Comparison of the Anabolic Effects of Reported Osteogenic Compounds on Human Mesenchymal Progenitor-Derived Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Compound Preparation
2.3. Metabolic Activity
2.4. Alkaline Phosphatase Activity and DNA Quantification
2.5. Calcium and Collagen Quantification
2.6. Statistical Analysis
3. Results
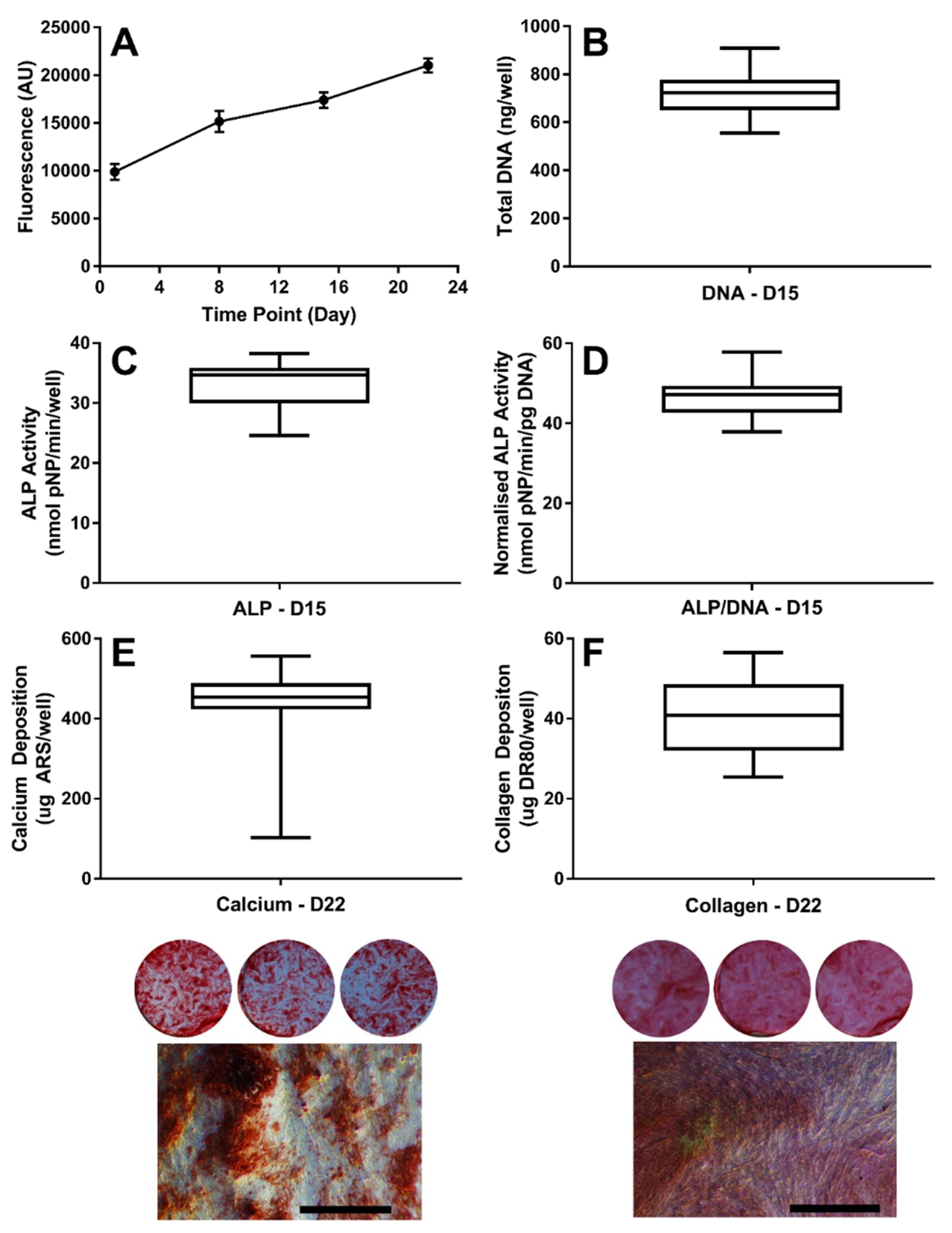
3.1. Establishing the Baseline Response of hES-MPs to Osteogenic Conditions
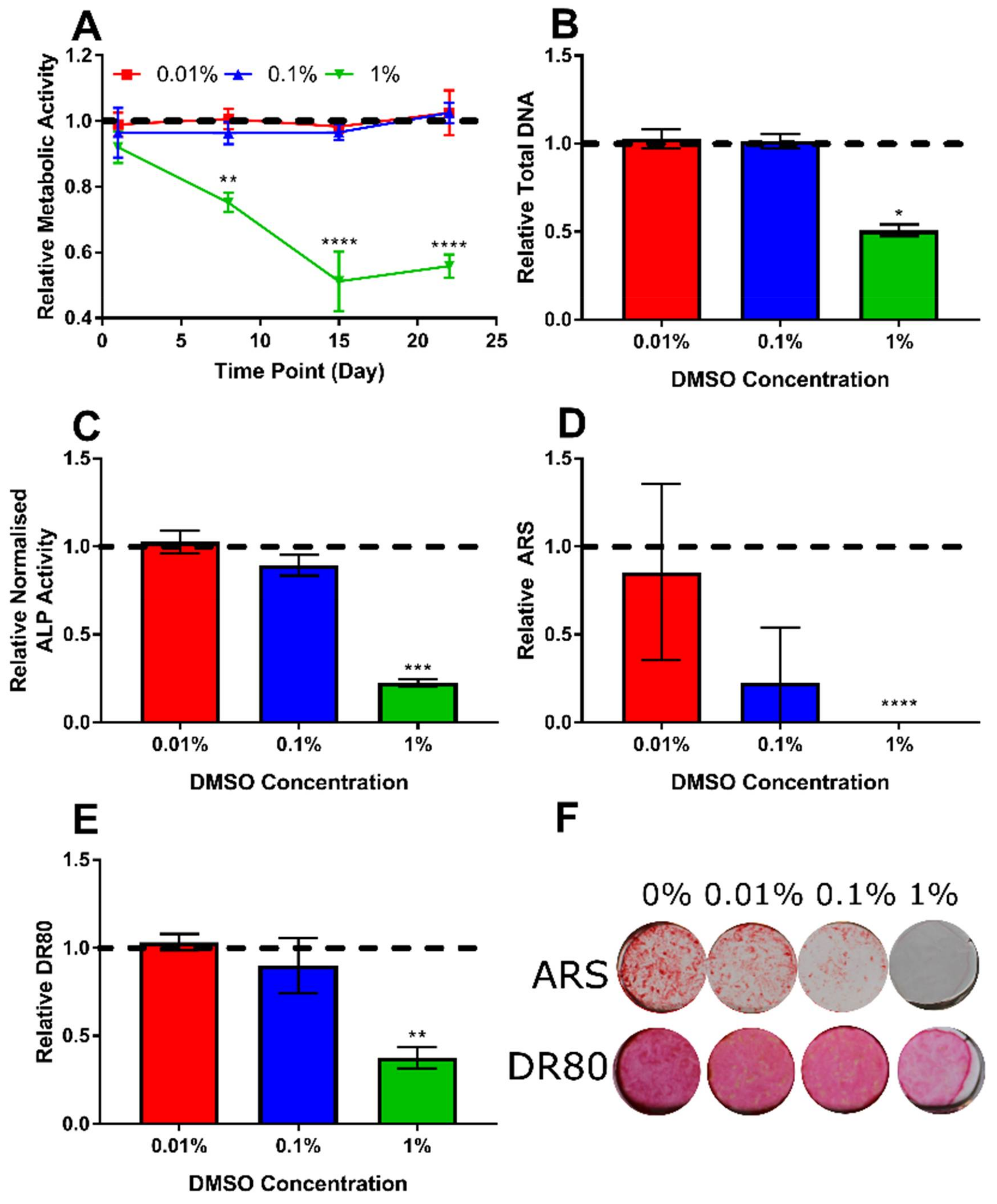
3.2. Determination of Appropriate Concentration of the DMSO Vehicle
3.3. The Highest Concentrations of Lactoferrin and Lithium Chloride SSignificantly Reduce Metabolic Activity
3.4. Total DNA Confirms High Concentrations of Lactoferrin and Lithium Chloride Reduce Cell Number
3.5. The Highest Concentrations of Lithium Chloride Significantly Enhance ALP Activity
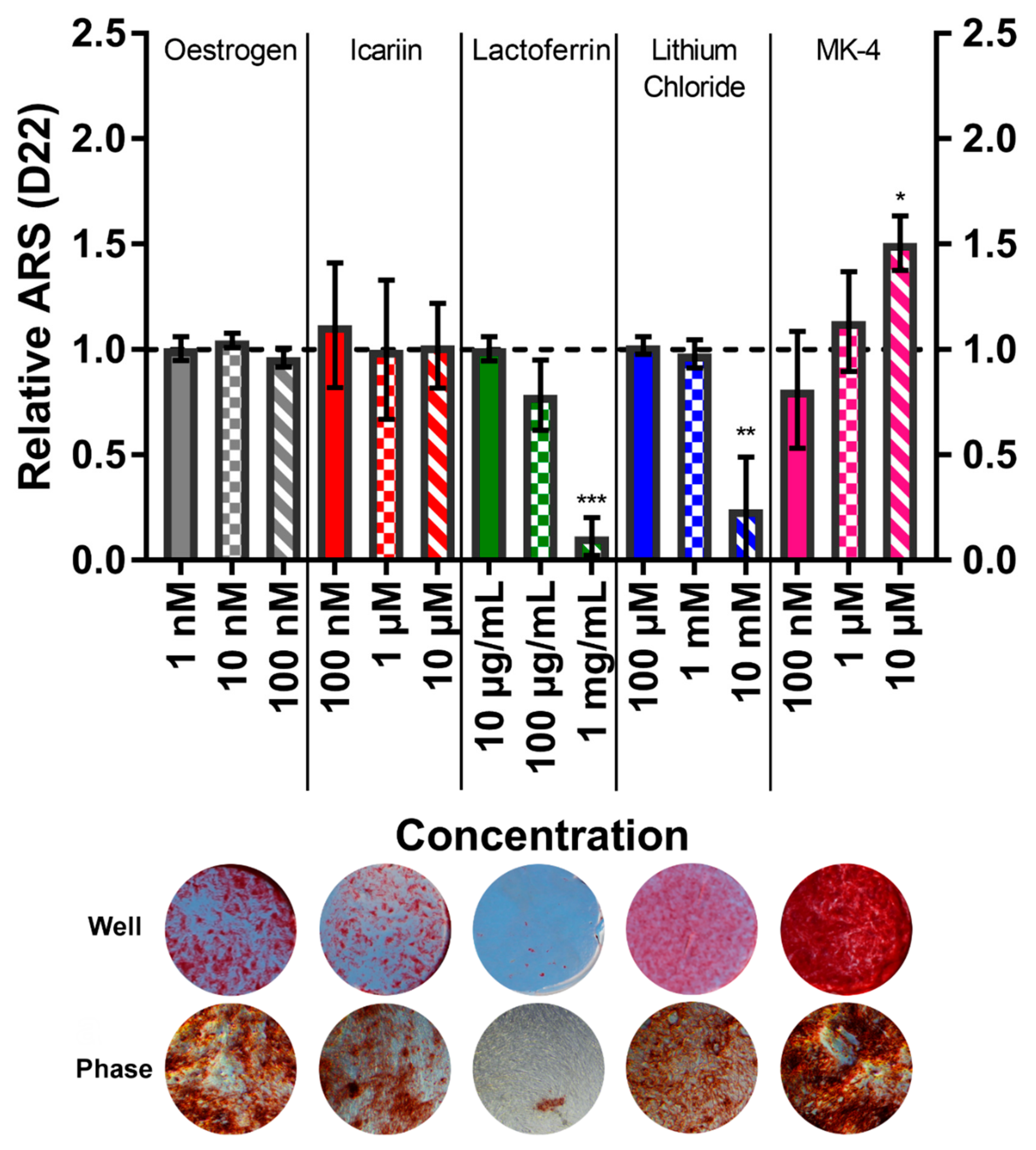
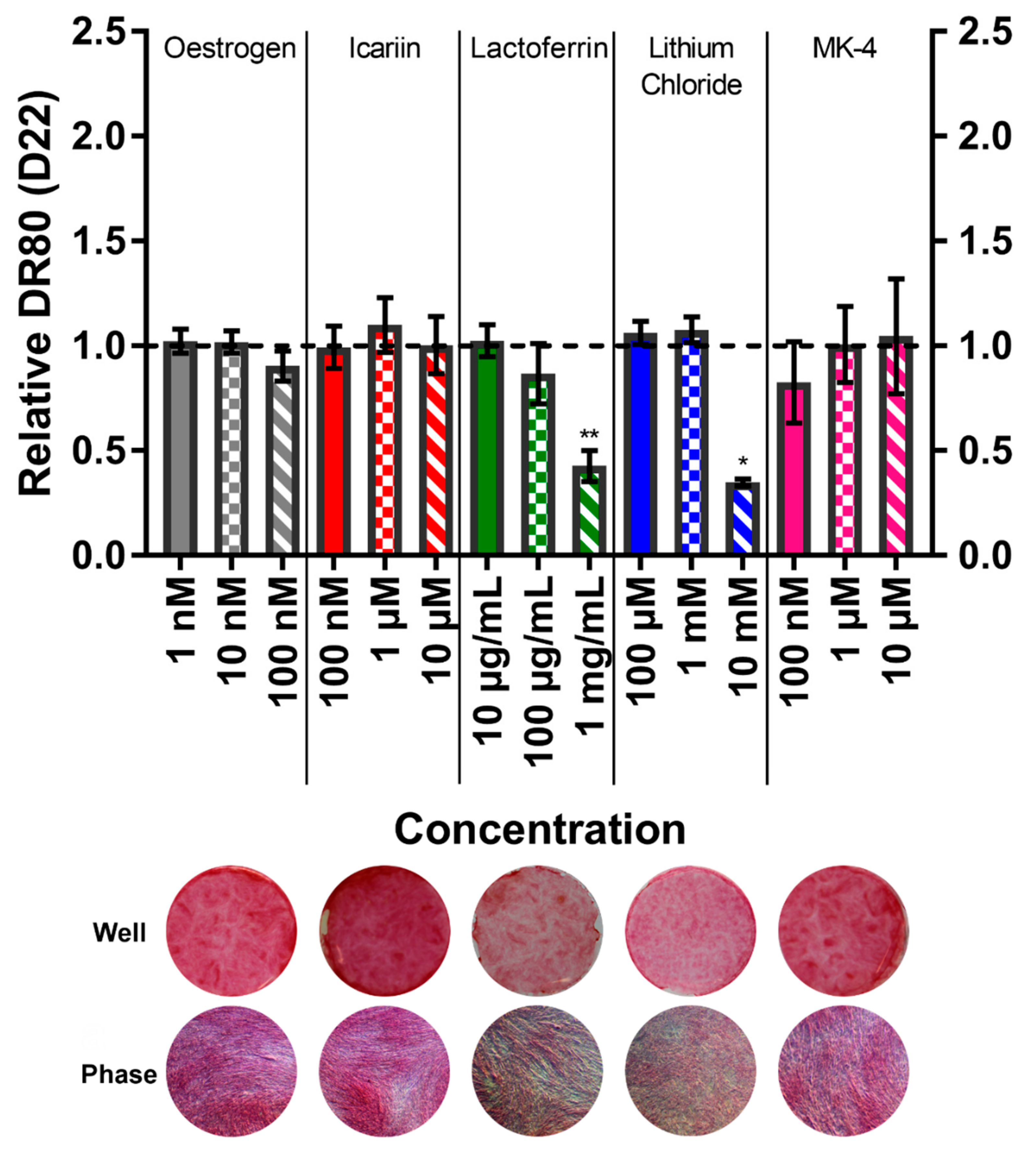
3.6. Menaquinone-4 Significantly Increases Mineral Deposition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Iordachescu, A.; Williams, R.L.; Hulley, P.A.; Grover, L.M. Organotypic Culture of Bone-Like Structures Using Composite Ceramic-Fibrin Scaffolds. Curr. Protoc. Stem Cell Biol. 2019, 48, e79. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K. and Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Diefenderfer, D.L.; Osyczka, A.M.; Reilly, G.C.; Leboy, P.S. BMP Responsiveness in Human Mesenchymal Stem Cells. Connect. Tissue Res. 2003, 44, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Liu, L.J.F.; Murray, T.M.; McDermott, E.; Zhang, X. Estrogen added intermittently, but not continuously, stimulates differentiation and bone formation in SaOS-2 cells. Biol. Pharm. Bull. 2003, 26, 936–945. [Google Scholar] [CrossRef]
- Robinson, J.A.; Harris, S.A.; Riggs, B.L.; Spelsberg, T.C. Estrogen regulation of human osteoblastic cell proliferation and differentiation. Endocrinology 1997, 138, 2919–2927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, F.P.; Lee, N.; Wang, K.C.; Soong, Y.K.; Huang, K.E. Effect of estrogen and 1α, 25 (OH) 2-vitamin D3 on the activity and growth of human primary osteoblast-like cells in vitro. Fertil. Steril. 2002, 77, 1038–1043. [Google Scholar] [CrossRef]
