Co-Networks Poly(hydroxyalkanoates)-Terpenes to Enhance Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
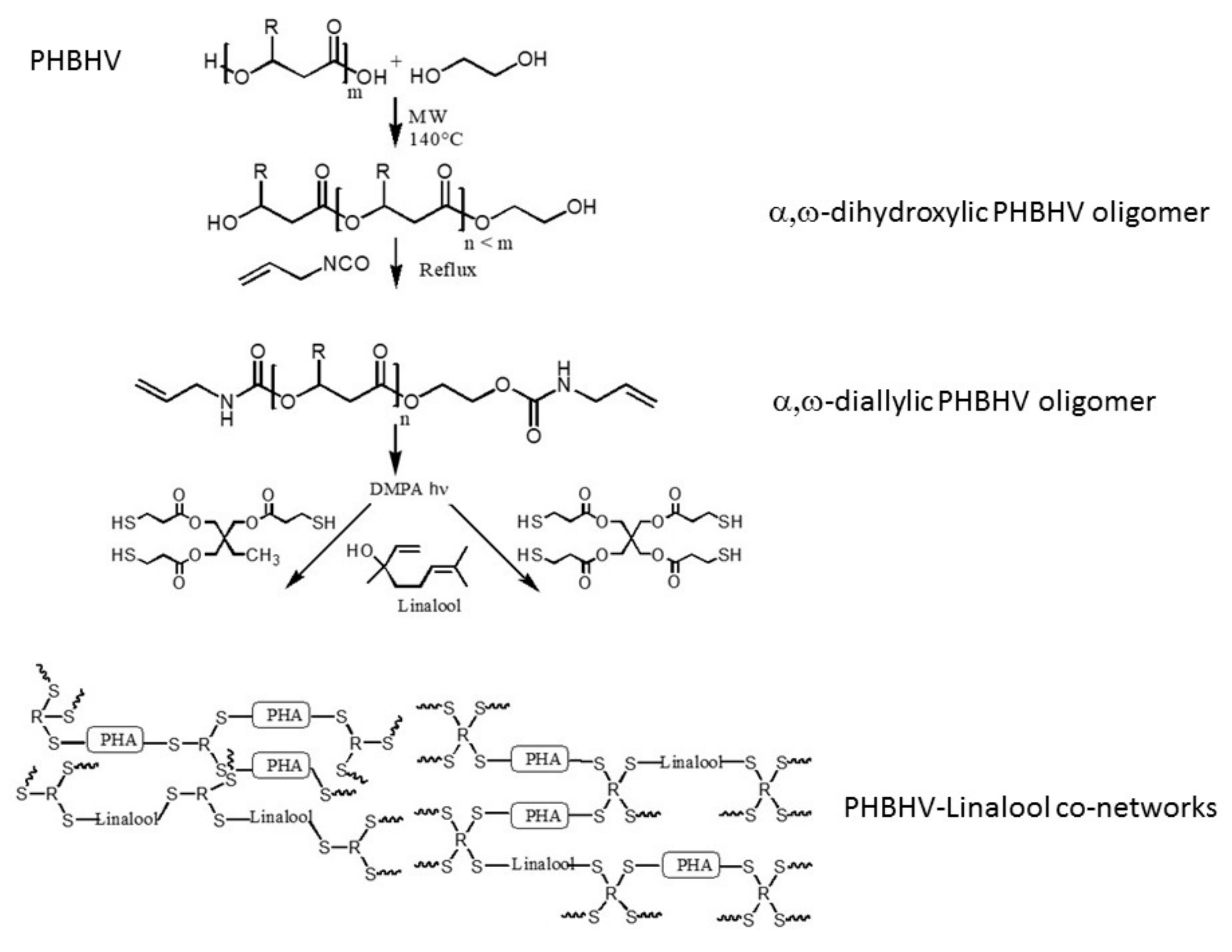
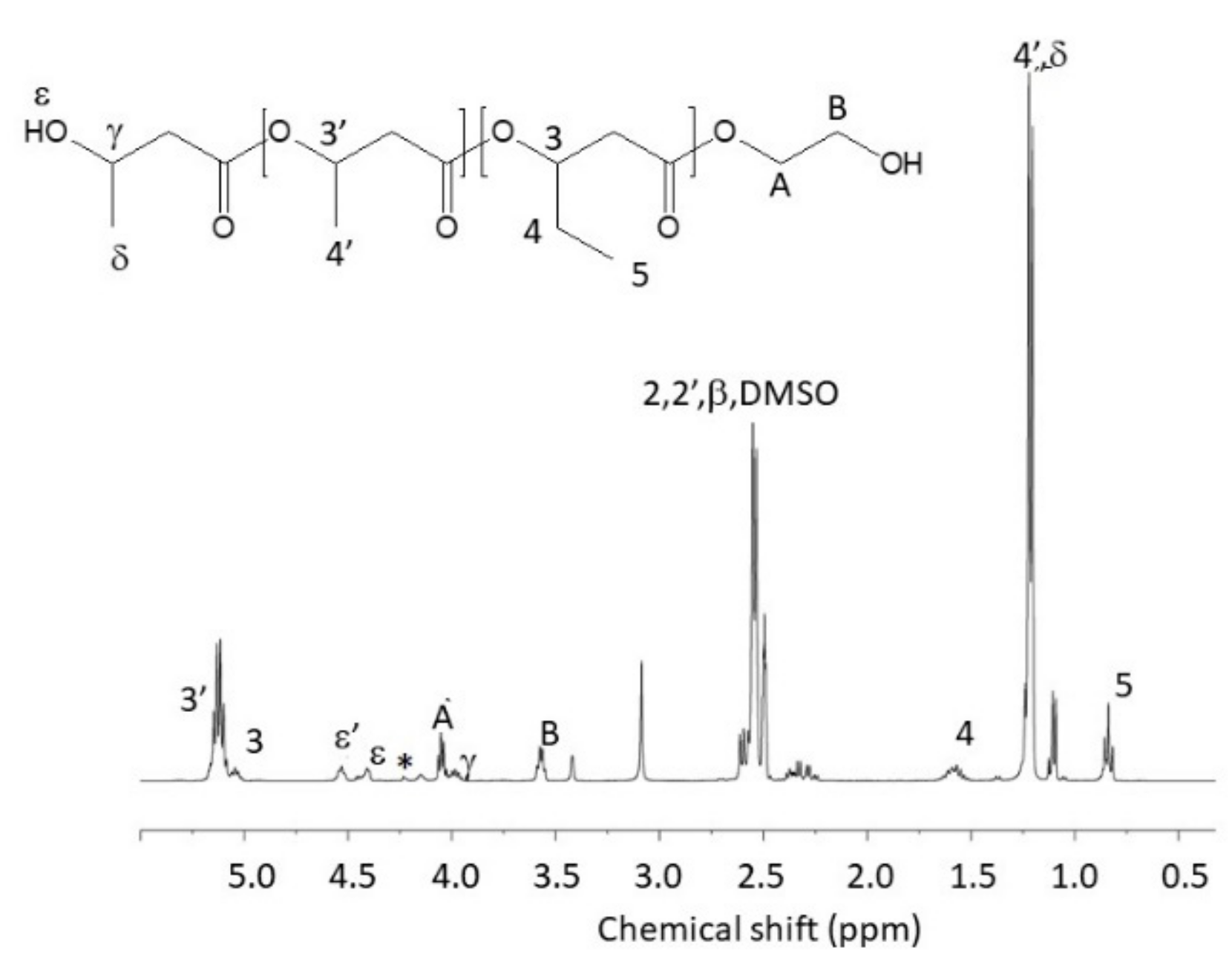
2.2. Synthesis of α,ω-Dihydroxylic PHBHV Oligomers under Microwave Irradiation
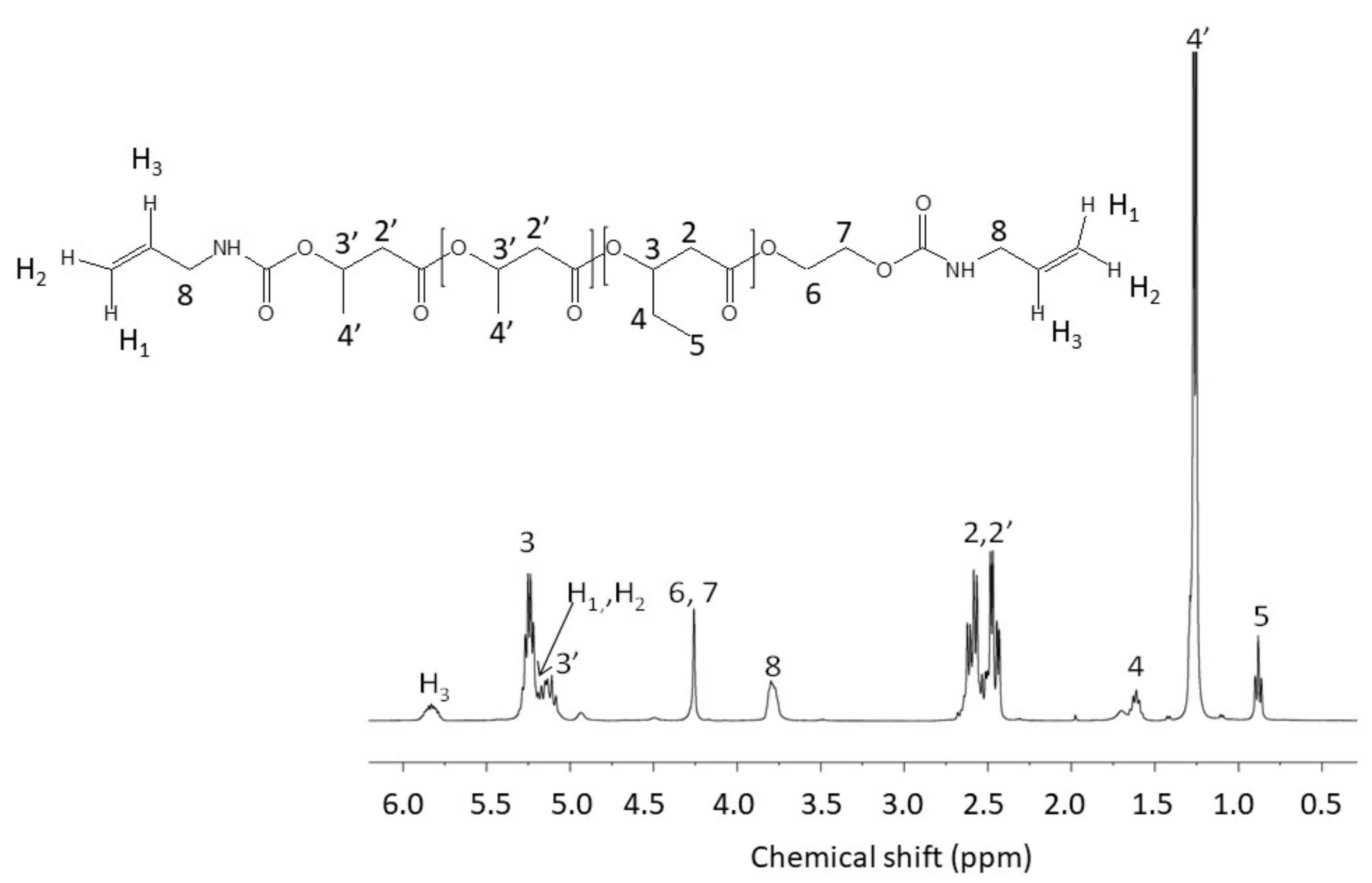
2.3. Synthesis of α,ω-Diallylic PHBHV Oligomers
2.4. Synthesis of PHBHV–Linalool (PHBHV-L) Co-networks
2.5. Characterization
3. Results and Discussion
3.1. Synthesis of α,ω-Diallylic PHBHV Oligomers
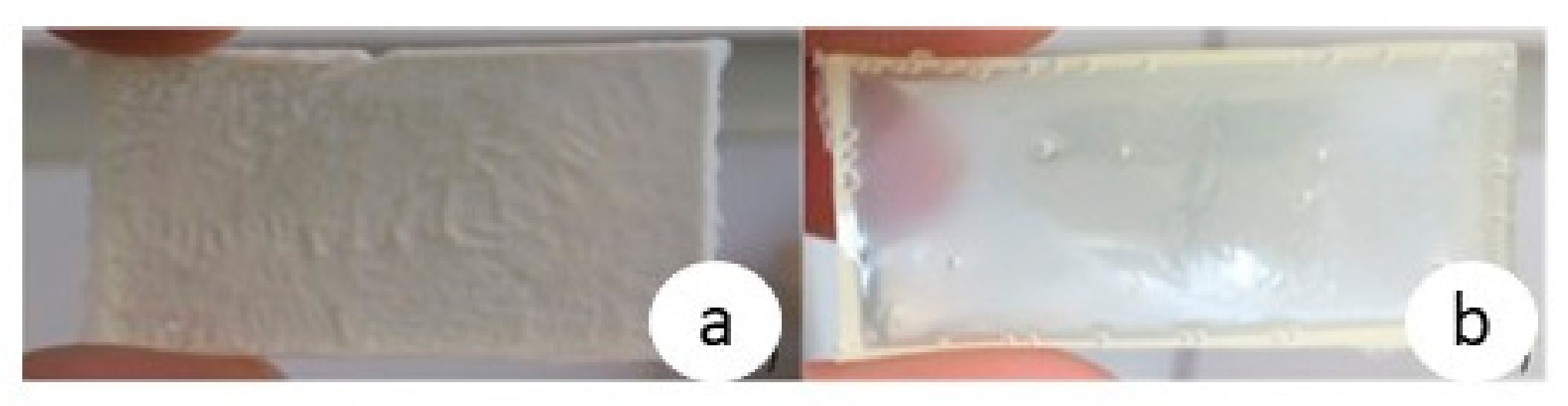
3.2. Preparation and Characterization of the PHBHV-L Co-Networks
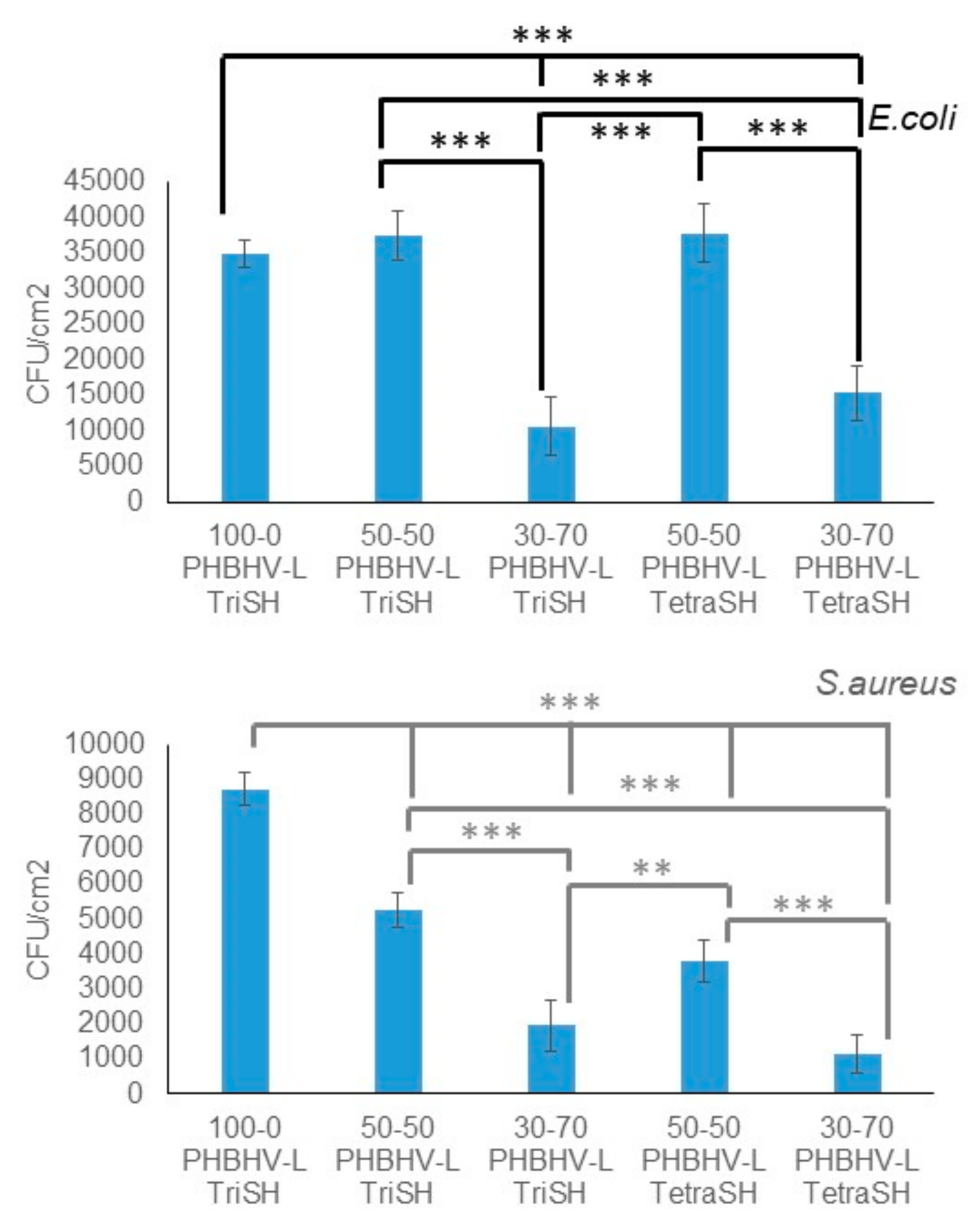
3.3. Bacterial Adherence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Evans, R.S.; Abouzelof, R.H.; Taylor, C.W.; Anderson, V.; Sumner, S.; Soutter, S.; Kleckner, R.; Lloyd, J.F. Computer Surveillance of Hospital-Acquired Infections: A 25 year Update. In Proceedings of the AMIA Symposium American Medical Informatics Association, San Francisco, CA, USA, 14–18 November 2009; Volume 2009, pp. 178–182. [Google Scholar]
- Centers for Disease Control (CDC and others). Public health focus: Surveillance, prevention, and control of nosocomial infections. MMWR Morb. Mortal. Wkly. Rep. 1992, 41, 783. [Google Scholar]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W.; Pogorzelska-Maziarz, M.; Herzig, C.T.; Weiner, L.M.; Furuya, E.Y.; Dick, A.; Larson, E. State of infection prevention in US hospitals enrolled in the National Health and Safety Network. Am. J. Infect. Control 2014, 42, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mussard, W.; Kebir, N.; Kriegel, I.; Estève, M.; Semetey, V. Facile and Efficient Control of Bioadhesion on Poly(Dimethylsiloxane) by Using a Biomimetic Approach. Angew. Chem. Int. Ed. 2011, 50, 10871–10874. [Google Scholar] [CrossRef]
