Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tissue Preparation
2.3. Nanoindentation
2.4. Immunohistochemistry
2.5. Confocal Microscopy
2.6. Statistical Analysis
3. Results
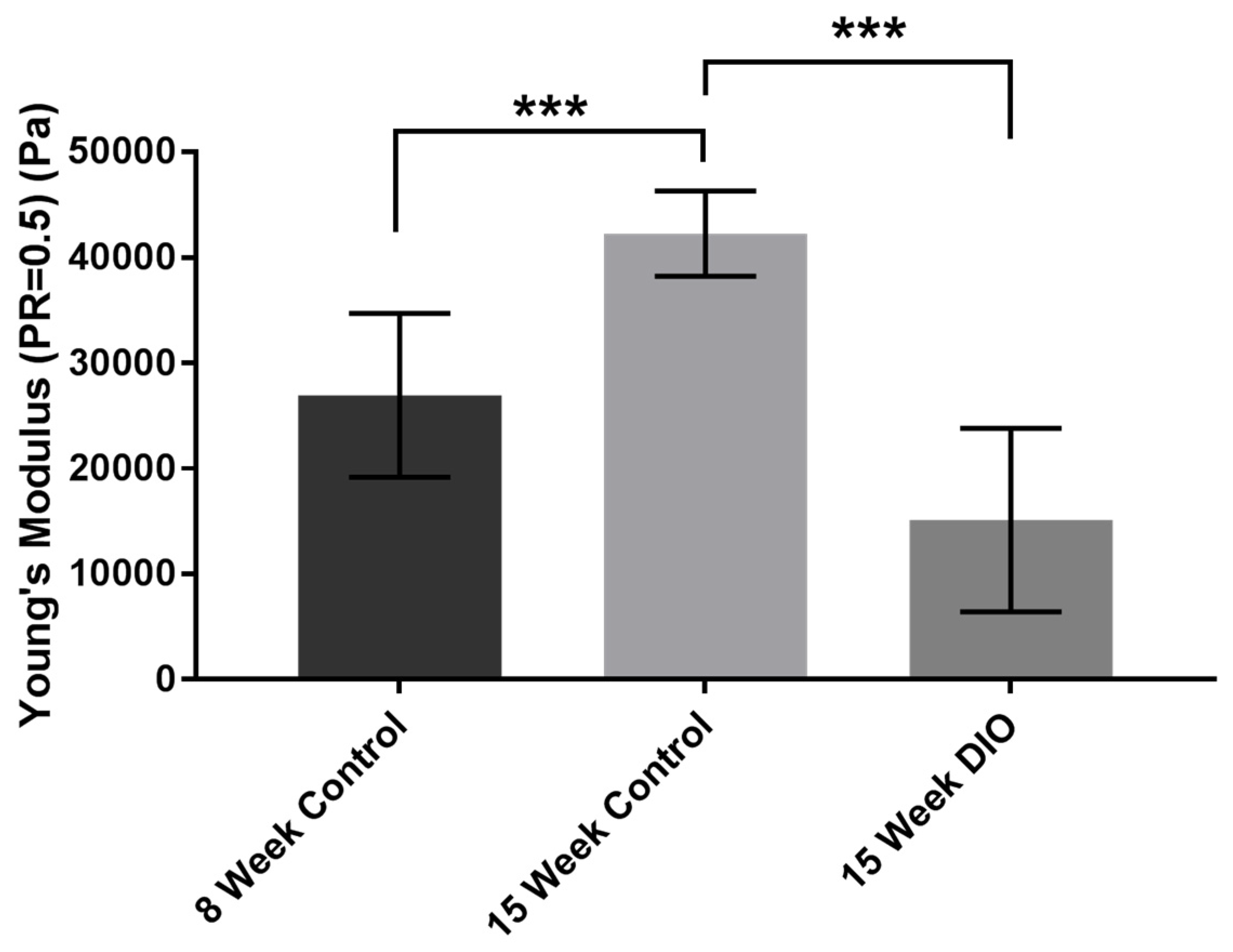
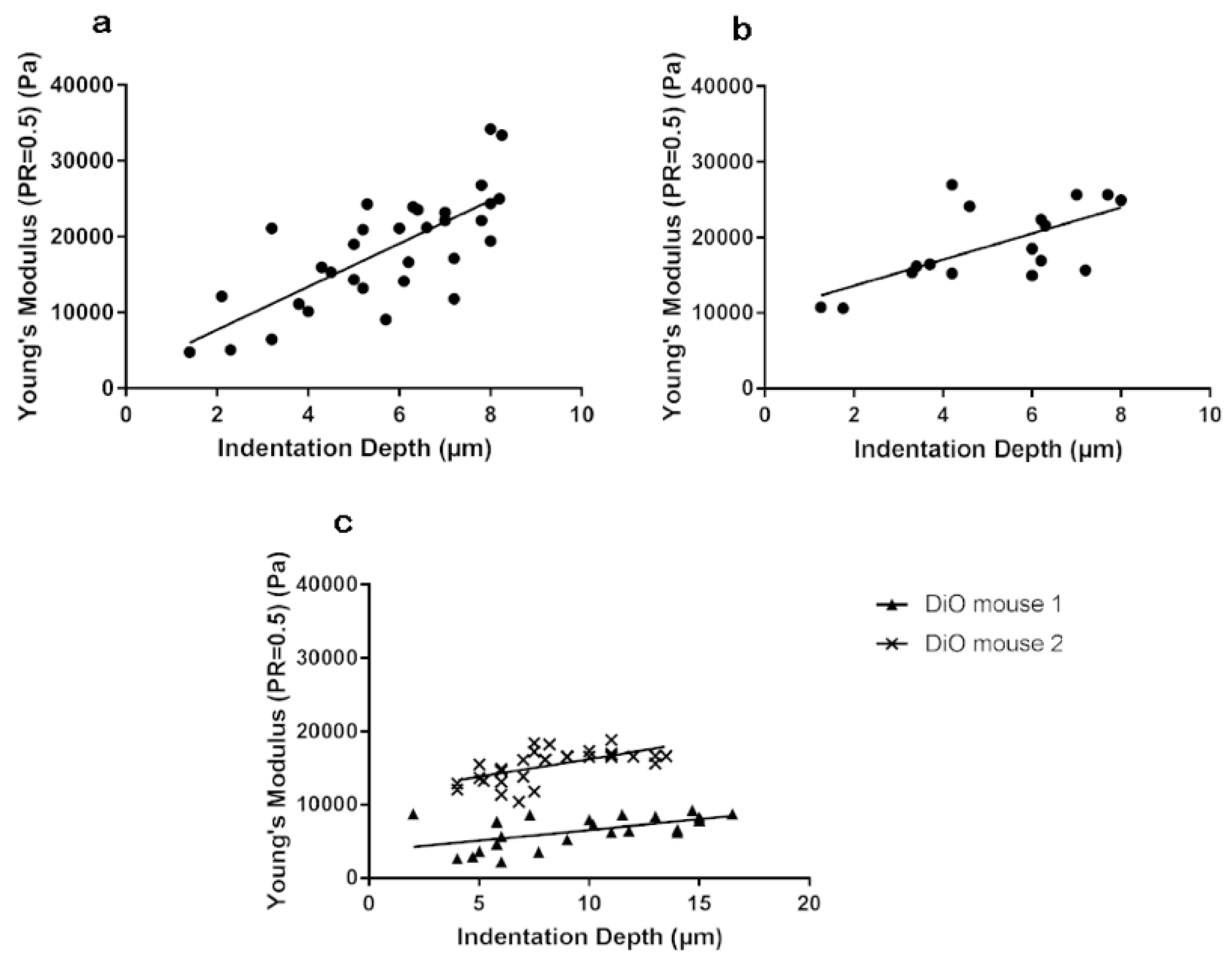
3.1. Stiffness Is Age and Obesity Dependent
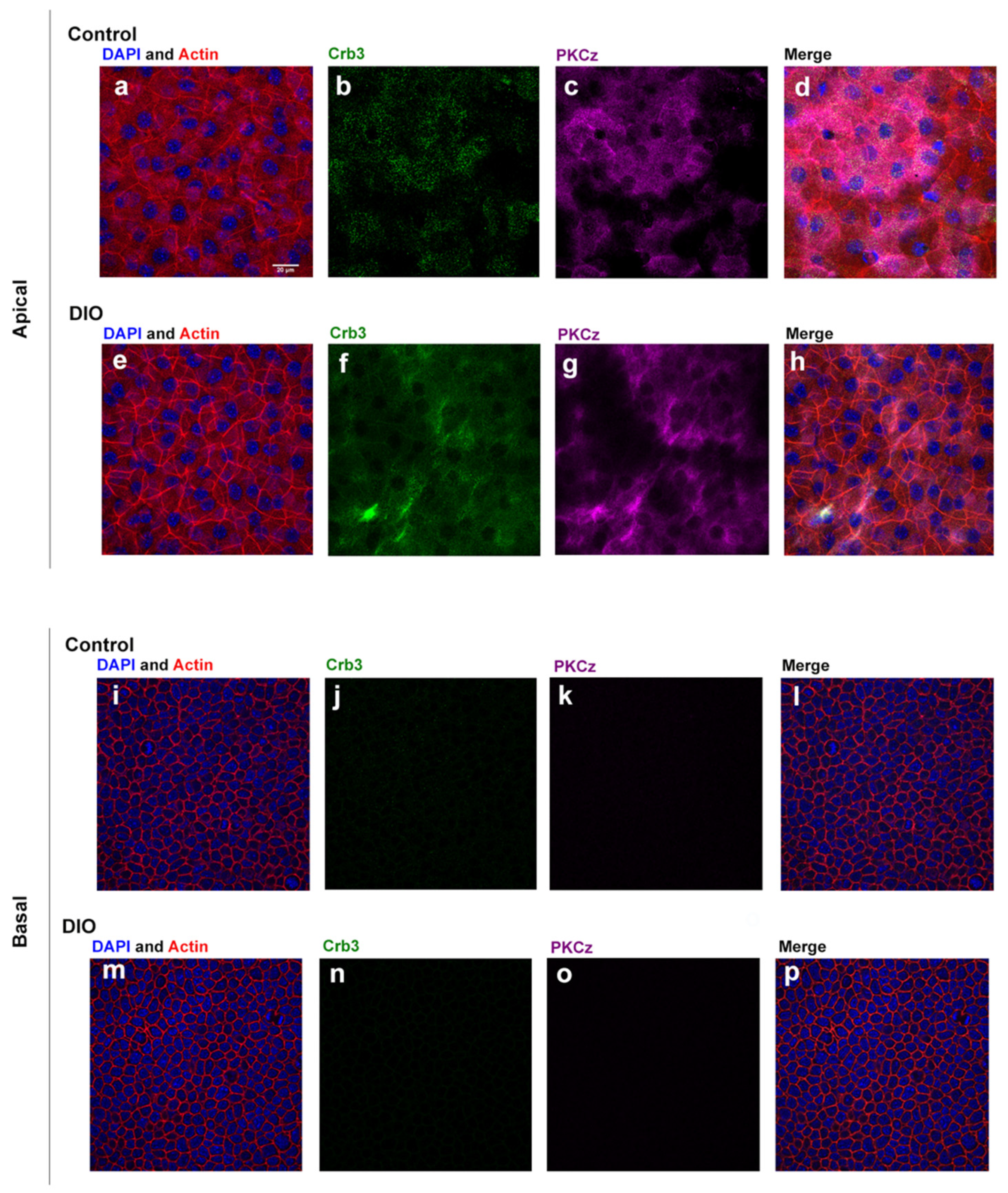
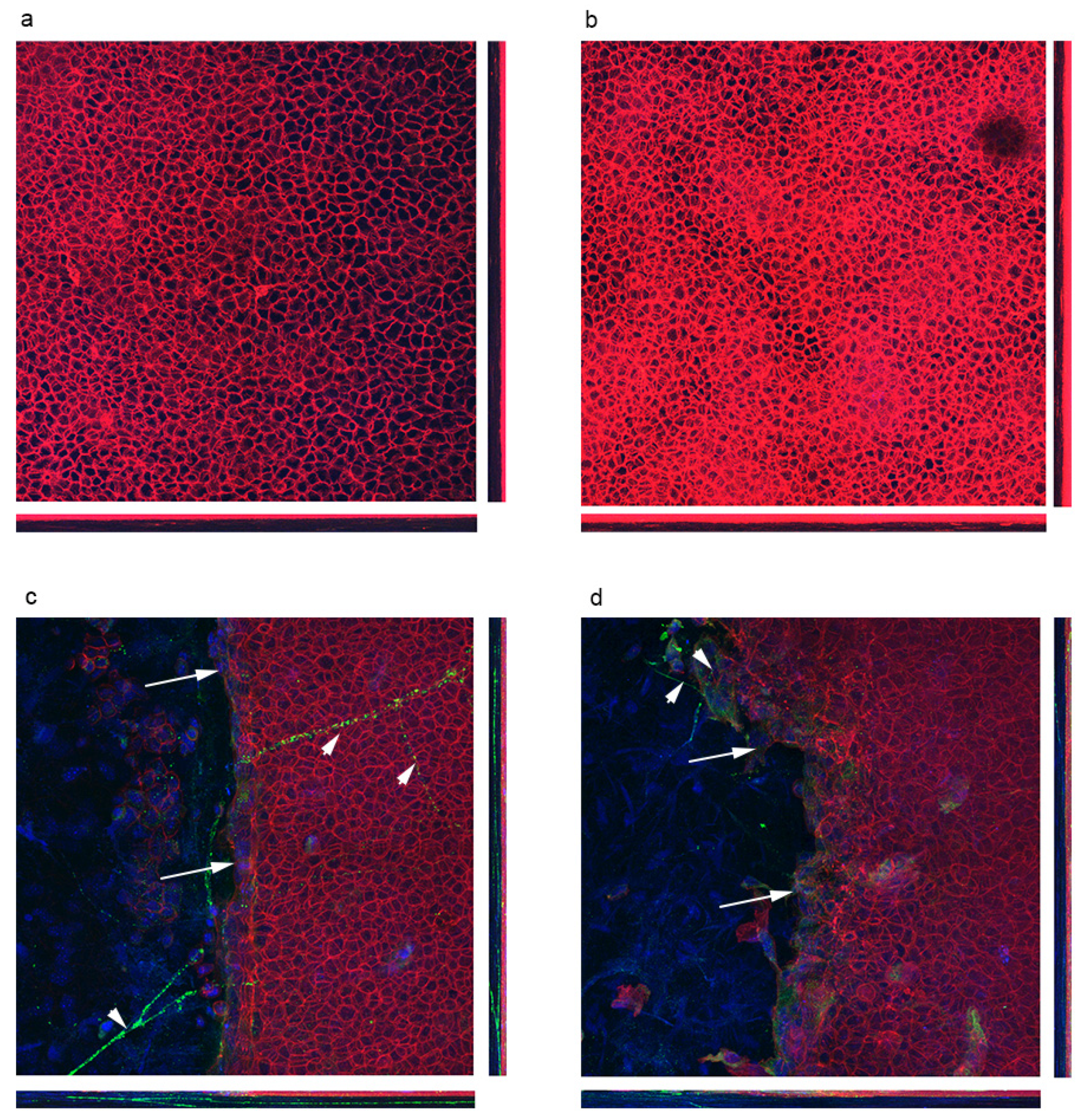
3.2. Localization of Polarity Proteins
3.3. Stiffness of Corneal Basement Membrane and Stroma
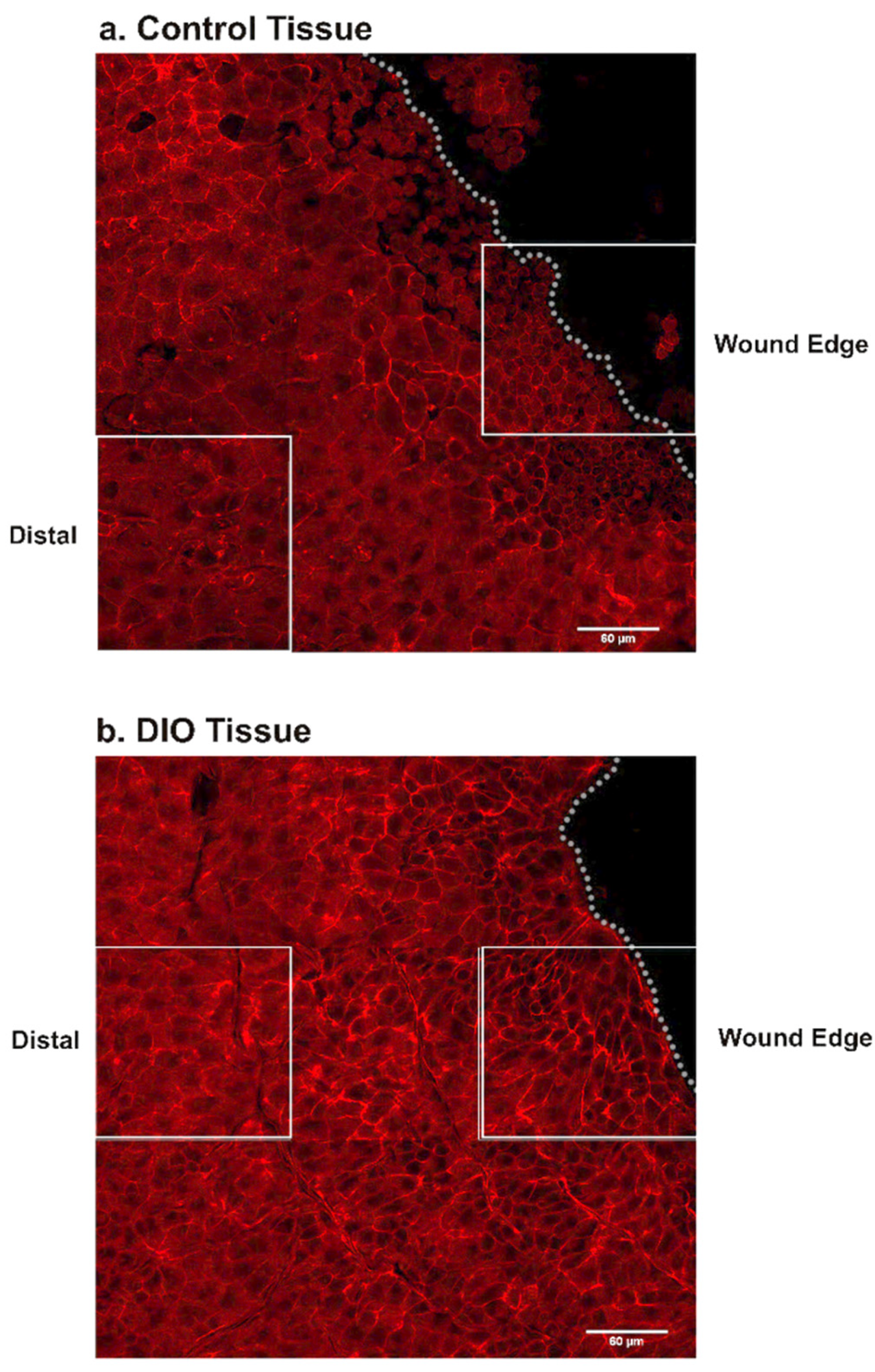
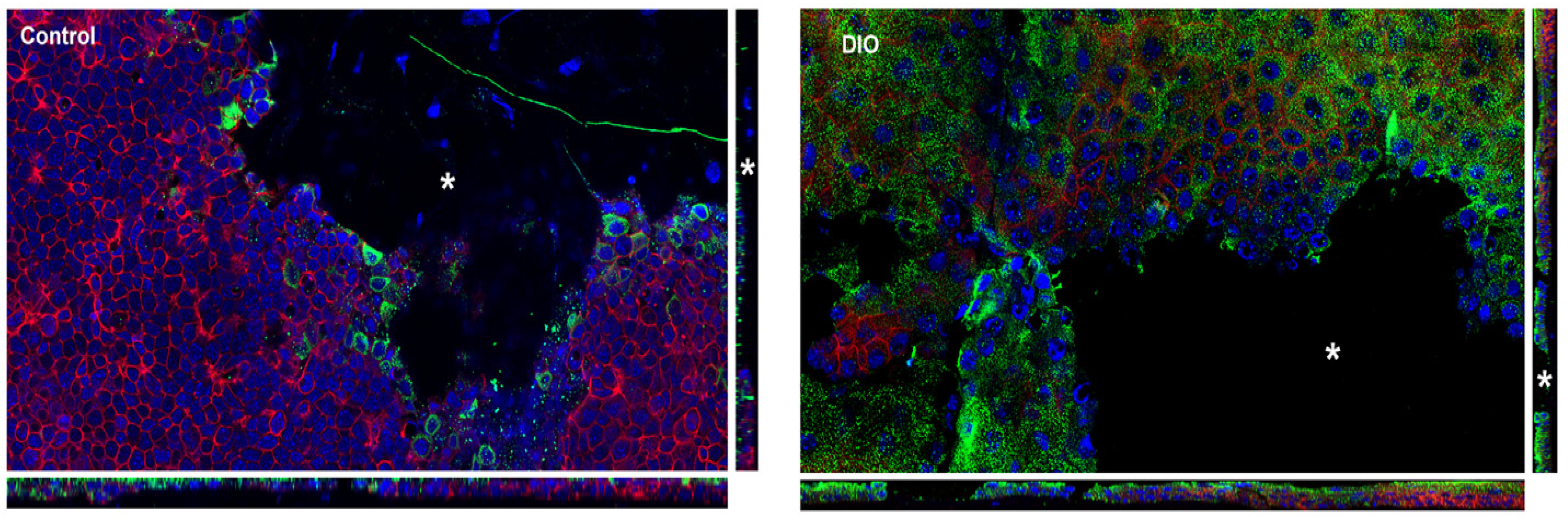
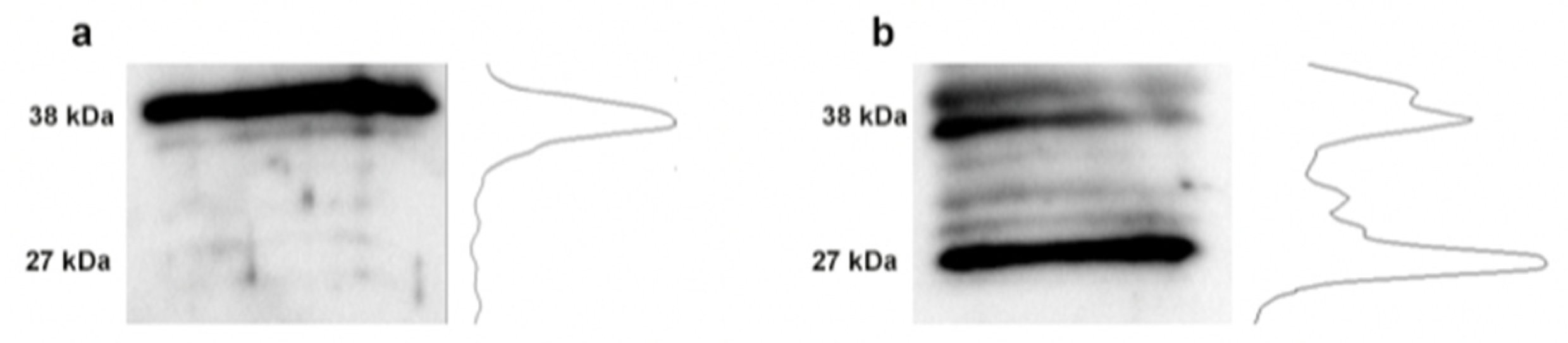
3.4. Changes in Fibronectin
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Resnick, N.; Yahav, H.; Shay-Salit, A.; Shushy, M.; Schubert, S.; Zilberman, L.C.; Wofovitz, E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog. Biophys. Mol. Biol. 2003, 81, 177–199. [Google Scholar] [CrossRef]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef]