- Patlas, N.; Zadik, Y.; Yaffe, P.; Patlas, M.; Schwartz, Z.; Ornoy, A. The response to sex steroid hormones and vitamin D of cultured osteoblasts derived from ovariectomized mice with and without 17β-estradiol pretreatment. Odontology 2005, 93, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B. Effects of low doses of estrone on the proliferation, differentiation and mineralization of osteoprecursor cells. Exp. Ther. Med. 2012, 4, 681–684. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- De Peppo, G.M.; Svensson, S.; Lennerås, M.; Synnergren, J.; Stenberg, J.; Strehl, R.; Hyllner, J.; Thomsen, P.; Karlsson, C. Human Embryonic Mesodermal Progenitors Highly Resemble Human Mesenchymal Stem Cells and Display High Potential for Tissue Engineering Applications. Tissue Eng. Part A 2010, 16, 2161–2182. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.R.E.; Zhang, R.; How, S.E.; Lilienkampf, A.; De Sousa, P.A.; Bradley, M. Long term mesenchymal stem cell culture on a defined synthetic substrate with enzyme free passaging. Biomaterials 2014, 35, 5998–6005. [Google Scholar] [CrossRef] [PubMed]
- De Peppo, G.M.; Sjovall, P.; Lennerås, M.; Strehl, R.; Hyllner, J.; Thomsen, P.; Karlsson, C. Osteogenic Potential of Human Mesenchymal Stem Cells and Human Embryonic Stem Cell-Derived Mesodermal Progenitors: A Tissue Engineering Perspective. Tissue Eng. Part A 2010, 16, 3413–3426. [Google Scholar] [CrossRef]
- Puwanun, S. Developing a tissue engineering strategy for cleft palate repair. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2014. [Google Scholar]
- Owen, R.; Sherborne, C.; Paterson, T.; Green, N.H.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds with tunable mechanical properties for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 54, 159–172. [Google Scholar] [CrossRef]
- Owen, R.; Sherborne, C.; Reilly, G.C.; Claeyssens, F. Data for the analysis of PolyHIPE scaffolds with tunable mechanical properties for bone tissue engineering. Data Brief 2015, 5, 616–620. [Google Scholar] [CrossRef]
- Paterson, T.E.; Gigliobianco, G.; Sherborne, C.; Green, N.H.; Dugan, J.M.; MacNeil, S.; Reilly, G.C.; Claeyssens, F. Porous microspheres support mesenchymal progenitor cell ingrowth and stimulate angiogenesis. APL Bioeng. 2018, 2, 026103. [Google Scholar] [CrossRef]
