Electrospun Nanofibers for Schwann Cell Proliferation. Colloids Surf. B Biointerfaces 2016, 144, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Puhl, D.L.; Funnell, J.L.; D’Amato, A.R.; Bao, J.; Zagorevski, D.V.; Pressman, Y.; Morone, D.; Haggerty, A.E.; Oudega, M.; Gilbert, R.J. Aligned Fingolimod-Releasing Electrospun Fibers Increase Dorsal Root Ganglia Neurite Extension and Decrease Schwann Cell Expression of Promyelinating Factors. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Leisz, S.; Simmermacher, S.; Prell, J.; Strauss, C.; Scheller, C. Nimodipine-Dependent Protection of Schwann Cells, Astrocytes and Neuronal Cells from Osmotic, Oxidative and Heat Stress Is Associated with the Activation of AKT and CREB. Int. J. Mol. Sci. 2019, 20, 4578. [Google Scholar] [CrossRef]
- Zech, J.; Leisz, S.; Göttel, B.; Syrowatka, F.; Greiner, A.; Strauss, C.; Knolle, W.; Scheller, C.; Mäder, K. Electrospun Nimodipine-Loaded Fibers for Nerve Regeneration: Development and in Vitro Performance. Eur. J. Pharm. Biopharm. 2020, 151, 116–126. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Xu, F.; Cong, H.; Li, Z.; Song, Y.; Wang, M. Spatio-Temporal Release of NGF and GDNF from Multi-Layered Nanofibrous Bicomponent Electrospun Scaffolds. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Thoenen, H.; Sendtner, M. Neurotrophins: From Enthusiastic Expectations through Sobering Experiences to Rational Therapeutic Approaches. Nat. Neurosci. 2002, 5, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.; Gander, B.; Madduri, S. Nerve Conduit Scaffolds for Discrete Delivery of Two Neurotrophic Factors. Eur. J. Pharm. Biopharm. 2013, 85, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Chan, J.R.; Shooter, E.M. Neurotrophin 3 Activation of TrkC Induces Schwann Cell Migration through the C-Jun N-Terminal Kinase Pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 14421–14426. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Chan, J.R.; Shooter, E.M. Neurotrophins Regulate Schwann Cell Migration by Activating Divergent Signaling Pathways Dependent on Rho GTPases. Proc. Natl. Acad. Sci. USA 2004, 101, 8774–8779. [Google Scholar] [CrossRef] [PubMed]
- Anton, E.S.; Weskamp, G.; Reichardt, L.F.; Matthew, W.D. Nerve Growth Factor and Its Low-Affinity Receptor Promote Schwann Cell Migration. Proc. Natl. Acad. Sci. USA 1994, 91, 2795–2799. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive Electrospun Scaffolds Delivering Growth Factors and Genes for Tissue Engineering Applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef]
- Puhl, S.; Li, L.; Meinel, L.; Germershaus, O. Controlled Protein Delivery from Electrospun Non-Wovens: Novel Combination of Protein Crystals and a Biodegradable Release Matrix. Mol. Pharm. 2014, 11, 2372–2380. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-Dimensional Aligned Nanofibers-Hydrogel Scaffold for Controlled Non-Viral Drug/Gene Delivery to Direct Axon Regeneration in Spinal Cord Injury Treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef]
- Liu, J.-J.; Wang, C.-Y.; Wang, J.-G.; Ruan, H.-J.; Fan, C.-Y. Peripheral Nerve Regeneration Using Composite Poly(Lactic Acid-Caprolactone)/Nerve Growth Factor Conduits Prepared by Coaxial Electrospinning. J. Biomed. Mater. Res. A 2011, 96A, 13–20. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein–Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Chan, B.P.; Chew, S.Y. Sustained Release of Neurotrophin-3 and Chondroitinase ABC from Electrospun Collagen Nanofiber Scaffold for Spinal Cord Injury Repair. J. Biomed. Mater. Res. A 2012, 100A, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Morano, M.; Fregnan, F.; Pugliese, P.; Crosio, A.; Tos, P.; Geuna, S.; Haastert-Talini, K.; Gambarotta, G. The Median Nerve Injury Model in Pre-Clinical Research – A Critical Review on Benefits and Limitations. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P.; Foy, C. Restoration of Neurological Function Following Peripheral Nerve Trauma. Int. J. Mol. Sci. 2020, 21, 1808. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.; Kim, J.; Kim, B.J.; Kim, E.; Park, H.Y.; Choi, B.-H.; Joo, K.I.; Cho, M.-L.; Rhie, J.W.; Lee, J.I.; et al. Multi-Dimensional Bioinspired Tactics Using an Engineered Mussel Protein Glue-Based Nanofiber Conduit for Accelerated Functional Nerve Regeneration. Acta Biomater. 2019, 90, 87–99. [Google Scholar] [CrossRef]
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned Chitosan Nanofiber Hydrogel Grafted with Peptides Mimicking Bioactive Brain-Derived Neurotrophic Factor and Vascular Endothelial Growth Factor Repair Long-Distance Sciatic Nerve Defects in Rats. Theranostics 2020, 10, 1590–1603. [Google Scholar] [CrossRef]
- Suzuki, K.; Tanaka, H.; Ebara, M.; Uto, K.; Matsuoka, H.; Nishimoto, S.; Okada, K.; Murase, T.; Yoshikawa, H. Electrospun Nanofiber Sheets Incorporating Methylcobalamin Promote Nerve Regeneration and Functional Recovery in a Rat Sciatic Nerve Crush Injury Model. Acta Biomater. 2017, 53, 250–259. [Google Scholar] [CrossRef]
- Case, L.C.; Tessier-Lavigne, M. Regeneration of the Adult Central Nervous System. Curr. Biol. 2005, 15, R749–R753. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the Glial Scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the Glial Scar for Spinal Cord Repair. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Colello, R.J.; Chow, W.N.; Bigbee, J.W.; Lin, C.; Dalton, D.; Brown, D.; Jha, B.S.; Mathern, B.E.; Lee, K.D.; Simpson, D.G. The Incorporation of Growth Factor and Chondroitinase ABC into an Electrospun Scaffold to Promote Axon Regrowth Following Spinal Cord Injury. J. Tissue Eng. Regen. Med. 2016, 10, 656–668. [Google Scholar] [CrossRef]
- Milbreta, U.; Lin, J.; Pinese, C.; Ong, W.; Chin, J.S.; Shirahama, H.; Mi, R.; Williams, A.; Bechler, M.E.; Wang, J.; et al. Scaffold-Mediated Sustained, Non-Viral Delivery of MiR-219/MiR-338 Promotes CNS Remyelination. Mol. Ther. 2019, 27, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial Fibrillary Acidic Protein: GFAP-Thirty-One Years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of Astrocytic Form and Function. In Astrocytes: Methods and Protocols; Methods in Molecular Biology; Milner, R., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 23–45. ISBN 978-1-61779-452-0. [Google Scholar]
- Hol, E.M.; Pekny, M. Glial Fibrillary Acidic Protein (GFAP) and the Astrocyte Intermediate Filament System in Diseases of the Central Nervous System. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Liddelow, S.A. Astrocytes and Microglia: Models and Tools. J. Exp. Med. 2019, 216, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Houle, J.D.; Xu, J.; Chan, B.P.; Chew, S.Y. Nanofibrous Collagen Nerve Conduits for Spinal Cord Repair. Tissue Eng. Part A 2012, 18, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Jung, S.M.; Ju, J.H.; Kwon, Y.S.; Yoon, G.H.; Shin, H.S. Regulation of Astrocyte Activity via Control over Stiffness of Cellulose Acetate Electrospun Nanofiber. Vitro Cell. Dev. Biol.—Anim. 2015, 51, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Kim, S.H.; Kim, C.R.; Paik, S.M.; Jung, S.M.; Shin, H.S. Effect of Topography of an Electrospun Nanofiber on Modulation of Activity of Primary Rat Astrocytes. Neurosci. Lett. 2013, 534, 80–84. [Google Scholar] [CrossRef]
- Gottipati, M.K.; D’Amato, A.R.; Ziemba, A.M.; Popovich, P.G.; Gilbert, R.J. TGFβ3 Is Neuroprotective and Alleviates the Neurotoxic Response Induced by Aligned Poly-l-Lactic Acid Fibers on Naïve and Activated Primary Astrocytes. Acta Biomater. 2020, S1742706120305821. [Google Scholar] [CrossRef]