- Baytukalov, T.A.; Bogoslovskaya, O.A.; Olkhovskaya, I.P.; Glushchenko, N.N.; Ovsyannikova, M.N.; Lopatin, S.A.; Varlamov, V.P. Regenerating Activity and Antibacterial Effect of Low-Molecular-Weight Chitosan. Biol. Bull. 2005, 32, 545–548. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Liang, M.N.; Meluleni, G.; Pier, G.; Ingber, N.E.; Whitesides, G.M. Self-Assembled Monolayers That Resist the Adsorption of Proteins and the Adhesion of Bacterial and Mammalian Cells. Langmuir 2001, 17, 6336–6343. [Google Scholar] [CrossRef]
- Krishnan, S.; Ward, R.J.; Hexemer, A.; Sohn, K.E.; Lee, K.L.; Angert, E.R.; Fischer, D.A.; Kramer, E.J.; Ober, C.K. Surfaces of Fluorinated Pyridinium Block Copolymers with Enhanced Antibacterial Activity. Langmuir 2006, 22, 11255–11266. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Bogliotti, N.; Brosseau, A.; Bourgon, J.; Mazeran, P.-E.; Lalevée, J.; Morlet-Savary, F.; Versace, D.-L. A (Triphenylphosphine)Silver (I) Complex as a New Performance Additive in Free-Radical Photopolymerization under Air. Macromol. Mater. Eng. 2018, 303, 1800101. [Google Scholar] [CrossRef]
- Wataha, J.; Lockwood, P.; Schedle, A. Effect of silver, copper, mercury, and nickel ions on cellular proliferation during extended, low-dose exposures. J. Biomed. Mater. Res. 2000, 52, 360–364. [Google Scholar] [CrossRef]