- Tetteh, G.; Khan, A.; Delaine-Smith, R.; Reilly, G.; Rehman, I. Electrospun polyurethane/hydroxyapatite bioactive Scaffolds for bone tissue engineering: The role of solvent and hydroxyapatite particles. J. Mech. Behav. Biomed. Mater. 2014, 39, 95–110. [Google Scholar] [CrossRef]
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, I.U. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef]
- Puwanun, S.; Bye, F.J.; Ireland, M.M.; MacNeil, S.; Reilly, G.C.; Green, N.H. Production and Characterization of a Novel, Electrospun, Tri-Layer Polycaprolactone Membrane for the Segregated Co-Culture of Bone and Soft Tissue. Polymers 2016, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Bye, F.J.; Bissoli, J.; Black, L.; Bullock, A.J.; Puwanun, S.; Moharamzadeh, K.; Reilly, G.C.; Ryan, A.J.; MacNeil, S. Development of bilayer and trilayer nanofibrous/microfibrous scaffolds for regenerative medicine. Biomater. Sci. 2013, 1, 942–951. [Google Scholar] [CrossRef]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Rao, P.S.; Reilly, G.C. Design and Assessment of a Dynamic Perfusion Bioreactor for Large Bone Tissue Engineering Scaffolds. Appl. Biochem. Biotechnol. 2018, 185, 555–563. [Google Scholar] [CrossRef]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef]
- Duffy, C.R.E.; Zhang, R.; How, S.-E.; Lilienkampf, A.; Tourniaire, G.; Hu, W.; West, C.C.; De Sousa, P.; Bradley, M. A high-throughput polymer microarray approach for identifying defined substrates for mesenchymal stem cells. Biomater. Sci. 2014, 2, 1683–1692. [Google Scholar] [CrossRef]
- Antonini, L.M.; Kothe, V.; Reilly, G.C.; Owen, R.; Marcuzzo, J.S.; Malfatti, C.D.F. Effect of Ti6Al4V surface morphology on the osteogenic differentiation of human embryonic stem cells. J. Mater. Res. 2017, 32, 3811–3821. [Google Scholar] [CrossRef]
- Delaine-Smith, R.M.; MacNeil, S.; Reilly, G.C. Matrix production and collagen structure are enhanced in two types of osteogenic progenitor cells by a simple fluid shear stress stimulus. Eur. Cell Mater. 2012, 24, 162–174. [Google Scholar] [CrossRef]