- Derricks, K.E.; Trinkaus-Randall, V.; Nugent, M.A. Extracellular matrix stiffness modulates VEGF calcium signaling in endothelial cells: Individual cell and population analysis. Integr. Biol. 2015, 7, 1011–1025. [Google Scholar] [CrossRef]
- Onochie, O.E.; Onyejose, A.J.; Rich, C.B.; Trinkaus-Randall, V. The Role of Hypoxia in Corneal Extracellular Matrix Deposition and Cell Motility. Anat. Rec. 2019. [Google Scholar] [CrossRef]
- Petrie, R.J.; Yamada, K.M. At the leading edge of three-dimensional cell migration. J. Cell Sci. 2012, 125, 5917–5926. [Google Scholar] [CrossRef]
- Sazonova, O.V.; Lee, K.L.; Isenberg, B.C.; Rich, C.B.; Nugent, M.A.; Wong, J.Y. Cell-cell interactions mediate the response of vascular smooth muscle cells to substrate stiffness. Biophys. J. 2011, 101, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Ingber, D.E. Mechanical control of tissue and organ development. Development 2010, 137, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; Iskratsch, T.; Sheetz, M.P. Finding the weakest link—Exploring integrin-mefiated mechanical molecular pathways. J. Cell Sci. 2012, 125, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Allen, S.G.; Ingram, P.N.; Buckanovich, R.; Merajver, S.D.; Yoon, E. Single-cell migration chip for chemotaxis-based microfluidic selection of heterogeneous cell populations. Sci. Rep. 2015, 5, 9980. [Google Scholar] [CrossRef]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef]
- Mak, M.; Spill, F.; Kamm, R.D.; Zaman, M.H. Single-cell migration in complex microenvironments: Mechanics and signaling dynamics. J. Biomech. Eng. 2016, 138, 021004. [Google Scholar] [CrossRef] [PubMed]
- Tambe, D.T.; Hardin, C.C.; Angelini, T.E.; Rajendran, K.; Park, C.Y.; Serra-Picamal, X.; Zhou, E.H.; Zaman, M.H.; Butler, J.P.; Weitz, D.A.; et al. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 2011, 10, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Brugues, A.; Anon, E.; Conte, V.; Veldhuis, J.H.; Gupta, M.; Colombelli, J.; Munoz, J.J.; Brodland, G.W.; Ladoux, B.; Trepat, X. Forces driving epithelial wound healing. Nat. Phys. 2014, 10, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Karamichos, D.; Onochie, O.E.; Hutcheon, A.E.K.; Rich, C.B.; Zieske, J.D.; Trinkaus-Randall, V. Hypoxia modulates the development of a corneal stromal matrix model. Exp. Eye Res. 2018, 170, 127–137. [Google Scholar] [CrossRef]
- Blanco-Mezquita, J.T.; Hutcheon, A.E.; Stepp, M.A.; Zieske, J.D. alphaVbeta6 integrin promotes corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8505–8513. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Minns, M.S.; Teicher, G.; Rich, C.B.; Trinkaus-Randall, V. Purinoreceptor P2X7 Regulation of Ca(2+) Mobilization and Cytoskeletal Rearrangement Is Required for Corneal Reepithelialization after Injury. Am. J. Pathol. 2016, 186, 285–296. [Google Scholar] [CrossRef]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
- Kneer, K.; Green, M.B.; Meyer, J.; Rich, C.B.; Minns, M.S.; Trinkaus-Randall, V. High fat diet induces pre-type 2 diabetes with regional changes in corneal sensory nerves and altered P2X7 expression and localization. Exp. Eye Res. 2018, 175, 44–55. [Google Scholar] [CrossRef]
- Szymaniak, A.D.; Mahoney, J.E.; Cardoso, W.V.; Varelas, X. Crumbs3-mediated polarity directs airway epithelial cell fate through the hippo pathway effector Yap. Dev. Cell 2015, 34, 283–296. [Google Scholar] [CrossRef]
- Payne, J.; Gong, H.; Trinkaus-Randall, V. Tyrosine phosphorylation: A critical component in the formation of hemidesmosomes. Cell Tissue Res. 2000, 300, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Derricks, K.; Minns, M.; Ji, S.; Chi, C.; Nugent, M.A.; Trinkaus-Randall, V. Hypoxia-induced changes in Ca(2+) mobilization and protein phosphorylation implicated in impaired wound healing. Am. J. Physiol. Cell Physiol. 2014, 306, C972–C985. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Żmijewska, A.; Piątkiewicz, P.; Śmigielska, B.; Sokołowska-Oracz, A.; Wawrzyniak, Z.M.; Romaniuk, D.; Szaflik, J.; Szaflik, J.P. Retinal photoreceptors and microvascular changes in prediabetes measured with adaptive optics (rtx1TM): A case-control study. J. Diabetes Res. 2017, 2017, 4174292. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Nelson, W.J. Running with neighbors: Coordinating cell migration and cell-cell adhesion. Curr. Opin. Cell Biol. 2015, 36, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Passini, F.S.; Silván, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaulli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of collagen crosslinking in diabetes and keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Pastel, E.; Price, E.; Sjöholm, K.; McCulloch, L.J.; Rittig, N.; Liversedge, N.; Knight, B.; Møller, N.; Svensson, P.A.; Kos, K. Lysyl oxidase and adipose tissue dysfunction. Metabolism 2018, 78, 118–127. [Google Scholar] [CrossRef]
- Mankus, C.; Chi, C.; Rich, C.; Ren, R.; Trinkaus-Randall, V. The P2X7 receptor regulates proteoglycan expression in the corneal stroma. Mol. Vis. 2012, 18, 128–138. [Google Scholar]
- Onochie, O.E.; Zollinger, A.; Rich, C.B.; Smith, M.; Trinkaus-Randall, V. Epithelial cells exert differential traction stress in response to substrate stiffness. Exp. Eye Res. 2019, 181, 25–37. [Google Scholar] [CrossRef]
- Foolen, J.; Shiu, J.Y.; Mitsi, M.; Zhang, Y.; Chen, C.S.; Vogel, V. Full-length fibronectin drives fibroblast accumulation at the surface of collagen microtissues during cell-induced tissue morphogenesis. PLoS ONE 2016, 11, e0160369. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Londregan, A.; Rich, C.; Trinkaus-Randall, V. Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity. Bioengineering 2020, 7, 14. https://doi.org/10.3390/bioengineering7010014
Xu P, Londregan A, Rich C, Trinkaus-Randall V. Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity. Bioengineering. 2020; 7(1):14. https://doi.org/10.3390/bioengineering7010014
Chicago/Turabian StyleXu, Peiluo, Anne Londregan, Celeste Rich, and Vickery Trinkaus-Randall. 2020. "Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity" Bioengineering 7, no. 1: 14. https://doi.org/10.3390/bioengineering7010014
APA StyleXu, P., Londregan, A., Rich, C., & Trinkaus-Randall, V. (2020). Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity. Bioengineering, 7(1), 14. https://doi.org/10.3390/bioengineering7010014