- Lau, C.L.; Kovacevic, M.; Tingleff, T.S.; Forsythe, J.S.; Cate, H.S.; Merlo, D.; Cederfur, C.; Maclean, F.L.; Parish, C.L.; Horne, M.K.; et al. 3D Electrospun Scaffolds Promote a Cytotrophic Phenotype of Cultured Primary Astrocytes. J. Neurochem. 2014, 130, 215–226. [Google Scholar] [CrossRef]
- Zhao, T.; Jing, Y.; Zhou, X.; Wang, J.; Huang, X.; Gao, L.; Zhu, Y.; Wang, L.; Gou, Z.; Liang, C.; et al. PHBV/PLA/Col-Based Nanofibrous Scaffolds Promote Recovery of Locomotor Function by Decreasing Reactive Astrogliosis in a Hemisection Spinal Cord Injury Rat Model. J. Biomed. Nanotechnol. 2018. [Google Scholar] [CrossRef]
- Chow, W.N.; Simpson, D.G.; Bigbee, J.W.; Colello, R.J. Evaluating Neuronal and Glial Growth on Electrospun Polarized Matrices: Bridging the Gap in Percussive Spinal Cord Injuries. Neuron Glia Biol. 2007, 3, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gerardo-Nava, J.; Führmann, T.; Klinkhammer, K.; Seiler, N.; Mey, J.; Klee, D.; Möller, M.; Dalton, P.D.; Brook, G.A. Human Neural Cell Interactions with Orientated Electrospun Nanofibers in Vitro. Nanomedicine 2009. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, C.; Min, S.K.; Jung, S.M.; Shin, H.S. In Vitro Evaluation of the Effects of Electrospun PCL Nanofiber Mats Containing the Microalgae Spirulina (Arthrospira) Extract on Primary Astrocytes. Colloids Surf. B Biointerfaces 2012, 90, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Schaub, N.J.; Gilbert, R.J. Controlled Release of 6-Aminonicotinamide from Aligned, Electrospun Fibers Alters Astrocyte Metabolism and Dorsal Root Ganglia Neurite Outgrowth. J. Neural Eng. 2011, 8, 46026. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Wang, H.; Dong, Y.; Zhang, F.; Zuo, B.; Zhang, H. Electrospun Silk Fibroin Nanofibers in Different Diameters Support Neurite Outgrowth and Promote Astrocyte Migration. J. Biomed. Mater. Res. Part A 2013, 101A, 2667–2678. [Google Scholar] [CrossRef]
- Xia, H.; Xia, Y. An in Vitro Study of Non-Aligned or Aligned Electrospun Poly(Methyl Methacrylate) Nanofibers as Primary Rat Astrocytes-Loading Scaffold. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Desmond, G.P.; Rivet, C.J.; Kearns, K.R.; Thompson, D.M.; Gilbert, R.J. Nebulized Solvent Ablation of Aligned PLLA Fibers for the Study of Neurite Response to Anisotropic-to-Isotropic Fiber/Film Transition (AFFT) Boundaries in Astrocyte-Neuron Co-Cultures. Biomaterials 2015, 46, 82–94. [Google Scholar] [CrossRef]
- Puschmann, T.B.; Zandén, C.; De Pablo, Y.; Kirchhoff, F.; Pekna, M.; Liu, J.; Pekny, M. Bioactive 3D Cell Culture System Minimizes Cellular Stress and Maintains the in Vivo-like Morphological Complexity of Astroglial Cells. Glia 2013, 61, 432–440. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Fioravanzo, L.; Bianco, A.; Grigioni, M.; Folin, M. In Vitro Astrocyte and Cerebral Endothelial Cell Response to Electrospun Poly(ε-Caprolactone) Mats of Different Architecture. J. Mater. Sci. Mater. Med. 2010, 21, 1353–1362. [Google Scholar] [CrossRef]
- Qi, D.; Wu, S.; Lin, H.; Kuss, M.A.; Lei, Y.; Krasnoslobodtsev, A.; Ahmed, S.; Zhang, C.; Kim, H.J.; Jiang, P.; et al. Establishment of a Human IPSC- and Nanofiber-Based Microphysiological Blood-Brain Barrier System. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Pensabene, V.; Crowder, S.W.; Balikov, D.A.; Lee, J.B.; Sung, H.J. Optimization of Electrospun Fibrous Membranes for in Vitro Modeling of Blood-Brain Barrier. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 16–20 August 2016. [Google Scholar]