- Dana, S.; Bettina, H.; Matthias, S.; Andreas, L.; Seiler, A.E. Osteogenic Differentiation of Human Embryonic Stem Cell-Derived Mesenchymal Progenitor Cells as a Model for Assessing Developmental Bone Toxicity In Vitro. Appl. In Vitro Toxicol. 2016, 2, 127–142. [Google Scholar] [CrossRef]
- Brennan, M.; Haugh, M.; O’Brien, F.; McNamara, L. Estrogen Withdrawal from Osteoblasts and Osteocytes Causes Increased Mineralization and Apoptosis. Horm. Metab. Res. 2014, 46, 537–545. [Google Scholar] [CrossRef]
- Hong, L.; Colpan, A.; Peptan, I.A. Modulations of 17-β Estradiol on Osteogenic and Adipogenic Differentiations of Human Mesenchymal Stem Cells. Tissue Eng. 2006, 12, 2747–2753. [Google Scholar] [CrossRef]
- Chen, K.; Ge, B.; Ma, H.; Liu, X.; Bai, M.; Wang, Y. Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharm. Int. J. Pharm. Sci. 2005, 60, 939–942. [Google Scholar]
- Huang, J.; Yuan, L.; Wang, X.; Zhang, T.L.; Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007, 81, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.P.; Ming, L.G.; Ge, B.F.; Zhai, Y.K.; Song, P.; Xian, C.J.; Chen, K.M. Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J. Cell. Biochem. 2011, 112, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.-I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.-M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin Is a Potent Regulator of Bone Cell Activity and Increases Bone Formation in Vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Banovic, T.; Zhu, Q.; Watson, M.; Callon, K.; Palmano, K.; Ross, J.; Naot, D.; Reid, I.R.; Cornish, J. The Low-Density Lipoprotein Receptor-Related Protein 1 Is a Mitogenic Receptor for Lactoferrin in Osteoblastic Cells. Mol. Endocrinol. 2004, 18, 2268–2278. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, J.; Hu, W.; Wang, J.; Yu, T.; Dai, Y.; Li, N. Effects of Recombinant Human Lactoferrin on Osteoblast Growth and Bone Status in Piglets. Anim. Biotechnol. 2017, 29, 90–99. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Tang, L.; Chen, Y.; Pei, F.; Zhang, H. Lithium Chloride Modulates Adipogenesis and Osteogenesis of Human Bone Marrow-Derived Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2015, 37, 143–152. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, L.; Li, J.; Ge, Z.; Dang, X.; Wang, K. Lithium chloride attenuates the abnormal osteogenic/adipogenic differentiation of bone marrow-derived mesenchymal stem cells obtained from rats with steroid-related osteonecrosis by activating the β-catenin pathway. Int. J. Mol. Med. 2015, 36, 1264–1272. [Google Scholar] [CrossRef]