- Arnt, L.; Nüsslein, K.; Tew, G.N. Nonhemolytic abiogenic polymers as antimicrobial peptide mimics. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3860–3864. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. AntimicrobialN-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Condat, M.; Babinot, J.; Tomane, S.; Malval, J.-P.; Kang, I.-K.; Spillebout, F.; Mazeran, P.-E.; Lalevée, J.; Andalloussi, S.A.; Versace, D.-L. Development of photoactivable glycerol-based coatings containing quercetin for antibacterial applications. RSC Adv. 2016, 6, 18235–18245. [Google Scholar] [CrossRef]
- Condat, M.; Mazeran, P.-E.; Malval, J.-P.; Lalevée, J.; Morlet-Savary, F.; Renard, E.; Langlois, V.; Andalloussi, S.A.; Versace, D.-L. Photoinduced curcumin derivative-coatings with antibacterial properties. RSC Adv. 2015, 5, 85214–85224. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Malval, J.P.; Weiss-Maurin, M.; Paul, J.; Blacha-Grzechnik, A.; Tomane, S.; Mazeran, P.E.; Lalevée, J.; Langlois, V.; Versace, D.L. Paprika, Gallic Acid, and Visible Light: The Green Combination for the Synthesis of Biocide Coatings. ACS Sustain. Chem. Eng. 2018, 6, 104–109. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Contreras, A.; Andaloussi, S.A.; Coradin, T.; Hélary, C.; Razza, N.; Sangermano, M.; Mazeran, P.-E.; Malval, J.-P.; Versace, D.-L. Eosin-mediated synthesis of polymer coatings combining photodynamic inactivation and antimicrobial properties. J. Mater. Chem. B 2017, 5, 7572–7582. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Development and characterization of essential oil component-based polymer films: A potential approach to reduce bacterial biofilm. Appl. Microbiol. Biotechnol. 2013, 97, 9515–9523. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A Review on Recent Research Results (2008–2010) on Essential Oils as Antimicrobials and Antifungals. A Review. Flavour Frangrance J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.