- Bischel, L.L.; Coneski, P.N.; Lundin, J.G.; Wu, P.K.; Giller, C.B.; Wynne, J.; Ringeisen, B.R.; Pirlo, R.K. Electrospun Gelatin Biopapers as Substrate for in Vitro Bilayer Models of Blood−brain Barrier Tissue. J. Biomed. Mater. Res. A 2016, 104, 901–909. [Google Scholar] [CrossRef]
- Gaston, J.D.; Bischel, L.L.; Fitzgerald, L.A.; Cusick, K.D.; Ringeisen, B.R.; Pirlo, R.K. Gene Expression Changes in Long-Term In Vitro Human Blood-Brain Barrier Models and Their Dependence on a Transwell Scaffold Material. J. Healthc. Eng. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Weightman, A.P.; Pickard, M.R.; Yang, Y.; Chari, D.M. An in Vitro Spinal Cord Injury Model to Screen Neuroregenerative Materials. Biomaterials 2014, 35, 3756–3765. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.J.; Low, W.C.; Lu, Q.R.; Chew, S.Y. Topographical Effects on Fiber-Mediated MicroRNA Delivery to Control Oligodendroglial Precursor Cells Development. Biomaterials 2015, 70, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, D.R.; Yu, L.M.Y.; Zahir, T.; Forsythe, J.S.; Shoichet, M.S. Characterization of Neural Stem Cells on Electrospun Poly(ε-Caprolactone) Submicron Scaffolds: Evaluating Their Potential in Neural Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2008, 19, 623–634. [Google Scholar] [CrossRef]
- Lee, S.; Leach, M.K.; Redmond, S.A.; Chong, S.Y.C.; Mellon, S.H.; Tuck, S.J.; Feng, Z.-Q.; Corey, J.M.; Chan, J.R. A Culture System to Study Oligodendrocyte Myelination Processes Using Engineered Nanofibers. Nat. Methods 2012, 9, 917–922. [Google Scholar] [CrossRef]
- Bechler, M.E.; Byrne, L.; ffrench-Constant, C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr. Biol. 2015, 25, 2411–2416. [Google Scholar] [CrossRef]
- Fu, M.; McAlear, T.S.; Nguyen, H.; Oses-Prieto, J.A.; Valenzuela, A.; Shi, R.D.; Perrino, J.J.; Huang, T.-T.; Burlingame, A.L.; Bechstedt, S.; et al. The Golgi Outpost Protein TPPP Nucleates Microtubules and Is Critical for Myelination. Cell 2019, 179, 132–146.e14. [Google Scholar] [CrossRef]
- Weightman, A.; Jenkins, S.; Pickard, M.; Chari, D.; Yang, Y. Alignment of Multiple Glial Cell Populations in 3D Nanofiber Scaffolds: Toward the Development of Multicellular Implantable Scaffolds for Repair of Neural Injury. Nanomedicine Nanotechnol. Biol. Med. 2014, 10, 291–295. [Google Scholar] [CrossRef]
- Salati, A.; Ahangari, G.; Keshvari, H.; Sanati, M.H. Modeling the Effect of Autoreactive T-Cells on Oligodendrocytes in Mutiple Sclerosis Patients Using Chitosan/Gelatin Nanofibrous Scaffolds. Biointerface Res. Appl. Chem. 2016, 6, 1214–1221. [Google Scholar]
- Dillenburg, A.; Ireland, G.; Holloway, R.K.; Davies, C.L.; Evans, F.L.; Swire, M.; Bechler, M.E.; Soong, D.; Yuen, T.J.; Su, G.H.; et al. Activin Receptors Regulate the Oligodendrocyte Lineage in Health and Disease. Acta Neuropathol. (Berl.) 2018, 135, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Elitt, M.S.; Shick, H.E.; Madhavan, M.; Allan, K.C.; Clayton, B.L.L.; Weng, C.; Miller, T.E.; Factor, D.C.; Barbar, L.; Nawash, B.S.; et al. Chemical Screening Identifies Enhancers of Mutant Oligodendrocyte Survival and Unmasks a Distinct Pathological Phase in Pelizaeus-Merzbacher Disease. Stem Cell Rep. 2018, 11, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, E.; Chen, X.; Chao, J.; Klein, J.; Qu, Q.; Sun, G.; Sun, G.; Huang, Y.; Warden, C.D.; et al. GFAP Mutations in Astrocytes Impair Oligodendrocyte Progenitor Proliferation and Myelination in an HiPSC Model of Alexander Disease. Cell Stem Cell 2018, 23, 239–251.e6. [Google Scholar] [CrossRef] [PubMed]