- Rasouli-Ghahroudi, A.A.; Akbari, S.; Najafi-Alishah, M.; Bohloli, M. The Effect of Vitamin K2 on Osteogenic Differentiation of Dental Pulp Stem Cells: An In Vitro Study. Regen. Reconstr. Restor. 2017, 2, 26–29. [Google Scholar]
- Mandatori, D.; Penolazzi, L.; Pipino, C.; Di Tomo, P.; Di Silvestre, S.; Di Pietro, N.; Trevisani, S.; Angelozzi, M.; Ucci, M.; Piva, R.; et al. Menaquinone-4 enhances osteogenic potential of human amniotic fluid mesenchymal stem cells cultured in 2D and 3D dynamic culture systems. J. Tissue Eng. Regen. Med. 2018, 12, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Puwanun, S.; Delaine-Smith, R.M.; Colley, H.; Yates, J.M.; MacNeil, S.; Reilly, G.C. A simple rocker-induced mechanical stimulus upregulates mineralization by human osteoprogenitor cells in fibrous scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 370–381. [Google Scholar] [CrossRef]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J. Bone Miner. Res. 2000, 15, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kawai, S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. Am. J. Med. 2004, 117, 549–555. [Google Scholar] [CrossRef]
- Koitaya, N.; Sekiguchi, M.; Tousen, Y.; Nishide, Y.; Morita, A.; Yamauchi, J.; Gando, Y.; Miyachi, M.; Aoki, M.; Komatsu, M.; et al. Low-dose vitamin K 2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. J. Bone Miner. Metab. 2014, 32, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.B.; Wan, S.L.; Lu, Y.J.; Ning, L.; Liu, C.; Fan, S.W. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporos. Int. 2015, 26, 1175–1186. [Google Scholar] [CrossRef]
- Orimo, H.; Nakamura, T.; Hosoi, T.; Iki, M.; Uenishi, K.; Endo, N.; Ohta, H.; Shiraki, M.; Sugimoto, T.; Suzuki, T.; et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—Executive summary. Arch. Osteoporos. 2012, 7, 3–20. [Google Scholar] [CrossRef]