-I.; Kubo, A.; Nihei, K.-I.; Ogura, T. Antibacterial Activity of Coriander Volatile Compounds against Salmonella choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial Activity of Cuminum Cyminum L. and Carum Carvi L. Essential Oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef]
- Shah, A.A.; Wang, C.; Chung, Y.R.; Kim, J.Y.; Choi, E.S.; Kim, S.W. Enhancement of Geraniol Resistance of Escherichia Coli by MarA over Expression. J. Biosci. Bioeng. 2013, 115, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.K. Antimicrobial Effect of Linalool and A-Terpineol against Periodontopathic and Cariogenic Bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Z.; Yousefi, M.; Mohammadi, M.; Eftekhar, F.; Biniyaz, T.; Rustaiyan, A. Chemical composition and antibacterial activity of the volatile oils from Artemisia turcomanica. Chem. Nat. Compd. 2010, 46, 819–821. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Iacobellis, N.S.; De Marco, A.; Capasso, F.; Senatore, F. Antibacterial Activity of Coriandrum Sativum L. and Foeniculum Vulgare Miller Var. Vulgare (Miller) Essential Oils. J. Agric. Food Chem. 2004, 52, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Ren, H.; Yu, M.; Li, X.; Li, D.; Mu, C. Using oxidized amylose as carrier of linalool for the development of antibacterial wound dressing. Carbohydr. Polym. 2017, 174, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061. [Google Scholar] [CrossRef]
- Lemoigne, M. Advances in Microbial Physiology. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Steinbüchel, A. PHB and Others Polyhydroxyalkanoic Acids; Rehm, H.J., Reed, G., Eds.; Biotechnology: Weinheim, Germany, 1991; pp. 403–464. [Google Scholar]