- Hubler, Z.; Allimuthu, D.; Bederman, I.; Elitt, M.S.; Madhavan, M.; Allan, K.C.; Shick, H.E.; Garrison, E.; T. Karl, M.; Factor, D.C.; et al. Accumulation of 8,9-Unsaturated Sterols Drives Oligodendrocyte Formation and Remyelination. Nature 2018, 560, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, A.R.; Correia, B.; da Silva, T.F.; Bessa-Neto, D.; Veldhoven, P.P.V.; Brites, P. Leukodystrophy Caused by Plasmalogen Deficiency Rescued by Glyceryl 1-Myristyl Ether Treatment. Brain Pathol. 2019, 29, 622–639. [Google Scholar] [CrossRef]
- Allimuthu, D.; Hubler, Z.; Najm, F.J.; Tang, H.; Bederman, I.; Seibel, W.; Tesar, P.J.; Adams, D.J. Diverse Chemical Scaffolds Enhance Oligodendrocyte Formation by Inhibiting CYP51, TM7SF2, or EBP. Cell Chem. Biol. 2019, 26, 593–599.e4. [Google Scholar] [CrossRef]
- Lee, S.; Chong, S.Y.C.; Tuck, S.J.; Corey, J.M.; Chan, J.R. A Rapid and Reproducible Assay for Modeling Myelination by Oligodendrocytes Using Engineered Nanofibers. Nat. Protoc. 2013, 8, 771–782. [Google Scholar] [CrossRef]
- Xu, Y.K.T.; Chitsaz, D.; Brown, R.A.; Cui, Q.L.; Dabarno, M.A.; Antel, J.P.; Kennedy, T.E. Deep Learning for High-Throughput Quantification of Oligodendrocyte Ensheathment at Single-Cell Resolution. Commun. Biol. 2019, 2, 116. [Google Scholar] [CrossRef]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.-T.D.; Yang, L.; Lee, K.-B. Guiding Stem Cell Differentiation into Oligodendrocytes Using Graphene-Nanofiber Hybrid Scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef]
- Li, Y.; Ceylan, M.; Shrestha, B.; Wang, H.; Lu, Q.R.; Asmatulu, R.; Yao, L. Nanofibers Support Oligodendrocyte Precursor Cell Growth and Function as a Neuron-Free Model for Myelination Study. Biomacromolecules 2014, 15, 319–326. [Google Scholar] [CrossRef]
- Diao, H.J.; Low, W.C.; Milbreta, U.; Lu, Q.R.; Chew, S.Y. Nanofiber-Mediated MicroRNA Delivery to Enhance Differentiation and Maturation of Oligodendroglial Precursor Cells. J. Control. Release 2015, 208, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Lin, J.; Bechler, M.E.; Wang, K.; Wang, M.; ffrench-Constant, C.; Chew, S.Y. Microfiber Drug/Gene Delivery Platform for Study of Myelination. Acta Biomater. 2018, 75, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Trapp, B.D. Microglia and Neuroprotection. J. Neurochem. 2016, 136, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.R.; Rocha, D.N.; Ambrosio, L.; Pêgo, A.P. The Role of the Surface on Microglia Function: Implications for Central Nervous System Tissue Engineering. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Suman, G.; Dhyani, V.; Swain, S.; Giri, L.; Samavedi, S. Three-Dimensional Imaging and Quantification of Real-Time Cytosolic Calcium Oscillations in Microglial Cells Cultured on Electrospun Matrices Using Laser Scanning Confocal Microscopy. Biotechnol. Bioeng. 2020. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishikawa, T.; Abe, R.; Nakayama, R.; Asada, A.; Matsuki, N.; Ikegaya, Y. Astrocyte Calcium Signalling Orchestrates Neuronal Synchronization in Organotypic Hippocampal Slices. J. Physiol. 2014, 592, 2771–2783. [Google Scholar] [CrossRef]
- Sieger, D.; Moritz, C.; Ziegenhals, T.; Prykhozhij, S.; Peri, F. Long-Range Ca2+ Waves Transmit Brain-Damage Signals to Microglia. Dev. Cell 2012, 22, 1138–1148. [Google Scholar] [CrossRef]
- Bartneck, M.; Heffels, K.-H.; Pan, Y.; Bovi, M.; Zwadlo-Klarwasser, G.; Groll, J. Inducing Healing-like Human Primary Macrophage Phenotypes by 3D Hydrogel Coated Nanofibres. Biomaterials 2012, 33, 4136–4146. [Google Scholar] [CrossRef]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M.; Visai, L. Effect of Electrospun Fiber Diameter and Alignment on Macrophage Activation and Secretion of Proinflammatory Cytokines and Chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage Functional Polarization (M1/M2) in Response to Varying Fiber and Pore Dimensions of Electrospun Scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Wu, T.; Pan, G.; Fan, C.; Xu, Y.; Cui, W. Macrophage Infiltration of Electrospun Polyester Fibers. Biomater. Sci. 2017, 5, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.