- Weng, S.J.; Xie, Z.J.; Wu, Z.Y.; Yan, D.Y.; Tang, J.H.; Shen, Z.J.; Li, H.; Bai, B.L.; Boodhun, V.; Dong, X.D.E.; et al. Effects of combined menaquinone-4 and PTH1-34 treatment on osetogenesis and angiogenesis in calvarial defect in osteopenic rats. Endocrine 2019, 63, 376–384. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Q.; Bai, B.L.; Weng, S.J.; Wu, Z.Y.; Xie, Z.J.; Feng, Z.H.; Cheng, L.; Boodhun, V.; Yang, L.; et al. Effects of combined human parathyroid hormone (1–34) and menaquinone-4 treatment on the interface of hydroxyapatite-coated titanium implants in the femur of osteoporotic rats. J. Bone Miner. Metab. 2018, 36, 691–699. [Google Scholar] [CrossRef]
- Sasaki, N.; Kusano, E.; Takahashi, H.; Ando, Y.; Yano, K.; Tsuda, E.; Asano, Y. Vitamin K2 inhibits glucocorticoid-induced bone loss partly by preventing the reduction of osteoprotegerin (OPG). J. Bone Miner. Metab. 2005, 23, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Seki, A.; Sato, Y.; Matsumoto, H.; Tadeda, T.; Yeh, J.K. Vitamin K2 Promotes Bone Healing in a Rat Femoral Osteotomy Model with or without Glucocorticoid Treatment. Calcif. Tissue Int. 2010, 86, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 Regulation of Bone Homeostasis Is Mediated by the Steroid and Xenobiotic Receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef]
- Frandsen, N.E.; Gordeladze, J.O. Vitamin K2-Vital for Health and Wellbeing; IntechOpen: London, UK, 2017; pp. 101–123. [Google Scholar] [CrossRef]
- Lloyd, A.A.; Gludovatz, B.; Riedel, C.; Luengo, E.A.; Saiyed, R.; Marty, E.; Lorich, D.G.; Lane, J.M.; Ritchie, R.O.; Busse, B.; et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 8722–8727. [Google Scholar] [CrossRef] [PubMed]
- Iordachescu, A.; Amin, H.D.; Rankin, S.M.; Williams, R.L.; Yapp, C.; Bannerman, A.; Pacureanu, A.; Addison, O.; Hulley, P.A.; Grover, L.M. Organotypic Bone Culture: An In Vitro Model for the Development of Mature Bone Containing an Osteocyte Network. Adv. Biosyst. 2018, 2, 1870012. [Google Scholar] [CrossRef]
- Follet, H.; Boivin, G.; Rumelhart, C.; Meunier, P. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone 2004, 34, 783–789. [Google Scholar] [CrossRef]
- Owen, R.; Reilly, G.C. In vitro Models of Bone Remodelling and Associated Disorders. Front Bioeng. Biotechnol. 2018, 6, 134, PubMed PMID: 30364287PubMed Central PMCID: PMCPMC6193121. [Google Scholar] [CrossRef]