- Brandl, H.; A Gross, R.; Lenz, R.W.; Fuller, R.C. Plastics from bacteria and for bacteria: Poly(beta-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. Adv. Biochem. Eng. 1990, 41, 77–93. [Google Scholar]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol-enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Wicks, D.A.; Hoyle, C.E. Thiourethane-Based Thiol-Ene High Tg Networks: Preparation, Thermal, Mechanical, and Physical Properties. J. Polym. Sci. Part Polym. Chem. 2007, 45, 5103–5111. [Google Scholar] [CrossRef]
- Lu, H.; Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent. Mater. 2005, 21, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Samuelsson, J.; Jönsson, M.; Brinck, T.; Johansson, M. Thiol-ene coupling reaction of fatty acid monomers. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6346–6352. [Google Scholar] [CrossRef]
- Black, M.; Rawlins, J.W. Thiol–ene UV-curable coatings using vegetable oil macromonomers. Eur. Polym. J. 2009, 45, 1433–1441. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Patani, R.; Wu, J.F.; Fernando, S.; Jogodzinski, K.; Webster, D.C. Soy-based UV-curable thiol–ene coatings. J. Coat. Technol. Res. 2010, 7, 603–613. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Monomers and polymers from plant oils via click chemistry reactions. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2111–2124. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. The Thiol-Ene (Click) Reaction for the Synthesis of Plant Oil Derived Polymers. Eur. J. Lipid Sci. Technol. 2013, 115, 41–54. [Google Scholar] [CrossRef]