-L.; et al. The Effect of Thick Fibers and Large Pores of Electrospun Poly(ε-Caprolactone) Vascular Grafts on Macrophage Polarization and Arterial Regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Y.; Wang, J.; Zhang, C.; Yan, H.; Zhu, M.; Wang, K.; Li, C.; Xu, Q.; Kong, D. Effect of Resveratrol on Modulation of Endothelial Cells and Macrophages for Rapid Vascular Regeneration from Electrospun Poly(ε-Caprolactone) Scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 19541–19551. [Google Scholar] [CrossRef] [PubMed]
- DePaula-Silva, A.B.; Gorbea, C.; Doty, D.J.; Libbey, J.E.; Sanchez, J.M.S.; Hanak, T.J.; Cazalla, D.; Fujinami, R.S. Differential Transcriptional Profiles Identify Microglial- and Macrophage-Specific Gene Markers Expressed during Virus-Induced Neuroinflammation. J. Neuroinflamm. 2019, 16, 152. [Google Scholar] [CrossRef]
- Zarruk, J.G.; Greenhalgh, A.D.; David, S. Microglia and Macrophages Differ in Their Inflammatory Profile after Permanent Brain Ischemia. Exp. Neurol. 2018, 301, 120–132. [Google Scholar] [CrossRef]
- Shemer, A.; Grozovski, J.; Tay, T.L.; Tao, J.; Volaski, A.; Süß, P.; Ardura-Fabregat, A.; Gross-Vered, M.; Kim, J.-S.; David, E.; et al. Engrafted Parenchymal Brain Macrophages Differ from Microglia in Transcriptome, Chromatin Landscape and Response to Challenge. Nat. Commun. 2018, 9, 5206. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S. Differences in the Phagocytic Response of Microglia and Peripheral Macrophages after Spinal Cord Injury and Its Effects on Cell Death. J. Neurosci. 2014, 34, 6316–6322. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Patel, A.R.; Grenier, J.M.; Crapser, J.; Verma, R.; Jellison, E.R.; McCullough, L.D. Functional Differences between Microglia and Monocytes after Ischemic Stroke. J. Neuroinflamm. 2015, 12, 106. [Google Scholar] [CrossRef]
- Nisbet, D.R.; Rodda, A.E.; Horne, M.K.; Forsythe, J.S.; Finkelstein, D.I. Neurite Infiltration and Cellular Response to Electrospun Polycaprolactone Scaffolds Implanted into the Brain. Biomaterials 2009, 30, 4573–4580. [Google Scholar] [CrossRef]
- Rivet, C.J.; Zhou, K.; Gilbert, R.J.; Finkelstein, D.I.; Forsythe, J.S. Cell Infiltration into a 3D Electrospun Fiber and Hydrogel Hybrid Scaffold Implanted in the Brain. Biomatter 2015, 5. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Shen, W.; Patel, S.; Zhang, R.; Young, W.L.; Li, S. Nanofibrous Patches for Spinal Cord Regeneration. Adv. Funct. Mater. 2010, 20, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Downing, T.L.; Wang, A.; Yan, Z.-Q.; Nout, Y.; Lee, A.L.; Beattie, M.S.; Bresnahan, J.C.; Farmer, D.L.; Li, S. Drug-Eluting Microfibrous Patches for the Local Delivery of Rolipram in Spinal Cord Repair. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Ni, S.; Li, X.; Yao, J.; Qi, H.; Fan, X.; Wang, J. Sustained Delivery of DbcAMP by Poly(Propylene Carbonate) Micron Fibers Promotes Axonal Regenerative Sprouting and Functional Recovery after Spinal Cord Hemisection. Brain Res. 2013, 1538, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.R.; Lopes, C.D.F.; Salvador, D.; Rocha, D.N.; Pêgo, A.P. Ibuprofen-Loaded Fibrous Patches—Taming Inhibition at the Spinal Cord Injury Site. J. Mater. Sci. Mater. Med. 2017, 28, 157. [Google Scholar] [CrossRef]
- Ni, S.; Xia, T.; Li, X.; Zhu, X.; Qi, H.; Huang, S.; Wang, J. Sustained Delivery of Chondroitinase ABC by Poly(Propylene Carbonate)-Chitosan Micron Fibers Promotes Axon Regeneration and Functional Recovery after Spinal Cord Hemisection. Brain Res. 2015, 1624, 469–478. [Google Scholar] [CrossRef]