- Kousteni, S. Nongenotropic, Sex-Nonspecific Signaling through the Estrogen or Androgen Receptors Dissociation from Transcriptional Activity. Cell 2001, 104, 719–730. [Google Scholar] [CrossRef]
- Krum, S.A.; Miranda-Carboni, G.A.; Lupien, M.; Eeckhoute, J.; Carroll, J.S.; Brown, M. Unique ERα cistromes control cell type-specific gene regulation. Mol. Endocrinol. 2008, 22, 2393–2406. [Google Scholar] [CrossRef]
- Glenske, K.; Schuler, G.; Arnhold, S.; Elashry, M.I.; Wagner, A.-S.; Barbeck, M.; Neumann, E.; Müller-Ladner, U.; Schnettler, R.; Wenisch, S. Effects of testosterone and 17β-estradiol on osteogenic and adipogenic differentiation capacity of human bone-derived mesenchymal stromal cells of postmenopausal women. Bone Rep. 2019, 11, 100226. [Google Scholar] [CrossRef]
- Liang, W.; Lin, M.; Li, X.; Li, C.; Gao, B.; Gan, H.; Yang, Z.; Lin, X.; Liao, L.; Yang, M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int. J. Mol. Med. 2012, 30, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Naot, D.; Grey, A.; Reid, I.R.; Cornish, J. Lactoferrin — A Novel Bone Growth Factor. Clin. Med. Res. 2005, 3, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, H.; Jing, H.; Li, Y.; Wang, X.; Zhang, H.; Jiang, L.; Ren, F. Lactoferrin Stimulates Osteoblast Differentiation Through PKA and p38 Pathways Independent of Lactoferrin’s Receptor LRP1. J. Bone Miner. Res. 2014, 29, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Schäck, L.M.; Noack, S.; Winkler, R.; Wißmann, G.; Behrens, P.; Wellmann, M.; Jagodzinski, M.; Krettek, C.; Hoffmann, A. The Phosphate Source Influences Gene Expression and Quality of Mineralization during In Vitro Osteogenic Differentiation of Human Mesenchymal Stem Cells. PLoS ONE 2013, 8, e65943. [Google Scholar] [CrossRef]
- Takayama, Y.; Mizumachi, K. Effect of Bovine Lactoferrin on Extracellular Matrix Calcification by Human Osteoblast-Like Cells. Biosci. Biotechnol. Biochem. 2008, 72, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Mizumachi, K. Effect of lactoferrin-embedded collagen membrane on osteogenic differentiation of human osteoblast-like cells. J. Biosci. Bioeng. 2009, 107, 191–195. [Google Scholar] [CrossRef]