- Hearon, K.; Nash, L.D.; Rodriguez, J.N.; Lonnecker, A.T.; Raymond, J.E.; Wilson, T.S.; Wooley, K.L.; Maitland, D.J. A High-Performance Recycling Solution for Polystyrene Achieved by the Synthesis of Renewable Poly(Thioether) Networks Derived from d-Limonene. Adv. Mater. 2014, 26, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Modjinou, T.; Versace, D.-L.; Abbad-Andallousi, S.; Bousserrhine, N.; Babinot, J.; Langlois, V.; Renard, E. Antibacterial Networks Based on Isosorbide and Linalool by Photoinitiated Process. ACS Sustain. Chem. Eng. 2015, 3, 1094–1100. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andallousi, S.; Bousserrhine, N.; Dubot, P.; Langlois, V.; Renard, E. Antibacterial and antioxidant bio-based networks derived from eugenol using photo-activated thiol-ene reaction. React. Funct. Polym. 2016, 101, 47–53. [Google Scholar] [CrossRef]
- Link, L.A.; Lonnecker, A.T.; Hearon, K.; Maher, C.A.; Raymond, J.E.; Wooley, K.L. Photo-cross-linked Poly(thioether-co-carbonate) Networks Derived from the Natural Product Quinic Acid. ACS Appl. Mater. Interfaces 2014, 6, 17370–17375. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.-J. The use of renewable feedstock in UV-curable materials – A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Theory of Rubber Elasticity. Available online: https://doi.org/10.1016/0032-3861(79)90268-4 (accessed on 17 January 2020).
- Lorenzini, C.; Versace, D.; Renard, E.; Langlois, V. Renewable epoxy networks by photoinitiated copolymerization of poly(3-hydroxyalkanoate)s and isosorbide derivatives. React. Funct. Polym. 2015, 93, 95–100. [Google Scholar] [CrossRef]
- Feng, X.; East, A.J.; Hammond, W.; Zhang, Y.; Jaffe, M. Overview of Advances in Sugar-Based Polymers. Polym. Adv. Technol. 2011, 22, 139–150. [Google Scholar] [CrossRef]