- Xi, K.; Gu, Y.; Tang, J.; Chen, H.; Xu, Y.; Wu, L.; Cai, F.; Deng, L.; Yang, H.; Shi, Q.; et al. Microenvironment-Responsive Immunoregulatory Electrospun Fibers for Promoting Nerve Function Recovery. Nat. Commun. 2020, 11, 4504. [Google Scholar] [CrossRef]
- Gelain, F.; Panseri, S.; Antonini, S.; Cunha, C.; Donega, M.; Lowery, J.; Taraballi, F.; Cerri, G.; Montagna, M.; Baldissera, F.; et al. Transplantation of Nanostructured Composite Scaffolds Results in the Regeneration of Chronically Injured Spinal Cords. ACS Nano 2011, 5, 227–236. [Google Scholar] [CrossRef]
- Liao, I.; Chew, S.; Leong, K. Aligned Core–Shell Nanofibers Delivering Bioactive Proteins. Nanomedicine 2006, 1, 465–471. [Google Scholar] [CrossRef]
- Sperling, L.E.; Reis, K.P.; Pranke, P.; Wendorff, J.H. Advantages and Challenges Offered by Biofunctional Core–Shell Fiber Systems for Tissue Engineering and Drug Delivery. Drug Discov. Today 2016, 21, 1243–1256. [Google Scholar] [CrossRef]
- Wang, C.; Yan, K.-W.; Lin, Y.-D.; Hsieh, P.C.H. Biodegradable Core/Shell Fibers by Coaxial Electrospinning: Processing, Fiber Characterization, and Its Application in Sustained Drug Release. Macromolecules 2010, 43, 6389–6397. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent Advances in Multiaxial Electrospinning for Drug Delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef] [PubMed]
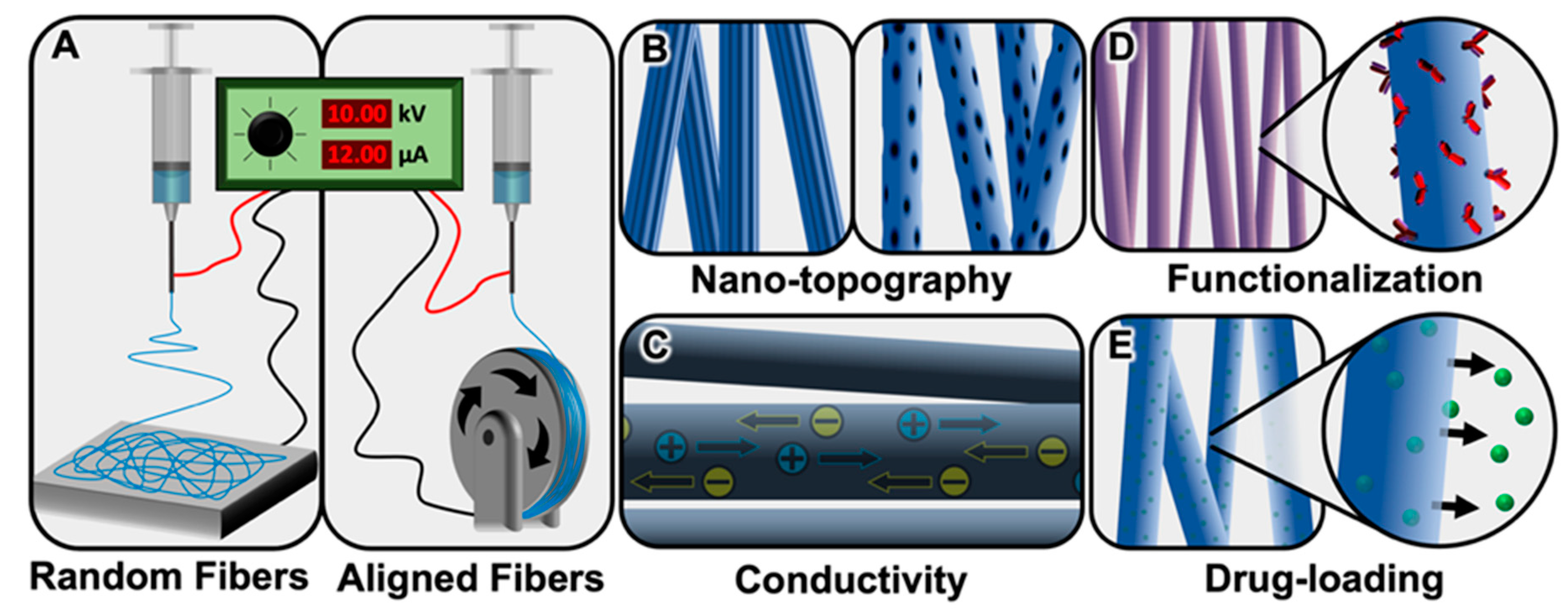
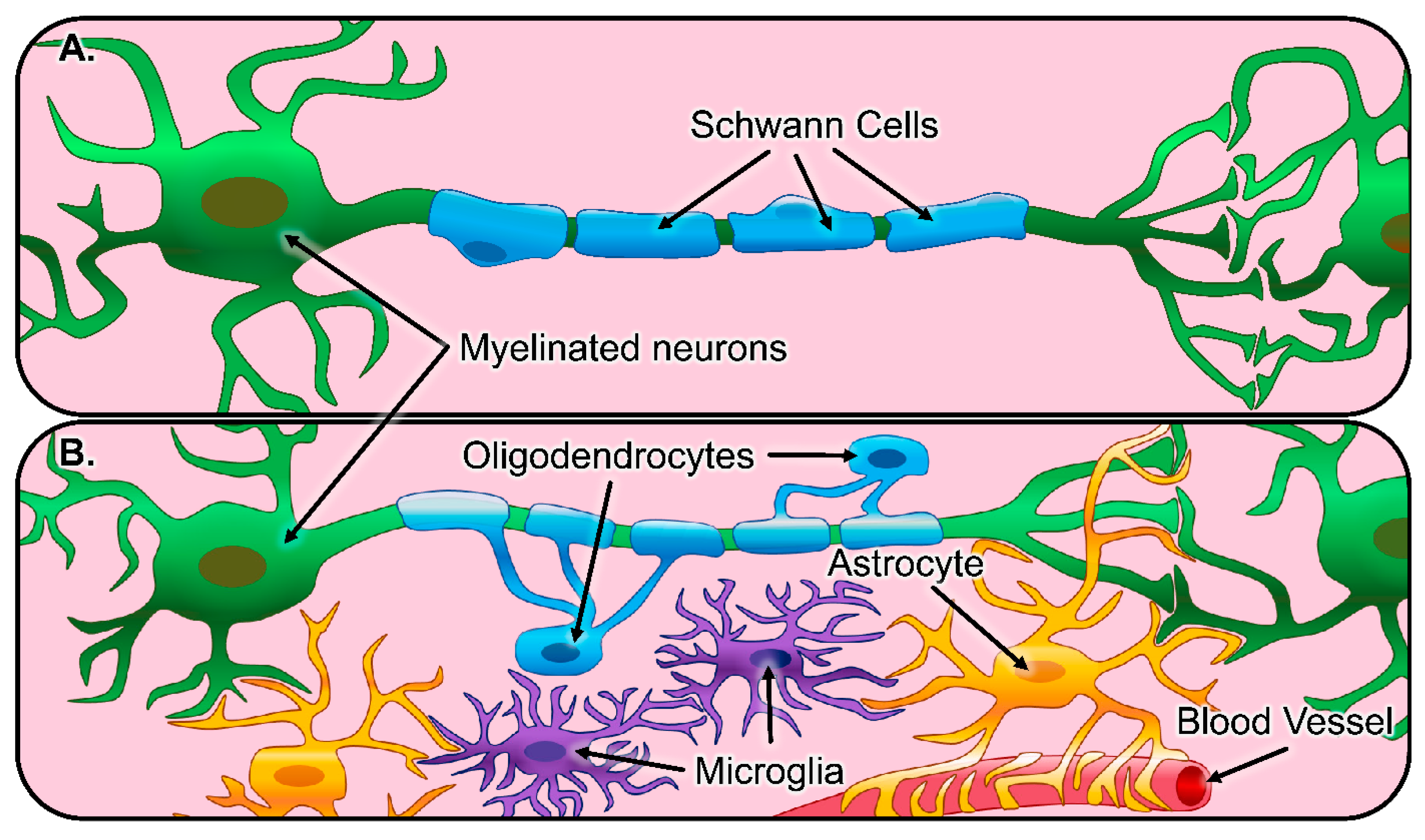
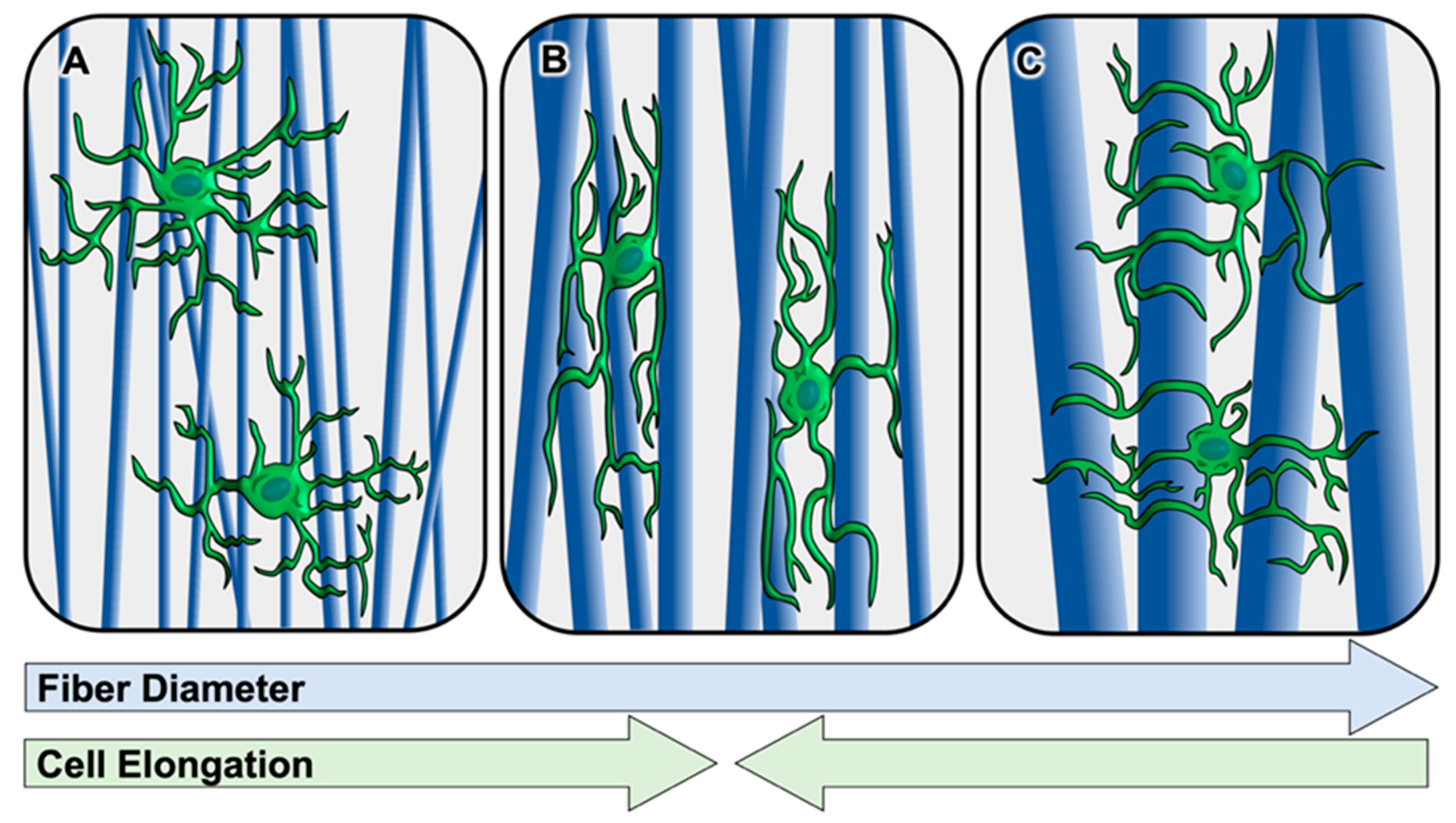

| Schwann Cells | Astrocytes | Oligodendroglia | Microglia |
|---|---|---|---|
| Reduce production of myelinating factors and demyelinate from axons Dedifferentiate into repair state Increase migration and production of neurotrophic factors Redifferentiate into mature myelinating state and remyelinate regenerated axons | Proliferate and migrate to injury site Produce neuroinhibitory molecules Generate glial scar around lesion | Immediately die from excitotoxicity Demyelinate damaged and nearby axons Oligodendrocyte precursor cells proliferate and migrate to lesion to stabilize dystrophic axons | Produce proinflammatory signals Remove cellular debris Activate nearby glia |
| Cell Response to Fiber: | Alignment | Diameter | Surface Nanotopography | Conductivity | Functionalization | Therapeutic Delivery |
|---|---|---|---|---|---|---|
| Schwann cells | Aligned improve stem and progenitor cell differentiation towards Schwann cell lineage [100,120,138]. Aligned improve Schwann cell maturation [105,106], elongation [14,15,64,105,106,107,108,111,112,113,141], and migration [114,115]. Random improve Schwann cell adhesion [108,109]. | Diameters of ~600 nm–1.6 µm improve stem and progenitor cell differentiation towards Schwann cell lineage [100,120]. Diameters of ~1–1.3 µm improve Schwann cell elongation and migration [28,125,126]. Diameters <600 nm improve Schwann cell adhesion [125]. Diameters ≥5 µm reduce Schwann cell elongation [126]. | Improve Schwann cell elongation [132] and migration [133]. | Support stem cell differentiation towards the Schwann cell lineage [138]. Can be toxic at high concentrations [139]. | Improve stem cell differentiation towards the Schwann cell lineage [120,121]. Improve Schwann cell adhesion/proliferation [146,147,148,152], migration [148,149,150], and maturation [150]. | Improve Schwann cell proliferation [112,143,156] and elongation [112], reduce Schwann cell maturation [157], and protects Schwann cells against stress and toxins [143,159]. |
| Cell Response to Fiber: | Alignment | Filler | Material Selection | Functionalization | Therapeutic Delivery |
|---|---|---|---|---|---|