- Zamani, A.; Omrani, G.R.; Nasab, M.M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Reduced Relative Risk of Fractures Among Users of Lithium. Calcif. Tissue Int. 2005, 77, 1–8. [Google Scholar] [CrossRef]
- Clément-Lacroix, P.; Ai, M.; Morvan, F.; Roman-Roman, S.; Vayssière, B.; Belleville, C.; Estrera, K.; Warman, M.L.; Baron, R.; Rawadi, G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 17406–17411. [Google Scholar] [CrossRef]
- Wheatley, D. Expression of primary cilia in mammalian cells. Cell Biol. Int. 1996, 20, 73–81. [Google Scholar] [CrossRef]
- Yao, R.; Sun, X.; Xie, Y.; Liu, L.; Han, D.; Yao, Y.; Li, H.; Li, Z.; Xu, K. Lithium chloride inhibits cell survival, overcomes drug resistance, and triggers apoptosis in multiple myeloma via activation of the Wnt/β-catenin pathway. Am. J. Transl. Res. 2018, 10, 2610–2618. [Google Scholar] [PubMed]



| Compound | Low | Medium | High | Citation |
|---|---|---|---|---|
| Oestrogen | 1 nM | 10 nM | 100 nM | [5,30,31] |
| Icariin | 100 nM | 1 µM | 10 µM | [32,33,34,35] |
| Lactoferrin | 10 µg/mL (~111 nM) | 100 µg/mL (~1.11 µM) | 1 mg/mL (~11.11 µM) | [36,37,38] |
| Lithium Chloride | 100 µM | 1 mM | 10 mM | [39,40,41] |
| Menaquinone-4 | 100 nM | 1 µM | 10 µM | [42,43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, R.; Bahmaee, H.; Claeyssens, F.; Reilly, G.C. Comparison of the Anabolic Effects of Reported Osteogenic Compounds on Human Mesenchymal Progenitor-Derived Osteoblasts. Bioengineering 2020, 7, 12. https://doi.org/10.3390/bioengineering7010012
Owen R, Bahmaee H, Claeyssens F, Reilly GC. Comparison of the Anabolic Effects of Reported Osteogenic Compounds on Human Mesenchymal Progenitor-Derived Osteoblasts. Bioengineering. 2020; 7(1):12. https://doi.org/10.3390/bioengineering7010012
Chicago/Turabian StyleOwen, Robert, Hossein Bahmaee, Frederik Claeyssens, and Gwendolen C. Reilly. 2020. "Comparison of the Anabolic Effects of Reported Osteogenic Compounds on Human Mesenchymal Progenitor-Derived Osteoblasts" Bioengineering 7, no. 1: 12. https://doi.org/10.3390/bioengineering7010012
APA StyleOwen, R., Bahmaee, H., Claeyssens, F., & Reilly, G. C. (2020). Comparison of the Anabolic Effects of Reported Osteogenic Compounds on Human Mesenchymal Progenitor-Derived Osteoblasts. Bioengineering, 7(1), 12. https://doi.org/10.3390/bioengineering7010012