- Lorenzini, C.; Versace, D.; Gaillet, C.; Lorthioir, C.; Boileau, S.; Renard, E.; Langlois, V. Hybrid networks derived from isosorbide by means of thiol-ene photoaddition and sol–gel chemistry. Polymer 2014, 55, 4432–4440. [Google Scholar] [CrossRef]
- Mangeon, C.; Modjinou, T.; De Anda, A.R.; Thevenieau, F.; Renard, E.; Langlois, V. Renewable Semi-Interpenetrating Polymer Networks Based on Vegetable Oils Used as Plasticized Systems of Poly(3-hydroxyalkanoate)s. ACS Sustain. Chem. Eng. 2018, 6, 5034–5042. [Google Scholar] [CrossRef]
- Franklin, T.J.; Snow, G.A. Biochemistry and Molecular Biology of Antimicrobial Drug Action; Chapman & Hall LTD: London, UK, 1981. [Google Scholar]
- Sussman, M. Escherichia Coli: Mechanisms of Virulence; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]

| Polymers | Yield (%) | Mn 1 (g·mol−1) | Mn 2 (g·mol−1) | Ð 2 | Tm 3 (°C) | Tg 3 (°C) | T5% 4 (°C) |
|---|---|---|---|---|---|---|---|
| PHBHV | - | - | 80,000 | 2.6 | 150 | 4 | 264 |
| α,ω-dihydroxylic PHBHV | 80 | 1000 | 1750 | 1.6 | 119 | −27 | 255 |
| α,ω-diallylic PHBHV | 90 | 1220 | 2070 | 1.5 | 121 | −5 | 243 |
| Cross-Linking Agent | TriSH | TetraSH | ||||
|---|---|---|---|---|---|---|
| PHBHV-L (w/w%) | 0–100 | 30–70 | 50–50 | 0–100 | 30–70 | 50–50 |
| C=C Conversion 1(%) | 94 | 99 | 98 | 77 | 70 | 75 |
| Reaction kinetics 1(s−1) | 12.2 | 11.9 | 13.7 | 3.0 | 1.7 | 2.1 |
| Composition PHBHV-L (w/w %) | Water Uptake 1 (%) | Soluble Extract 2 (%) | Θ3 (°) | |
|---|---|---|---|---|
| 0–100 | 2.9 | 6.2 ± 0.7 | - | |
| TriSH | 30–70 | 2.7 | 7.0 ± 0.6 | 82 |
| 50–50 | 2.9 | 3.5 ± 0.6 | 87 | |
| 0–100 | 1.5 | 2.6 ± 0.5 | 80 | |
| TetraSH | 30–70 | 1.1 | 1.4 ± 0.2 | 97 |
| 50–50 | 1.6 | 5.2 ± 0.7 | 99 |