| Schwann cells | Aligned improve Schwann cell infiltration of nerve grafts [63,64,66] and uniaxial elongation [15,63]. | Filling hollow nerve grafts with electrospun fibers improves Schwann cell adhesion to grafts [62], uniform infiltration of Schwann cells throughout grafts [15,62,63], and remyelination of regenerated axons [62]. | Fibers fabricated from natural biopolymers improve Schwann cell response to nerve grafts [62]. Conductive fibers improve stem cell differentiation towards the Schwann cell lineage following graft implantation and improve remyelination of regenerated axons [138]. | Improve Schwann cell infiltration of nerve grafts [175] and remyelination of regenerated axons [175,176]. | Improve Schwann cell remyelination of regenerated axons [170,177]. |
| Cell Response to Fiber: | Alignment | Diameter | Surface Nanotopography | Conductivity | Functionalization | Therapeutic Delivery |
|---|---|---|---|---|---|---|
| Astrocytes | Aligned enhance astrocyte migration and elongation [29]. | Larger fibers (808 vs. 386 nm) enhance astrocyte regenerative properties [27]. | Pitted and divoted fibers broaden astrocyte morphology and restrict regenerative properties. Smooth fibers enhance astrocyte regenerative properties [25]. | Unknown | Enhance astrocyte viability and adhesion. Decrease astrocyte reactivity [200]. | Decrease astrocyte metabolic activity [189,190]. Increase astrocyte survival after insult [159]. |
| Oligodendrocytes | Random enhance OPC differentiation and maturation [207] | >0.4 µm induces myelination [209]. >0.5 µm improves OPC maturation and myelination [209,210]. | Unknown | Unknown | Promote neural stem cell differentiation towards oligodendrocyte lineage [222]. Improve oligodendrocyte myelination [204,217]. | Enhance OPC differentiation and maturation [176,201,218,219]. |
| Microglia | Aligned promote microglial elongation and yield coordinated responses [221,222]. | Unknown | Unknown | Unknown | Unknown | Unknown |
| Cell Response to Fiber: | Alignment | Density | Material Selection | Therapeutic Delivery |
|---|---|---|---|---|
| Astrocytes | Aligned increase astrocyte infiltration into the scaffold [16,69]. | High-density fiber scaffolds reduce glial scarring [67,237]. High-density fiber scaffolds increase astrocyte infiltration into the scaffold [243]. | Decrease astrocyte reactivity and accumulation at the lesion [68,186]. | Increase astrocyte infiltration into the scaffold [175,221,244]. Reduce glial scarring [240,242,243,244]. |
| Oligodendrocytes | Aligned increase oligodendrocyte precursor cell (OPC) infiltration into the scaffold [69]. | Unknown | Unknown | Enhance OPC differentiation and maturation [182]. Increase oligodendrocyte infiltration into the scaffold [175,176]. |
| Microglia | Aligned promote microglial infiltration into the scaffold [187]. | Unknown | Unknown | Decrease inflammation [221,243]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puhl, D.L.; Funnell, J.L.; Nelson, D.W.; Gottipati, M.K.; Gilbert, R.J. Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration. Bioengineering 2021, 8, 4. https://doi.org/10.3390/bioengineering8010004
Puhl DL, Funnell JL, Nelson DW, Gottipati MK, Gilbert RJ. Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration. Bioengineering. 2021; 8(1):4. https://doi.org/10.3390/bioengineering8010004
Chicago/Turabian StylePuhl, Devan L., Jessica L. Funnell, Derek W. Nelson, Manoj K. Gottipati, and Ryan J. Gilbert. 2021. "Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration" Bioengineering 8, no. 1: 4. https://doi.org/10.3390/bioengineering8010004
APA StylePuhl, D. L., Funnell, J. L., Nelson, D. W., Gottipati, M. K., & Gilbert, R. J. (2021). Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration. Bioengineering, 8(1), 4. https://doi.org/10.3390/bioengineering8010004