| Cross-Linking Agent | TriSH | TetraSH | |
|---|---|---|---|
| Composition PHBHV-L | 50–50 | 30–70 | 50–50 |
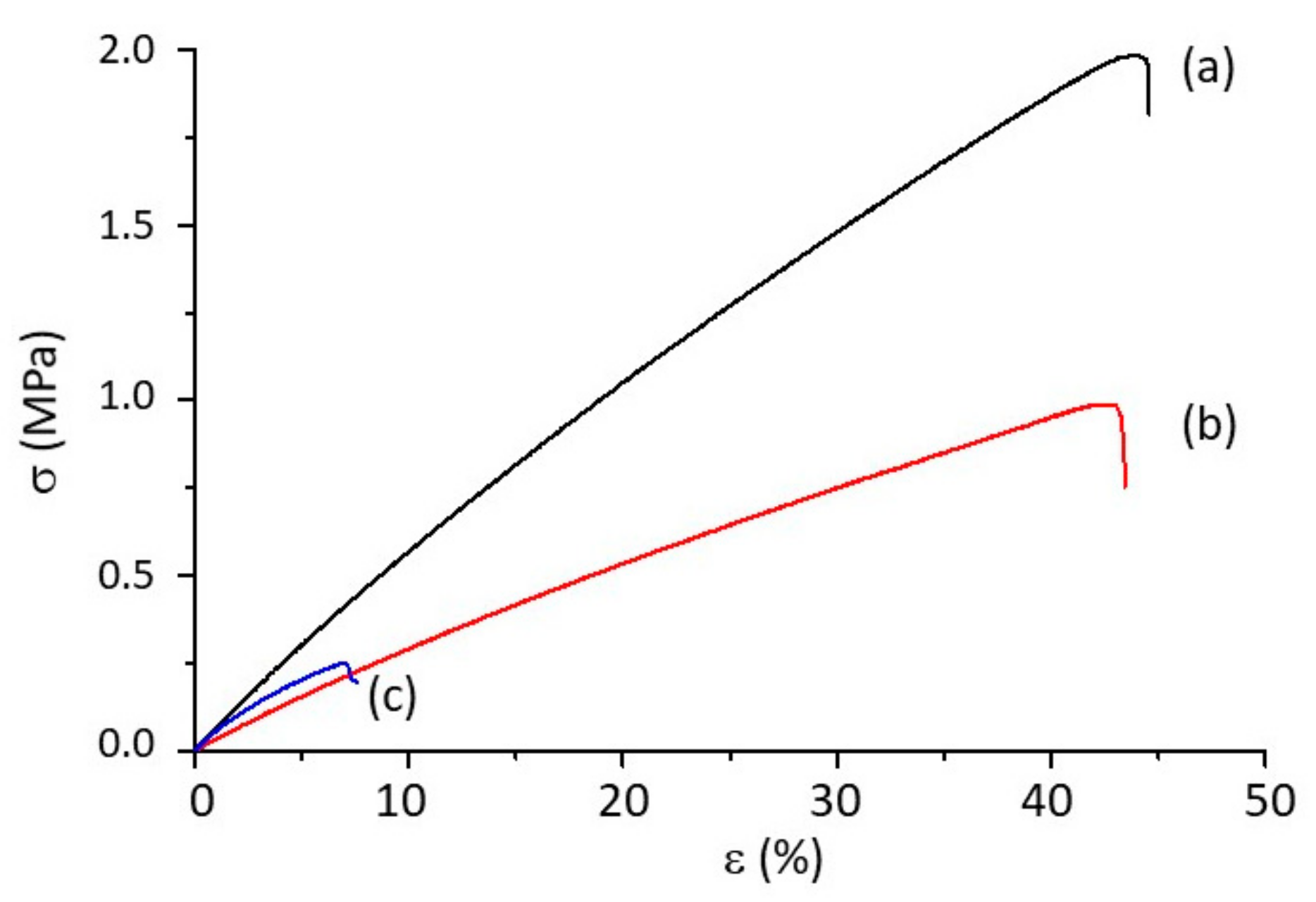
| E 1 (MPa) | 4.7 ± 0.7 | 2.9 ± 0.1 | 5.8 ± 0.4 |
| σr 1 (MPa) | 0.3 ± 0.1 | 1.0 ± 0.1 | 1.8 ± 0.1 |
| εr 1 (%) | 6.9 ± 0.4 | 42.7 ± 4.3 | 41.1 ± 1.7 |
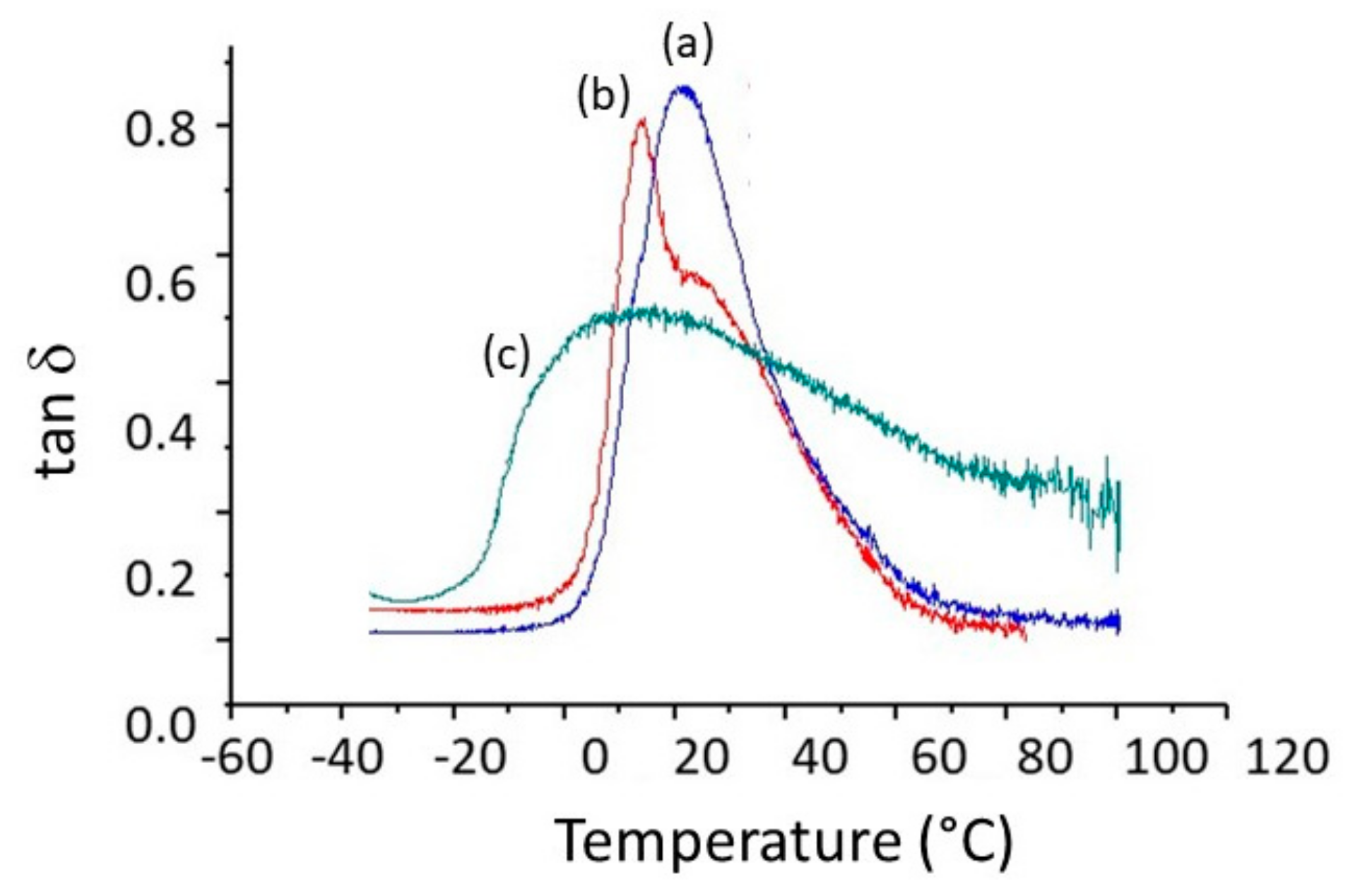
| E’ 2 (MPa) | 3.1 ± 0.1 | 1.6 ± 0.7 | 2.6 ± 0.8 |
| tanδ 2 (°C) | 14 ± 2 | 15 ± 1 | 21 ± 1 |
| Lα 3 (°C) | 84 ± 2 | 28 ± 1 | 28 ± 1 |
| hα 4 | 0.4 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| νe 5 (mol.cm−3) × 10 | 0.40 ± 0.1 | 0.21 ± 0.1 | 0.33 ± 0.1 |
| Ts 6 (°C) | 151.7 | 152.0 | 155.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modjinou, T.; Versace, D.L.; Abbad Andaloussi, S.; Langlois, V.; Renard, E. Co-Networks Poly(hydroxyalkanoates)-Terpenes to Enhance Antibacterial Properties. Bioengineering 2020, 7, 13. https://doi.org/10.3390/bioengineering7010013
Modjinou T, Versace DL, Abbad Andaloussi S, Langlois V, Renard E. Co-Networks Poly(hydroxyalkanoates)-Terpenes to Enhance Antibacterial Properties. Bioengineering. 2020; 7(1):13. https://doi.org/10.3390/bioengineering7010013
Chicago/Turabian StyleModjinou, Tina, Davy Louis Versace, Samir Abbad Andaloussi, Valérie Langlois, and Estelle Renard. 2020. "Co-Networks Poly(hydroxyalkanoates)-Terpenes to Enhance Antibacterial Properties" Bioengineering 7, no. 1: 13. https://doi.org/10.3390/bioengineering7010013
APA StyleModjinou, T., Versace, D. L., Abbad Andaloussi, S., Langlois, V., & Renard, E. (2020). Co-Networks Poly(hydroxyalkanoates)-Terpenes to Enhance Antibacterial Properties. Bioengineering, 7(1), 13. https://doi.org/10.3390/bioengineering7010013





